Single-incision laparoscopic distal gastrectomy for early gastric cancer
Introduction
Laparoscopic gastrectomy for early gastric cancer has been widely adopted as an alternative treatment option and has been reported to be beneficial for patients, with better early postoperative outcomes and also with compatible long-term oncologic outcomes to open gastrectomy (1,2).
In the meanwhile, efforts are underway to reduce the minimal invasiveness of laparoscopy. Natural orifice transluminal endoscopic surgery (NOTES) and single incision laparoscopic surgery (SILS) is the representative of this effort. While NOTES is still on the research area because of the limitation of equipment and difficulty of closure of incised lumen, there have been explosive reports on single ports surgery in the various clinical surgical fields (3).
Single-incision laparoscopic surgery (SILS) has been introduced to reduce the abdomen wall incision and trauma and the embryonic scar of the umbilicus is easily accessed to the peritoneum without any visible scar. SILS procedures decrease postoperative pain and hospital stay and lead to faster postoperative recovery than conventional laparoscopy apart from better cosmetic aspects (4). Although long-term data are still lacking to prove the outcome SILS, the reported data of oncologic patients undergoing SILS provide the technical feasibility of a single port surgery (5).
However, the feasibility of single-incision laparoscopic distal gastrectomy (SIDG) for early gastric cancer comparing with laparoscopic distal gastrectomy (LDG) has not been demonstrated in the field of gastrectomy.
There have been only 3 reports describing this procedure for patients with early gastric cancer (EGC) (6-8). Furthermore, all of these reports use 1 or 2 additional assistant ports in SIDG (Table 1). The technical difficulty of this operation could be showed by a long operation time comparing with conventional LDG, although the number of retrieved lymph node was not less.
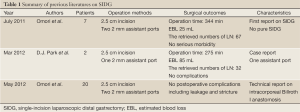
Full Table
Herein, we present briefly our clinical experience of SIDG in Seoul National University Bundang Hospital.
Methods
Study design and data collection
Prospectively maintained database from patients who underwent laparoscopic distal gastrectomy (LDG) due to gastric cancer were reviewed. From October 2010 to January 2013, 30 consecutive patients underwent SIDG for early gastric cancer at Seoul National University Bundang Hospital in Korea. A 2 mm assistant port was used in the initial 10 cases of SIDG. Last 20 cases of SIDG were done without any assistant port (Pure SIDG).
All 30 operations except initial 7 cases of SIDG were done by a single surgeon who had experience of 100 cases of LDG before starting the single port surgery and more than 50 cases of conventional open gastrectomy. In this study, we included patients with a preoperative diagnosis of stage I (7th edition AJCC), in whom no lymph node (LN) enlargement.
Surgical technique
Pure single-incision distal gastrectomy with D1+beta lymph node dissection
The patient was placed in a lithotomy position with reverse Trendelenburg. The operator and a scopist were positioned between the patient’s legs. A longitudinal 2.5-cm long transumbilical skin incision was made. A commercial 4-holes single port (Glove port; Nelis, Bucheon-si, Gyeonggi-do, Korea) was then placed in the umbilical incision, and the abdominal cavity was insufflated with carbon dioxide at a pressure of 13 mmHg. There was no additional assistant trocar. A 10-mm flexible high-definition scope (Endoeye flexible HD camera system; Olympus Medical Systems Corp., Tokyo, Japan) and a Harmonic Scalpel (Ethicon Endo-Surgery Inc., Cincinnati, OH) were used to visualize every corner of the operative field and facilitate dissection. We used the conventional laparoscopic grasper when operating in the greater curvature side and the curved instruments for single port surgery (Olympus Medical Systems Corp.) when operating in the lesser curvature side, including the suprapancreatic LND. Modified combined suture retraction of the falciform ligament and the left lobe of the liver was performed using 2-0 prolene on a straight needle and 5-mm hemoclips (6). Partial omentectomy was initiated distally approximately 3 to 4 cm away from the gastroepiploic arcade, which included the LN 4 d. To prevent omental infarction, the left gastroepiploic vessels were ligated distal to the omental branch. Then, the omentum was dissected and taken down from the mesocolon to the head of the pancreas and duodenum. The right gastroepiploic arcade was approached in a retrograde fashion. We first dissected the space between the duodenum and the basin including the right gastroepiploic vessels and LN station 6 and then detached these from the duodenum and distal stomach. Thus, we could easily dissect and divide the right gastroepiploic area without any significant bleeding. After dissecting LN 6, the right gastric artery and the proper hepatic artery were adequately exposed to dissect LNs 5 and 12a, and the operator exchanged the grasper for the prototype curved instruments. The right gastric artery was then divided at its origin. The duodenum was divided 2 cm distal to the pylorus using a laparoscopic linear stapler (Echelon 60 mm -3.5 and 4.5; Ethicon). LNs 8a and 9, located on the right side of the left gastric artery, were dissected along each artery. The left gastric vein and artery were exposed, individually clipped, and divided to allow dissection of LN 11p. However, we do not expose the portal vein and splenic vein for D2 lymph node dissection. LNs 1, including the vagus nerve, were dissected and the lesser curvature side was cleared up for transecting the stomach. After the transecting the stomach by linear staplers, the specimen was removed in a plastic bag from the single umbilical incision without any extension.
Uncut Roux-en Y Gastro-jejunostomy
For intracorporeal anastomosis, we used laparoscopic flexible linear stapler (Echelon flex 60-3.5 and 4.5; Ethicon Endo-Surgery Inc., Cincinnati, OH). To make an antiperistaltic gastro-jejunostomy, 2 small holes were made on the greater curvature side of the stomach and the jejunum 20 cm distal from Treitz ligament. After the formation of gastro-jejunostomy (G-Jstomy), we checked bleeding in the stapler line and lumen and the common opening was closed with a linear stapler. Next, side-to-side jejuno-jejunostomy (J-Jstomy), 25 cm below the G-Jstomy was performed in an intracorporeal fashion using 2 linear staplers. Finally, a linear stapler with no knife was applied in the afferent loop between the G-Jstomy and J-Jstomy in order to prevent bile reflux. The abdominal cavity was checked, 1 Jackson-Pratt (J-P) drainage tubes was placed through the umbilical wound around the subhepatic area, and the incision was closed.
Results
Patient demographics and clinical characteristics
The demographics of patients are described in Table 2. There were 23 males and 7 females in the SIDG group. The mean age of both groups was 56.2±12.8.
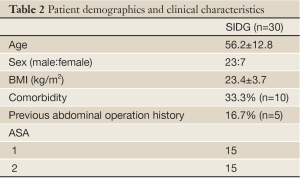
Full Table
Operative data
All the surgeries, involving D1+beta or D2 lymphadenectomy without any laparoscopic or open conversion, were performed by a single surgeon and done in R0 status. The surgical parameters of the both group are shown in Table 3. The mean operation time was calculated from the start of the incision to the closure of the wound and was 175.5±46.6 in the SIDG group. The estimated blood loss was 49.3±40.9 in the SIDG group. There were initial 10 cases of SIDG with one 2 mm assistant port and 20 cases of pure SIDG without any assistant port in the SIDG group. No serious intraoperative events or complications were observed.
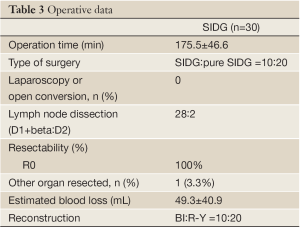
Full Table
Postoperative outcomes
The postoperative outcomes were described in Table 4.
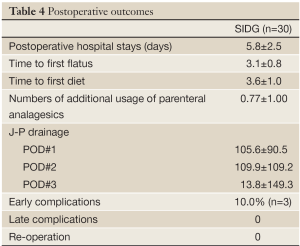
Full Table
The overall early complication occurred in 3 patients (10.0%) in the SIDG group. The early complications included 1 case each of wound seroma, delayed gastric emptying and anastomotic stenosis. The wound complication was improved by conservative management. The delayed gastric emptying was improved by fasting for 5 days. A major complication, defined by a grade higher than Clavien-Dindo IIIa, was observed in 1 patient (3.3%), which was treated by temporary stent insertion.
Pathologic findings
The pathologic findings of this study are shown in Table 5. All the patients were diagnosed with EGC during the preoperative examinations. The number of retrieved lymph nodes was 46.9±12.2.
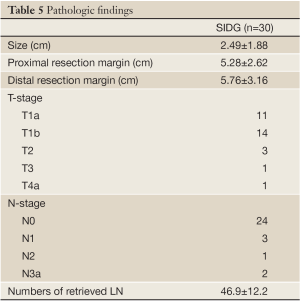
Full Table
Operation time and learning curve
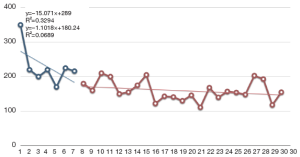
Figure 1 shows that the time taken for an operation gradually decreased in the SIDG group. To examine the learning process of SIDG, SIDG cases were divided into 3 groups based on the serial number of surgery (0-10, 11-20 and 21-30, respectively). The operation time in the 11-20 and 21-30 cases groups were significantly shorter than in the 0-10 cases group (P=0.007).
Conclusions
In this study, we evaluated the surgical outcomes of SIDG in 30 patients with EGC, which shows excellent postoperative outcomes. This procedure was found to have acceptable oncologic outcome, surgical time, and complications rates.
Thus, we conclude that SIDG is a likely acceptable treatment for EGC; furthermore, it is a feasible, safe, and useful method for reducing postoperative pain and facilitating cosmesis.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 2010;251:417-20.
- Hwang SH, Park do J, Jee YS, et al. Actual 3-year survival after laparoscopy-assisted gastrectomy for gastric cancer. Arch Surg 2009;144:559-64; discussion 565.
- Pfluke JM, Parker M, Stauffer JA,et al. Laparoscopic surgery performed through a single incision: a systematic review of the current literature. J Am Coll Surg 2011;212:113-8.
- Velthuis S, van den Boezem PB, Lips DJ, et al. Comparison of Short-Term Surgical Outcomes after Single-Incision Laparoscopic versus Multiport Laparoscopic Right Colectomy: A Two-Center, Prospective Case-Controlled Study of 100 Patients. Dig Surg 2013;29:477-83.
- Kim SJ, Ryu GO, Choi BJ, et al. The short-term outcomes of conventional and single-port laparoscopic surgery for colorectal cancer. Ann Surg 2011;254:933-40.
- Omori T, Oyama T, Akamatsu H, et al. Transumbilical single-incision laparoscopic distal gastrectomy for early gastric cancer. Surg Endosc 2011;25:2400-4.
- Park do J, Lee JH, Ahn SH, et al. Single-port laparoscopic distal gastrectomy with D1+β lymph node dissection for gastric cancers: report of 2 cases. Surg Laparosc Endosc Percutan Tech 2012;22:e214-6.
- Omori T, Tanaka K, Tori M, et al. Intracorporeal circular-stapled Billroth I anastomosis in single-incision laparoscopic distal gastrectomy. Surg Endosc 2012;26:1490-4.