Unveiling the role of tumor reactive stroma in cholangiocarcinoma: an opportunity for new therapeutic strategies
Introduction
Cholangiocarcinoma (CCA), although considered a rare disease, is the second most frequent primary tumor of the liver, accounting for 10-25% of all hepatobiliary cancers and for 10-20% of liver cancer-related deaths (1). Broadly, CCA is defined as the malignant transformation of biliary epithelial cells (cholangiocytes) even though recent evidence suggests CCA may originate from hepatic progenitor cells located at the canals of Hering and/or peribiliary glands or even originate from transformed hepatocytes (2,3). Based on well-established anatomic criteria, CCA is conventionally divided into intrahepatic CCA (iCCA) and extrahepatic CCA (eCCA). iCCA arises from ducts proximal to the second order bile ducts and accounts for 8% of all CCAs, eCCA develops from the second order ducts to the pancreatico-duodenal ampulla, which tumors are not considered eCCA; eCCA includes perihilar (Klatskin) CCA which arises in the biliary segment from the second order ducts to the origin of the cystic duct and represents the most common form of CCA (50% of all CCA) (4). Incidence of CCA has increased in the last decade particularly in Europe ranging between 0.45 (Switzerland) and 3.36 (Italy) per 100,000, and in the US (1.67 per 100,000); these figures are still lower with respect to South-East Asia, where the highest incidence is 85 per 100,000 in North-East Thailand (5). The increased incidence of this neoplasm is substantially attributable to iCCA, whereas incidence of eCCA has remained stable (6,7).
In addition to the emerging epidemiological relevance, the growing interest in CCA is strictly related to its highly aggressive behavior responsible for an extremely poor prognosis. In fact, therapeutic approaches with a curative intent in CCA are still very limited due to the frequent extensive spread of the tumor at the time of diagnosis (8,9). CCA metastasizes mostly via lymphatic vessels and along the route of bile ducts to areas deeper within the liver, to regional lymph nodes, or to distant sites such as the lung (10). Surgery remains the only curative treatment for patients with CCA, but often with disappointing results. In fact, less than 30% of patients with iCCA are eligible for curative resection because of its early metastasization to regional lymph nodes, and of those, only 60% survive for 5 years, which is more than for those with eCCA (median survival of 36 months) (11,12). Liver transplantation may be offered only to a highly selected group of patients; however, tumor recurrence within 5 years still affects 12-32% of cases (13,14), taking advantage of a multimodality protocol (Mayo Protocol), which combines neoadjuvant external beam radiotherapy, brachytherapy, chemotherapy, and finally a staging laparotomy to rule out metastatic disease (13). The lack of effective treatments reflects the uncertainty of the mechanisms underlying the invasiveness of CCA.
An important histological feature of CCA is the highly desmoplastic microenvironment where the neoplastic bile ducts lay densely embedded. Multiple autocrine and paracrine signals are exchanged between the many cell types of the stroma and the neoplastic cells. There is a strong likelihood that these interactions greatly influence the aggressive behavior of CCA. Given the rise in CCA incidence, in the face of a still disappointing prognosis, better therapeutic strategies for patients with CCA are eagerly awaited. The stromal microenvironment surrounding CCA will be the focus of this review: we will first discuss the multiple cell types involved in the tumor stroma and then some of the molecular interactions responsible for the aggressive nature of CCA. The possibility of exploiting these interactions as therapeutic targets will also be discussed.
Tumor reactive stroma: a close cellular collaboration that modulates tumor behavior
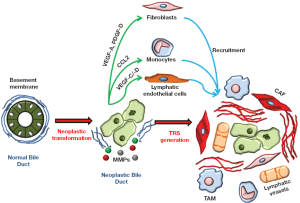
Cholangiocarcinoma cells are surrounded by a specialized mesenchyma that includes an inflammatory infiltrate, which releases cytokines, chemokines and growth factors, and activated fibroblasts, which also secrete collagen, both of which are drawn to the site of the tumor; in addition, blood and lymphatic vessels increase and feed the growth of the tumor (Figure 1). These histopathological changes have been collectively termed as tumor reactive stroma (TRS), formerly referred to as desmoplasia. In contrast with the common mechanisms governing tissue repair and remodeling, the role of the TRS is not to heal the wound, but rather to provide an environment that favors cancer growth and likely metastasization. Evidently, during cancer development, immunomodulatory and metabolic functions of neoplastic cells overcome the ability of the hosting tissue to dictate a reparative response. A number of human malignances, including prostate (15,16), pancreatic (17-21), ovarian (22), and skin (23) cancers characterized by strong invasive properties exhibit an abundant TRS. Other studies in different malignancies featuring abundant desmoplasia have shown the pro-oncogenic effects of the TRS, both in vitro and in vivo. A preliminary study on a murine model of breast carcinoma (24) demonstrated that the TRS is necessary for the implant of neoplastic cells and that the extent of the TRS correlates with a poor prognosis (25). In vitro experiments using mouse and human samples from hepatocellular carcinoma (HCC), as well as an in vivo HCC mouse model, have highlighted the concerted efforts of stromal cells and neoplastic cells in promoting tumor progression (26). Recent data also support the role of stromal cells in favoring cancer cell resistance to chemotherapy (27). Based on these observations, it can be speculated that a reduction in the TRS may represent a highly desirable effect to hamper cancer invasiveness.
Cell elements populating the tumor reactive stroma in cholangiocarcinoma
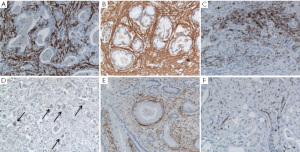
The TRS is host to a number of stromal cell types and structures variably contributing to CCA progression, including cancer-associated fibroblasts (CAF), tumor-associated macrophages (TAM), and blood and lymphatic vessels (28,29) (Figure 2). Data on the specific role of each component of the tumor stroma in CCA are still limited by the lack of experimental models of this type of tumor. Therefore, data discussed hereafter are also derived from studies performed in different cancers with rich TRS.
Cancer-associated fibroblasts
Fibroblasts within the TRS are in a state of activation (myofibroblasts or activated fibroblasts). CAF may originate from the recruitment of hepatic stellate cells resident within the liver, or from fibroblasts that reside within the portal tract, and are recruited to the tumor area by pro-inflammatory chemokines and cytokines released by inflammatory cells and by the tumoral cells themselves. CAF can also be recruited from circulating bone marrow-derived mesenchymal cells that co-express both hematopoietic and fibroblast markers (30,31).
Alternatively, several studies proposed that CAF may derive from the epithelial-to-mesenchymal transition (EMT) of tumoral epithelial cells that would undergo a morphological and functional switch towards a mesenchymal phenotype, which enhance cell motility and the ability to remodel the surrounding extracellular matrix (ECM). However, the ability of cancer cells to undergo complete EMT and serve as a potential source of CAF is controversial (32-34). In CCA, CAF share the same phenotypic markers of hepatic myofibroblasts, such as vimentin, Thy-1, α-smooth muscle actin (α-SMA) (Figure 2A), intercellular adhesion molecule-1 and laminin (35). High expression of -SMA in CCA correlates with larger tumor size and poor survival of the patients (36). Regardless of the cell origin, cross-talk mechanisms with neighboring cell populations play a relevant role in promoting activation of fibroblasts in the TRS. Fibroblasts may become activated in response to cytokines released by cancer cells and inflammatory cells such as platelet-derived growth factor (PDGF), transforming growth factor (TGF)-β and fibroblast growth factor (FGF)-2 (28,31,37). In HCC, tumor cells have been found to release lysophostatidic acid that targets its receptor on resident fibroblasts and induce their activation to CAF; treatment with an inhibitor of lysophostatic acid blocks generation of CAF coupled with a reduction in HCC growth and progression (26). Interleukin (IL)-6 is overexpressed in CCA cells in response to hypoxia, and represents a key determinant of cancer invasiveness (38,39). Elevated levels of IL-6 have been linked with enhanced recruitment of mesenchymal stem cells to the hypoxic TRS via the signal transducer and activator of transcription (STAT)3 pathway which in turn, promote survival of the tumor by releasing anti-apoptotic proteins (39).
A defining feature of CAF is their tendency to maintain a persistent state of activation as a result of their ability to release cytokines and ECM components in a self-perpetuating autocrine loop (40). CAF isolated from different types of neoplasia, such as breast cancer, melanoma and Wilms tumor, exhibit a high proliferation rate in vitro (28) and are able to promote tumor growth by hyper-expressing TGF-β and hepatocyte growth factor (HGF) (24). In addition, CAF secrete several growth factors [vascular endothelial growth factor (VEGF), FGF, connective tissue growth factor], cytokines and chemokines [CCL2 (monocyte chemotactic protein; MCP-1), stromal cell-derived growth factor (SDF)-1, also known as CXCL12, CXCL14], that are able to recruit monocytes and macrophages, endothelial cells and other inflammatory cells (41). CAF are also able to modify the structure of the matrix supporting the TRS. To favor the tumor cell spread, the TRS needs a highly stiff ECM. This is achieved by transforming soluble fibronectin, a major component of the ECM, into a stable, insoluble complex where exposed integrins coordinate and bind fibronectin fibrils (42). These changes in the fibronectin structure allow collagen to anchor to the fibrils, further augmenting the tensile strength of the ECM. In this process, CAF release neuropilin-1, which promotes fibronectin matrix assembly via regulation of integrin expression (43).
Tumor-associated macrophages
TAM are polarized M2 macrophages that possess properties that support the tumor environment (44) (Figure 2C,D). In contrast with M1 macrophages that are more involved in immune response, M2 macrophages modulate tissue remodeling and angiogenesis, and suppress T cell activity/proliferation. Phenotypically, TAM are characterized by a constitutive high expression of the chemokines CCL17 and CCL18, of the interleukins IL-1ra, IL-6, IL-10, and of arginase-1 (45), and by the low expression of CD51, carbopeptidase M and IL-12. Derived from circulating monocytes, TAM are recruited by MCP-1 expressed by tumoral cells and/or several cellular components of the TRS (46). Besides MCP-1, several other chemokines can direct monocyte homing, including CCL3, CCL4, CCL5, CCL8, macrophage inhibitory protein-1, macrophage migration inhibitory factor, as well as the growth factors VEGF and monocyte-colony stimulating factor (M-CSF) (46). Once attracted to the neoplastic area, monocytes differentiate into M2 macrophages under the influence of soluble factors, such as prostaglandin E2 (PGE2), and cytokines, such as IL-2, IL10 and TGF-β1 (47). In addition to CAF, TAM could play an important role in CCA invasiveness as they correlate with poor disease-free survival in CCA (48). Similarly, in HCC as well as in several other epithelial cancers (49), there is a strong correlation between macrophage density and poor survival (50). However, molecular effects of TAM in CCA are still largely unknown. TAMs effects on tumor invasiveness are mostly derived from studies performed in cancers featuring a rich TRS, such as in breast and colon carcinomas. In a mouse model of breast cancer, expression of colony stimulating factor-1 (CSF-1) is highest at the invasive edge of tumor cells, a site highly populated with macrophages (50). While CSF-1 released by breast tumor cells has been associated with recruitment of TAM, TAM reciprocate by releasing epidermal growth factor (EGF) stimulating tumor cells to migrate and metastasize. Similar findings have been observed in a genetic model of colon cancer (50). TAM’s ability to support cancer cell migration is also related to the secretion of several other growth factors, including VEGF, FGF1 and 2, PDGF, GM-CSF, insulin-like growth factor (IGF)-1 and TGF-β, and pro-inflammatory mediators, such as IL-1, -6, -8, prostaglandins, interferon-γ and tumor necrosis factor (TNF)-α (46). In breast cancer, TAM also over-express non-canonical Wnt 5a which induces tumor invasiveness via the Jun-N-terminal kinase (JNK) pathway. However, the role of Wnt 5a in cancer is controversial, since in other studies it has been reported to behave as a tumor suppressor (51). Moreover, TAM can secrete a number of cytokines involved in angiogenesis and ECM remodeling, which include VEGF, IL-8, TNF- and matrix metalloproteinases (MMPs), namely MMP-2 and -9 (44). In particular, TAM may promote lymphangiogenesis by secreting VEGF-C (52). These secretory abilities are potentially further enhanced by hypoxic conditions present in most desmoplastic tumors (49,53). TAM are also thought to increase the invasive potential of tumoral cells by releasing MMPs, and indeed, they have been found in close vicinity to areas of basement membrane breakdown (44).
Lymphatic vessels
The lymphatic system is a network of vessels and nodes necessary to maintain tissue fluid homeostasis, and for immunosurveillance by modulating leukocyte traffic. Akin to veins, lymphatic vessels possess valves protruding into the lumen lined by a specialized endothelium (lymphatic endothelial cells, LEC) and surrounded by a scant presence of -SMA-positive mural cells (54). The lymphatic vasculature is characterized by the expression of VEGFR-3, the specific receptor for the main lymphangiogenic growth factors, VEGF-C and VEGF-D, but also by a number of peculiar markers such as lymphatic vessel endothelial hyaluronan receptor-1 (Lyve-1) (55), podoplanin (a membrane sialoglycoprotein) (56), and the transcription factor prospero homeobox 1 (PROX1) (57).
The lymphatic vasculature is a critical element of the TRS and one of the main routes of metastatic spread, particularly in CCA, a malignancy in which metastasis to regional lymph nodes is the main cause of ineligibility for patients to surgical resection (6,10,58,59). In CCA, the presence and extent of lymph node metastasis after surgical resection is a predictor of poor prognosis (60). A highly developed lymphatic bed typically forms in close proximity to CCA neoplastic tissue, embedded in a dense TRS. Interestingly, within the TRS, not only do LEC appear to be in close relationship with neoplastic cells, but also in contiguity with CAF. The extent of CAF appears to correlate with lymphatic microvessel density and lymph node metastasis in the early stages of invasive colorectal carcinoma (61) and in ovarian carcinoma (62). These observations are consistent with the hypothesis that CAF are capable of generating a pro-oncogenic microenvironment conducive of metastatic behavior by expressing a number of angiogenic factors (28). The most studied growth factors active on the lymphatic vessels are VEGF-C and -D, their receptor VEGFR-3 and the co-receptor neuropilin (NRP)-2 (63-65), along with Ang-1 and -2 and their receptors Tie-1 and -2 (66). Although explored to a much lesser extent, other growth factors that may exert pro-lymphangiogenic effects include VEGF-A (67), PDGF-B (68), IGF (69), HGF (70), and FGF (71). Noteworthy, the expression in TRS of lymphangiogenic growth factors and receptors such as VEGF-C and VEGFR-3, respectively, correlate with a poor prognosis in iCCA (72-74).
In CCA, the large expansion of the lymphatic vasculature contrasts with the significant reduction in blood vessels (Figure 2 E,F). The resulting hypoxic tumoral microenvironment may represent a critical determinant of CCA invasiveness (75). In fact, hypoxia is known to promote tumor progression by providing a selective pressure that favors the survival of the most aggressive malignant cells (76). The vast majority of hypoxic effects observed in tumors is mediated by hypoxia inducible factor-1 alpha (HIF-1), a transcription factor expressed at both cytoplasmic and nuclear levels. Several studies have shown that HIF-1 promotes tumor lymphangiogenesis, by inducing the secretion of VEGF-A (77), VEGF-C (78) and VEGF-D (79,80), Ang-1 and -2 (81), and PDGF-B (82). HIF-1 expression positively correlates with poor prognosis and VEGF-C secretion in non-small cell lung cancer (83), oral squamous cell carcinoma (84), and breast carcinoma (52,85); HIF-1 expression also correlates with VEGF-D in esophageal squamous cell carcinoma (86) and breast carcinoma (85). In iCCA (87), HIF-1 expression correlates with the size of the tumor and with overall and disease-free survival. Besides hypoxia, the local concentration of pro-lymphangiogenic growth factors can be modulated by signals released by the inflammatory cells populating the TRS. Leukocytes and in particular macrophages, may induce lymphatic vessel growth through LEC activation or recruitment of bone marrow-derived cells (81). It is worth noting that hematopoietic stem cells may serve as an additional source of LEC as they account for 1-3% of all lymphatic cells in mice transplanted with hematopoietic stem cells. Macrophages are able to secrete high amounts of VEGF-C, also in response to TGF-β and TNF-α (88). The amount of TAM correlates with lymphatic microvessel density in cervix cancer (89). In patients with CCA, macrophages (90), or M2 macrophages (48), strongly contribute to the local secretion of VEGF-C, and their accumulation in TRS correlates with a poor prognosis (48).
The different cell components of the tumor reactive stroma promote cholangiocarcinoma invasiveness by modulating the phenotype of tumoral cells
Strong evidence suggests that the progression of CCA is influenced by molecular factors secreted by stromal cells, as well as by the neoplastic cholangiocytes themselves. These autocrine and paracrine cues acting on the tumor may influence its survival, proliferation, migration and ultimately its invasive potential.
EMT has been suggested by many studies as an alternative, albeit not a mutually exclusive, mechanism promoting cancer invasiveness. EMT is characterized by the loss of the epithelial phenotype in exchange of mesenchymal properties. Down-regulation of E-cadherin, a major component of the adherens junction complex, and of occludins and claudin, necessary for tight junctions and epithelial cell polarity, results in a reduced intercellular adhesion and epithelial layer cohesion that could allow a more enhanced invasiveness. In turn, tumor cells acquire a range of mesenchymal phenotypic traits that are characterized by the enrichment of integrin receptors responsible for the interactions with the ECM, a leading/trailing edge asymmetry, and up-regulation of cytoskeletal proteins within the mesenchymal lineage including vimentin, src kinase, N-cadherin, -SMA and MMPs (32). Transcription factors analogous to those involved in embryogenesis, such as Twist, Slug, Snail, Zeb-1 and Zeb-2, which regulate E-cadherin expression, are involved in cell motility. They represent the molecular basis of EMT-changes typically featuring tumor cells at the invasive front compared to those within the core of the tumor (91). Overall, EMT imposes plasticity upon the tumor cells thereby allowing them to detach from their primary site of growth. However, recent studies from our group have demonstrated that despite the ability of human CCA cells to express some mesenchymal markers, such as S100A4 and vimentin, a complete transdifferentiation of CCA cells to -SMA-positive cells was not found after xenotransplantation of human CCA cells into SCID mice (92,93). Therefore instead of a full EMT, the presence of mesenchymal features in transformed cholangiocytes represent only the acquisition of EMT-like changes. This suggests that interactions with the neighboring stromal cells contribute to the mesenchymal-like phenotype observed in the tumor cells. In CCA cell lines, In CCA cell lines, TGF-β1 induces the activation of Snail, a negative regulator of E-cadherin expression, whose expression correlates with reduced expression of Keratin-19, a cholangiocyte marker, and with increased expression of vimentin, a mesenchymal cell marker (94). TGF-β1/Snail activation has also been associated with increased invasive capabilities of CCA cholangiocytes both in vitro and in vivo (94).
Phenotypic changes of tumoral cells may also include the gain of stem cell-like properties. These changes not only induce more invasive characteristics but even imposes a heightened resistance to apoptosis allowing the transformed cells to withstand chemotherapeutic agents (95).
Cell behavior is also strongly influenced by interactions with ECM proteins. ECM proteins modulate a variety of cellular functions, including differentiation, proliferation and secretion. In particular, two ECM components, periostin and tenascin-C, are released by CAF and function together to activate integrins on iCCA cholangiocytes, ultimately stimulating their migration and invasion (96). Interestingly, periostin, a protein regulated by TGF-β, is present within the fibrous stoma of CCA in higher amounts than any other hepatic malignancy (97). The ECM also serves as a reservoir of CAF-derived growth factors, such as HGF and SDF-1, which can therefore be persistently supplied to cancer cells. HGF and SDF-1 signal via their receptors, c-MET and CXCR4 respectively, on CCA cholangiocytes to stimulate their proliferation and migratory activities (96). Not only does the TRS provide a communication exchange between tumor cells and stromal cells but also among the stromal cells themselves; for example, SDF-1 can regulate recruitment of TAM (50). Periostin, HGF and SDF-1 are coincidently up-regulated by hypoxia, which is a characteristic of CCA, as previously discussed (96).
Proteases and MMPs released by both tumoral and stromal cells direct the remodeling of the TRS matrix. CAF, for example, secrete MMP-1, -2 and -9 as well as seprase, which are all important for the dissemination of cancer cells (96).
Enhanced cell survival is another mechanism strongly involved in tumor invasiveness which is favored by the interactions within the TRS. Signals released by CAF, such as periostin, PGE2 and sphingosine-1-phosphate, enable CCA cholangiocytes to resist chemotherapeutic agents (97). In HCC, PGE2 as well as indoleamine 2,3-dioxygenase 1 secreted by CAF have been found to suppress the activation and cytotoxic effect of NK cells (98). Recently, PDGF-B secreted by CAF has been discovered to protect CCA cholangiocytes from tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) by cross-talking with the Hedgehog signaling cascade (99). When PDGF-B binds to PDGFR-, its cognate receptor expressed by neoplastic cholangiocytes, the Hedgehog receptor, Smoothened, is trafficked to the plasma membrane in a protein kinase A-dependent fashion leading to activation of GLI transcription factors, critical regulators of several survival pathways (51).
To focus attention to another PDGF-mediated mechanism, CCA cholangiocytes can secrete PDGF-D, which acting on its cognate receptor PDGFR- expressed by CAF, enables them to be recruited into the TRS (93). This finding confirms that CCA cells are major players of TRS generation and indicates PDGF-D as a pivotal paracrine signal. Activation of PDGF-D signaling in CAF involves Rho GTPases, mainly Rac1 and Cdc42, as well as JNK, as molecular effectors underpinning CAF migration.
Molecular factors released by tumor cells such as IL-10, VEGF-A, TGF-β and MMP-2 are also relevant to induce recruitment and differentiation of macrophages towards the TAM M2 phenotype acting via STAT3 (48). TAM are also particularly attracted to the hypoxic microenvironment which they respond to by up-regulating HIF-1α and HIF-2α (44), mechanisms that can be of great relevance in CCA. Activation of HIF-1α in TAM leads to the hypersecretion of a number of cytokines, including VEGF, glucose transporter 1, phosphoglucokinase and inducible nitric oxide synthase (100). The M1-M2 shift that occurrs during tumorigenesis is driven by HIF-1 in concert with a balanced down-regulation of NF-kB. Whereas M1 are characterized by a high expression of NF-kB, M2 macrophages express high amounts of p50-NF-kB, that inhibits the transcriptional activity of NF-kB, as shown in murine fibrosarcoma and human ovarian cancer (101).
Possibility of new therapeutic strategies in cholangiocarcinoma by interfering with the tumor reactive stroma
When CCA is diagnosed early, i.e. before the development of metastasis to regional lymph nodes, a limited set of curative options can be considered, encompassing surgical resection and, in highly selected cases, liver transplantation. In the majority of patients with CCA, especially iCCA, which can remain asymptomatic until advanced stages, the disease will have already extended outside the liver by the time of diagnosis. In these patients, palliative approaches mainly relying on endoscopic treatments or percutaneous biliary drainage to relieve biliary obstruction can be offered. In recent years, photodynamic therapy has been proposed as neoadjuvant treatment for the palliative management of hilar CCA (102). In patients ineligible for curative therapies, chemotherapy is still largely ineffective (103); the standard of care for advanced CCA therapy is a combination of Cisplatin and Gemcitabine, which has increased survival time by 3 months compared with Gemcitabine alone (104).
The disappointing results of the treatments of CCA are a consequence of the uncertainty on the mechanisms of carcinogenesis and the absence of known molecular signatures of CCA invasiveness, which has, so far, precluded the possibility to develop molecularly targeted therapies. Furthermore, there are no validated biomarkers to test a specific targeted agent in CCA at present (105).
In the last few years, growing interest has been drawn on the TRS as a potential therapeutic target in a number of malignancies, including CCA. It is supposed that stromal cells have a putative role in the progression of cancers with high desmoplasia, and hypothesized that interfering with the TRS and with the recruitment of its different cell components may be of therapeutic relevance (106,107). In CCA, validation of the effectiveness of this approach is still limited by the current lack of experimental models. Hypothetically, several pharmacological opportunities to inhibit target proteins critical for the recruitment of cellular components of the TRS can be considered and may represent a step forwards in anticancer treatment, particularly in CCA; these are summarized in Table 1.
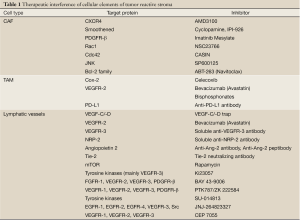
Full Table
Strategies aimed at cancer-associated fibroblasts
A multitude of interactions are exchanged between CCA cells and CAF. A number of agents are available to interrupt these communications by specifically targeting CAF, and preliminary studies indicate that these compounds are also effective in reducing tumor mass and improving patient survival.
Hepatic stellate cells are known to secrete CXCL12 [or SDF-1], the ligand for CXCR4, a receptor highly expressed in CCA including human CCA cell lines such as HuCCT-1 and CCKS-1, but not in normal cholangiocytes (108). As a potential therapeutic intervention to reduce the extent of CAF, treatment of cultured CCA cells with the specific CXCR4 inhibitor (AMD3100) hampered the CCA-mediated migration of hepatic stellate cells. Furthermore, IL-6 secreted by tumor cells within a hypoxic environment has been linked with recruitment of mesenchymal cells and an antibody against IL-6 has resulted in attenuation of mesenchymal cell migration (38).
Cyclopamine, an inhibitor of the Hedgehog signaling pathway acting on Smoothened, unleashed TRAIL on neoplastic cholangiocytes and induced their apoptosis reversing the effects of PDGF-B released by CAF (99). In vivo experiments have found that Cyclopamine reduces tumor size but also the metastatic potential of CCA cholangiocytes (99). IPI-926, a novel derivate of Cyclopamine, can potentially improve the efficacy of Gemcitabine in ductal pancreatic cancer, by inhibiting the Hedgehog signaling pathway and therefore depleting the TRS (109).
In a different study, the tyrosine kinase inhibitor, Imatinib mesylate, was a more direct approach to target the CAF-emitted-PDGF-B that is signaled to CCA. By blocking the PDGFR- expressed by CCA cholangiocytes, Imatinib resulted in increases of TRAIL and apoptosis of tumoral cholangiocytes (110). A recent paper published by our group (93) outlined the inverse role of PDGFR- in mediating the recruitment of myofibroblasts following stimulation by PDGF-D secreted by CCA cholangiocytes. In this model, PDGFR- can be interfered directly using Imatinib mesylate or indirectly by inhibiting the downstream effectors of this pathway, in particular the Rho GTPases with NSC23766 (Rac1 inhibitor) and CASIN (Cdc42 inhibitor) and/or JNK signaling with SP600125 (93); a strong reduction in CAF migration was similarly obtained with both approaches. As with Cyclopamine, Imatinib also reduced CCA tumor size and metastasis in rodents (110).
Pro-apoptotic agents, such as Navitoclax, have been shown to act selectively on CAF. By activating Bax, Navitoclax reduced the number of -SMA-positive CAF as well as CCA tumor mass, which is associated with an improved survival in rodents (111).
Theoretically, MMP inhibition may also be of potential interest to modulate cell communications within the TRS. However, MMP inhibition does not seem to improve patient outcome in many epithelial cancers, even though to date, MMP or Seprase inhibition have not been studied in CCA (112). A vaccine against Seprase that reduces collagen type I expression has shown positive outcomes in survival in mice with mammary and colon carcinomas (112).
Strategies aimed at tumor-associated macrophages
There is growing evidence indicating that TAM are fundamental in mediating tumor cell proliferation and invasion in various carcinomas and that their presence correlates with a poor prognosis, however, drugs specifically targeting TAM are lacking (46). The most promising therapy aimed at antagonizing TAM function is the use of an anti-programmed death (PD)-L1 antibody. PD-L1 is a ligand for PD-1, whose secretion is induced in circulating monocytes by the pro-inflammatory cytokines, TNF- and IL-10, released by the tumoral microenvironment. PD-L1 exerts two pro-oncogenic effects: it induces apoptosis in T-cells (113) and enhances the survival of tumoral cells in HCC (114), where overexpression of PD-L1 corresponds with a poor prognosis and increased mortality (113,114). Actually, an anti-PD-L1 antibody was approved for phase I clinical trials in the US for several types of cancers, including melanoma and non-small cell lung, colorectal, and renal cell cancers (115).
Another possibility to target TAM function is related to the inhibition of some biological processes dependent upon the presence of macrophages. For example, the use of specific Cyclooxygenase-2 inhibitors is able to reduce macrophage-induced secretion of VEGF-C subsequently suppressing lymphangiogenesis and lymph node metastasis in two different murine models engrafted with either human gastric carcinoma or human lung adenocarcinoma cell lines (116,117). On the other hand, Bevacizumab (Avastatin) is a VEGF-A neutralizing antibody that can inhibit, not only neoangiogenesis and development of the lymphangiogenic network (see below), but also the recruitment of macrophages (118,119).
The inorganic compound family of bisphosphonates (such as Zoledronic acid), commonly used for the treatment of metabolic bone diseases, are also able to modulate a range of macrophage functions, by reducing cell migration, infiltration, proliferation (120), and to revert macrophage polarization from the pro-metastatic M2 phenotype to that of an anti-tumoral M1 phenotype (120,121). Whether their effects are therapeutically relevant in CCA is a matter worth being investigated.
Strategies aimed at tumoral lymphangiogenesis
Currently there are no reports of therapies acting on tumoral lymphangiogenesis in CCA, although the use of various anti-lymphangiogenic compounds (drugs, soluble receptors, blocking antibodies) are under evaluation in other malignancies. Specific targeting of tumoral lymphatic vessels has not been developed yet, so one major disabling side-effect in tumoral lymphangiogenic therapy is lymphedema.
Several studies in animal models have been conducted to directly inhibit the main pro-lymphangiogenic growth factors with different inhibitors. Since the VEGF-C/VEGF-D/VEGFR-3/NRP-2 axis is fundamental in this process, several strategies to block its signaling were explored by generating monoclonal antibodies against the ligands, and soluble antibodies against the receptors (122,123). Originally used to hamper tumoral neoangiogenesis, Bevacizumab, a VEGF trap antibody able to interfere with activation of the VEGFR-2 signaling cascade, can function to reduce also lymphangiogenesis (118). The angiopoietin/Tie pathway is another cascade that can be targeted to suppress lymphatic tumoral angiogenesis. To interfere with this signaling, generation of Ang-1, -2, and Tie-2 neutralizing antibodies were able to reduce the LEC sprouting, extent of the lymphatic bed, and metastasization in different rodent models.
Recent data support the use of Rapamycin to suppress lymphangiogenesis and lymphatic metastasis in pancreatic cancer (124). In a pancreatic tumor cell line and in cholangiocytes, the AKT/mTOR pathway is a critical mediator of VEGF-A and -C overexpression (124,125), along with an autocrine effect on proliferation and likely, sprouting of the epithelial cells.
Furthermore, a recent study outlined the usefulness of tyrosine kinase inhibitors to interfere with the lymphangiogenic signaling, in particular Ki23057, an inhibitor of VEGFR-3 autophosphorylation. Ki23057 appears to hamper metastasization and lymphangiogenesis in an experimental model of gastric carcinoma. Other less specific inhibitors of tyrosine kinases are actually in phase I to III clinical trials for different malignancies (i.e. breast, ovarian, pancreatic, prostate, lung and advanced solid tumors), among them are BAY 43-9006 (126), PTK787/ZK 222584 (127,128), SU-014813 (129), JNJ-264823327 (130), and CEP 7055 (131).
Conclusions
CCA is a very aggressive cancer, with limited therapeutic chances, whose mechanisms of progression are hitherto largely unknown. Although rare, the epidemiological impact of CCA is increasing. All these aspects make CCA a hotspot in liver research. An important morphological feature of CCA is the presence of a striking desmoplastic reaction, also termed as the tumor reactive stroma, closely embedding the neoplastic bile ducts. An abundant reactive stroma is reflective of various carcinomas similarly characterized by strong invasiveness. Functionally, the TRS is an aberrant reparative complex, populated by different cell elements, including CAF, TAM and lymphatic vessels, strictly interacting with the cancer cell compartment. Recent evidence suggests that the TRS is a key driver of CCA progression. In fact, the large variety of signals and mediators reciprocally exchanged between stromal and neoplastic cells provides the tumor microenvironment with invasiveness-promoting properties. These paracrine communications have started to be elucidated only recently and may represent a putative target amenable of therapeutic interference. Although there are no current data on the use of drugs able to target the paracrine communications in the TRS of CCA, some compounds seem to be of great potential interest. The lack of experimental models of CCA makes the possibility to actually test the effects of these compounds difficult, even though the model of human CCA cells xenografted into SCID mice seems to provide valuable readouts (92,93). Therefore, a deeper knowledge of the molecular factors regulating the epithelial/stromal interactions in TRS is necessary to open new therapeutic avenues in CCA.
Acknowledgements
Progetto di Ricerca Ateneo 2008 (grant #CPDA083217) and 2011 (grant #CPD113799/11) to MC and LF. Associazione Scientifica Gastroenterologica di Treviso (ASGET, associazione di promozione sociale senza scopo di lucro) to SDM and LF. Fondazione Amici Dell Epatologia (FADE) to LF. NIH Grant DK34989: Silvio O. Conte Digestive Diseases Research Core Centers C 5P30DK034989 and a grant from PSC partners for a care to MS.
Disclosure: The authors declare no conflict of interest.
References
- Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology 2011;54:173-84. [PubMed]
- Komuta M, Govaere O, Vandecaveye V, et al. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology 2012;55:1876-88. [PubMed]
- Fan B, Malato Y, Calvisi DF, et al. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest 2012;122:2911-5. [PubMed]
- DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg 2007;245:755-62. [PubMed]
- Cardinale V, Semeraro R, Torrice A, et al. Intra-hepatic and extra-hepatic cholangiocarcinoma: New insight into epidemiology and risk factors. World J Gastrointest Oncol 2010;2:407-16. [PubMed]
- Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2002;2:10. [PubMed]
- Khan SA, Thomas HC, Davidson BR, et al. Cholangiocarcinoma. Lancet 2005;366:1303-14. [PubMed]
- Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology 2008;48:308-21. [PubMed]
- Gatto M, Bragazzi MC, Semeraro R, et al. Cholangiocarcinoma: update and future perspectives. Dig Liver Dis 2010;42:253-60. [PubMed]
- Fabris L, Alvaro D. The prognosis of perihilar cholangiocarcinoma after radical treatments. Hepatology 2012;56:800-2. [PubMed]
- Razumilava N, Gores GJ, Lindor KD. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology 2011;54:1842-52. [PubMed]
- Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol 2013;11:13-21. [PubMed]
- Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg 2005;242:451-8. [PubMed]
- Darwish Murad S, Kim WR, Therneau T, et al. Predictors of pretransplant dropout and posttransplant recurrence in patients with perihilar cholangiocarcinoma. Hepatology 2012;56:972-81. [PubMed]
- Cunha GR, Hayward SW, Wang YZ, et al. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer 2003;107:1-10. [PubMed]
- Chung LW, Baseman A, Assikis V, et al. Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J Urol 2005;173:10-20. [PubMed]
- Hartel M, Di Mola FF, Gardini A, et al. Desmoplastic reaction influences pancreatic cancer growth behavior. World J Surg 2004;28:818-25. [PubMed]
- Bachem MG, Schünemann M, Ramadani M, et al. Pancreatic carcinoma cells induce fibrosis by stimulating proliferation and matrix synthesis of stellate cells. Gastroenterology 2005;128:907-21. [PubMed]
- Korc M. Pancreatic cancer-associated stroma production. Am J Surg 2007;194:S84-86. [PubMed]
- Pandol S, Edderkaoui M, Gukovsky I, et al. Desmoplasia of pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol 2009;7:S44-47. [PubMed]
- Cousin B, Ravet E, Poglio S, et al. Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PLoS One 2009;4:e6278. [PubMed]
- Parrott JA, Nilsson E, Mosher R, et al. Stromal-epithelial interactions in the progression of ovarian cancer: influence and source of tumor stromal cells. Mol Cell Endocrinol 2001;175:29-39. [PubMed]
- Skobe M, Fusenig NE. Tumorigenic conversion of immortal human keratinocytes through stromal cell activation. Proc Natl Acad Sci U S A 1998;95:1050-5. [PubMed]
- Kuperwasser C, Chavarria T, Wu M, et al. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci USA 2004;101:4966-71. [PubMed]
- Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle 2006;5:1597-601. [PubMed]
- Mazzocca A, Dituri F, Lupo L, et al. Tumor-secreted lysophostatidic acid accelerates hepatocellular carcinoma progression by promoting differentiation of peritumoral fibroblasts in myofibroblasts. Hepatology 2011;54:920-30. [PubMed]
- Rebucci M, Michiels C. Molecular aspects of cancer cell resistance to chemotherapy. Biochem Pharmacol 2013;85:1219-26. [PubMed]
- Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer 2006;6:392-401. [PubMed]
- Leyva-Illades D, McMillin M, Quinn M, et al. Cholangiocarcinoma pathogenesis: Role of the tumor microenvironment. Transl Gastrointest Cancer 2012;1:71-80. [PubMed]
- Okabe H, Beppu T, Hayashi H, et al. Hepatic stellate cells accelerate the malignant behavior of cholangiocarcinoma cells. Ann Surg Oncol 2011;18:1175-84. [PubMed]
- Fabris L, Strazzabosco M. Epithelial-mesenchymal interactions in biliary diseases. Semin Liver Dis 2011;31:11-32. [PubMed]
- Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res 2006;66:8319-26. [PubMed]
- Anderberg C, Pietras K. On the origin of cancer-associated fibroblasts. Cell Cycle 2009;8:1461-2. [PubMed]
- Kisseleva T, Brenner DA. Is it the end of the line for the EMT? Hepatology 2011;53:1433-5. [PubMed]
- Mueller L, Goumas FA, Affeldt M, et al. Stromal fibroblasts in colorectal liver metastases originate from resident fibroblasts and generate an inflammatory microenvironment. Am J Pathol 2007;171:1608-18. [PubMed]
- Chuaysri C, Thuwajit P, Paupairoj A, et al. Alpha-smooth muscle actin-positive fibroblasts promote biliary cell proliferation and correlate with poor survival in cholangiocarcinoma. Oncol Rep 2009;21:957-69. [PubMed]
- Bremnes RM, Dønnem T, Al-Saad S, et al. The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J Thorac Oncol 2011;6:209-17. [PubMed]
- Okada K, Shimizu Y, Nambu S, et al. Interleukin-6 functions as an autocrine growth factor in a cholangiocarcinoma cell line. J Gastroenterol Hepatol 1994;9:462-7. [PubMed]
- Rattigan Y, Hsu JM, Mishra PJ, et al. Interleukin 6 mediated recruitment of mesenchymal stem cells to the hypoxic tumor milieu. Exp Cell Res 2010;316:3417-24. [PubMed]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 2009;119:1420-8. [PubMed]
- Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth--bystanders turning into key players. Curr Opin Genet Dev 2009;19:67-73. [PubMed]
- Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol 2010;26:397-419. [PubMed]
- Yaqoob U, Cao S, Shergill U, et al. Neuropilin-1 stimulates tumor growth by increasing fibronectin fibril assembly in the tumor microenvironment. Cancer Res 2012;72:4047-59. [PubMed]
- Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res 2006;66:605-12. [PubMed]
- Shirabe K, Mano Y, Muto J, et al. Role of tumor-associated macrophages in the progression of hepatocellular carcinoma. Surg Today 2012;42:1-7. [PubMed]
- Siveen KS, Kuttan G. Role of macrophages in tumour progression. Immunol Lett 2009;123:97-102. [PubMed]
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010;11:889-96. [PubMed]
- Hasita H, Komohara Y, Okabe H, et al. Significance of alternatively activated macrophages in patients with intrahepatic cholangiocarcinoma. Cancer Sci 2010;101:1913-9. [PubMed]
- Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 2002;196:254-65. [PubMed]
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell 2010;141:39-51. [PubMed]
- Pukrop T, Klemm F, Hagemann T, et al. Wnt 5a signaling is critical for macrophage-induced invasion of breast cancer cell lines. Proc Natl Acad Sci U S A 2006;103:5454-9. [PubMed]
- Schoppmann SF, Birner P, Stöckl J, et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol 2002;161:947-56. [PubMed]
- Porta C, Riboldi E, Sica A. Mechanisms linking pathogens-associated inflammation and cancer. Cancer Lett 2011;305:250-62. [PubMed]
- Yamauchi Y, Michitaka K, Onji M. Morphometric analysis of lymphatic and blood vessels in human chronic viral liver diseases. Am J Pathol 1998;153:1131-7. [PubMed]
- Banerji S, Ni J, Wang SX, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol 1999;144:789-801. [PubMed]
- Sleeman JP, Krishnan J, Kirkin V, et al. Markers for the lymphatic endothelium: in search of the holy grail? Microsc Res Tech 2001;55:61-9. [PubMed]
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell 1999;98:769-78. [PubMed]
- Patel T. increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology 2001;33:1353-7. [PubMed]
- Lazaridis KN, Gores GJ. Cholangiocarcinoma. Gastroenterology 2005;128:1655-67. [PubMed]
- Malhi H, Gores GJ. Cholangiocarcinoma: modern advances in understanding a deadly old disease. J Hepatol 2006;45:856-67. [PubMed]
- Liang P, Hong JW, Ubukata H, et al. Myofibroblasts correlate with lymphatic microvessel density and lymph node metastasis in early-stage invasive colorectal carcinoma. Anticancer Res 2005;25:2705-12. [PubMed]
- Zhang Y, Tang H, Cai J, et al. Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett 2011;303:47-55. [PubMed]
- Yuan L, Moyon D, Pardanaud L, et al. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development 2002;129:4797-806. [PubMed]
- Xu Y, Yuan L, Mak J, et al. Neuropilin-2 mediates VEGF-C-induced lymphatic sprouting together with VEGFR3. J Cell Biol 2010;188:115-30. [PubMed]
- Francis H, Alpini G, DeMorrow S. Recent advances in the regulation of cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol 2010;299:G1-9. [PubMed]
- Augustin HG, Koh GY, Thurston G, et al. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol 2009;10:165-77. [PubMed]
- Nagy JA, Vasile E, Feng D, et al. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med 2002;196:1497-506. [PubMed]
- Cao R, Björndahl MA, Religa P, et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell 2004;6:333-45. [PubMed]
- Björndahl M, Cao R, Nissen LJ, et al. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci U S A 2005;102:15593-8. [PubMed]
- Kajiya K, Hirakawa S, Ma B, et al. Hepatocyte growth factor promotes lymphatic vessel formation and function. EMBO J 2005;24:2885-95. [PubMed]
- Larrieu-Lahargue F, Welm AL, Bouchecareilh M, et al. Blocking Fibroblast Growth Factor receptor signaling inhibits tumor growth, lymphangiogenesis, and metastasis. PLoS One 2012;7:e39540. [PubMed]
- Park BK, Paik YH, Park JY, et al. The clinicopathologic significance of the expression of vascular endothelial growth factor-C in intrahepatic cholangiocarcinoma. Am J Clin Oncol 2006;29:138-42. [PubMed]
- Aishima S, Nishihara Y, Iguchi T, et al. Lymphatic spread is related to VEGF-C expression and D2-40-positive myofibroblasts in intrahepatic cholangiocarcinoma. Mod Pathol 2008;21:256-64. [PubMed]
- Fava G, Demorrow S, Gaudio E, et al. Endothelin inhibits cholangiocarcinoma growth by a decrease in the vascular endothelial growth factor expression. Liver Int 2009;29:1031-42. [PubMed]
- Rosmorduc O, Housset C. Hypoxia: a link between fibrogenesis, angiogenesis, and carcinogenesis in liver disease. Semin Liver Dis 2010;30:258-70. [PubMed]
- Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell 2007;129:465-72. [PubMed]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer 2003;3:721-32. [PubMed]
- Simiantonaki N, Jayasinghe C, Michel-Schmidt R, et al. Hypoxia-induced epithelial VEGF-C/VEGFR-3 upregulation in carcinoma cell lines. Int J Oncol 2008;32:585-92. [PubMed]
- Teng X, Li D, Johns RA. Hypoxia up-regulates mouse vascular endothelial growth factor D promoter activity in rat pulmonary microvascular smooth-muscle cells. Chest 2002;121:82S-83S. [PubMed]
- Currie MJ, Hanrahan V, Gunningham SP, et al. Expression of vascular endothelial growth factor D is associated with hypoxia inducible factor (HIF-1alpha) and the HIF-1alpha target gene DEC1, but not lymph node metastasis in primary human breast carcinomas. J Clin Pathol 2004;57:829-34. [PubMed]
- Albrecht I, Christofori G. Molecular mechanisms of lymphangiogenesis in development and cancer. Int J Dev Biol 2011;55:483-94. [PubMed]
- Nilsson I, Shibuya M, Wennström S. Differential activation of vascular genes by hypoxia in primary endothelial cells. Exp Cell Res 2004;299:476-85. [PubMed]
- Zuo S, Ji Y, Wang J, et al. Expression and clinical implication of HIF-1alpha and VEGF-C in non-small cell lung cancer. J Huazhong Univ Sci Technolog Med Sci 2008;28:674-76. [PubMed]
- Liang X, Yang D, Hu J, et al. Hypoxia inducible factor-alpha expression correlates with vascular endothelial growth factor-C expression and lymphangiogenesis/angiogenesis in oral squamous cell carcinoma. Anticancer Res 2008;28:1659-66. [PubMed]
- Okada K, Osaki M, Araki K, et al. Expression of hypoxia-inducible factor (HIF-1alpha), VEGF-C and VEGF-D in non-invasive and invasive breast ductal carcinomas. Anticancer Res 2005;25:3003-9. [PubMed]
- Tzao C, Lee SC, Tung HJ, et al. Expression of hypoxia-inducible factor (HIF)-1alpha and vascular endothelial growth factor (VEGF)-D as outcome predictors in resected esophageal squamous cell carcinoma. Dis Markers 2008;25:141-8. [PubMed]
- Morine Y, Shimada M, Utsunomiya T, et al. Hypoxia inducible factor expression in intrahepatic cholangiocarcinoma. Hepatogastroenterology 2011;58:1439-44. [PubMed]
- Lee AS, Lee JE, Jung YJ, et al. Vascular endothelial growth factor-C and -D are involved in lymphangiogenesis in mouse unilateral ureteral obstruction. Kidney Int 2013;83:50-62. [PubMed]
- Utrera-Barillas D, Castro-Manrreza M, Castellanos E, et al. The role of macrophages and mast cells in lymphangiogenesis and angiogenesis in cervical carcinogenesis. Exp Mol Pathol 2010;89:190-6. [PubMed]
- Subimerb C, Pinlaor S, Khuntikeo N, et al. Tissue invasive macrophage density is correlated with prognosis in cholangiocarcinoma. Mol Med Rep 2010;3:597-605. [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [PubMed]
- Fabris L, Cadamuro M, Moserle L, et al. Nuclear expression of S100A4 calcium-binding protein increases cholangiocarcinoma invasiveness and metastasization. Hepatology 2011;54:890-9. [PubMed]
- Cadamuro M, Nardo G, Indraccolo S, et al. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology 2013. [Epub ahead of print]. [PubMed]
- Sato Y, Harada K, Itatsu K, et al. Epithelial-mesenchymal transition induced by transforming growth factor-{beta}1/Snail activation aggravates invasive growth of cholangiocarcinoma. Am J Pathol 2010;177:141-52. [PubMed]
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 2009;9:265-73. [PubMed]
- Sirica AE. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2011;9:44-54. [PubMed]
- Fujimoto K, Kawaguchi T, Nakashima O, et al. Periostin, a matrix protein, has potential as a novel serodiagnostic marker for cholangiocarcinoma. Oncol Rep 2011;25:1211-6. [PubMed]
- Li T, Yang Y, Hua X, et al. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett 2012;318:154-61. [PubMed]
- Fingas CD, Bronk SF, Werneburg NW, et al. Myofibroblast-derived PDGF-BB promotes Hedgehog survival signaling in cholangiocarcinoma cells. Hepatology 2011;54:2076-88. [PubMed]
- Rius J, Guma M, Schachtrup C, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 2008;453:807-11. [PubMed]
- Saccani A, Schioppa T, Porta C, et al. p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res 2006;66:11432-40. [PubMed]
- Quyn AJ, Ziyaie D, Polignano FM, et al. Photodynamic therapy is associated with an improvement in survival in patients with irresectable hilar cholangiocarcinoma. HPB (Oxford) 2009;11:570-7. [PubMed]
- Alvaro D, Bragazzi MC, Benedetti A, et al. Cholangiocarcinoma in Italy: A national survey on clinical characteristics, diagnostic modalities and treatment. Results from the “Cholangiocarcinoma” committee of the Italian Association for the Study of Liver disease. Dig Liver Dis 2011;43:60-5. [PubMed]
- Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 2010;362:1273-81. [PubMed]
- Zhu AX, Hezel AF. Development of molecularly targeted therapies in biliary tract cancers: reassessing the challenges and opportunities. Hepatology 2011;53:695-704. [PubMed]
- Achen MG, Stacker SA. Targeting tumor stroma. Curr Cancer Drug Targets 2008;8:446. [PubMed]
- Ahmed F, Steele JC, Herbert JM. Tumor stroma as a target in cancer. Curr Cancer Drug Targets 2008;8:447-53. [PubMed]
- Gentilini A, Rombouts K, Galastri S, et al. Role of the stromal-derived factor-1 (SDF-1)-CXCR4 axis in the interaction between hepatic stellate cells and cholangiocarcinoma. J Hepatol 2012;57:813-20. [PubMed]
- Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009;324:1457-61. [PubMed]
- Fingas CD, Mertens JC, Razumilava N, et al. Targeting PDGFR-beta in Cholangiocarcinoma. Liver Int 2012;32:400-9. [PubMed]
- Mertens JC, Fingas CD, Christensen JD, et al. Therapeutic Effects of Deleting Cancer-Associated Fibroblasts in Cholangiocarcinoma. Cancer Res 2013;73:897-907. [PubMed]
- Loeffler M, Krüger JA, Niethammer AG, et al. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest 2006;116:1955-62. [PubMed]
- Kuang DM, Zhao Q, Peng C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 2009;206:1327-37. [PubMed]
- Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009;15:971-9. [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [PubMed]
- Iwata C, Kano MR, Komuro A, et al. Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via reduction of lymphangiogenesis. Cancer Res 2007;67:10181-9. [PubMed]
- Liu H, Yang Y, Xiao J, et al. Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via VEGF-C. Anat Rec (Hoboken) 2009;292:1577-83. [PubMed]
- Whitehurst B, Flister MJ, Bagaitkar J, et al. Anti-VEGF-A therapy reduces lymphatic vessel density and expression of VEGFR-3 in an orthotopic breast tumor model. Int J Cancer 2007;121:2181-91. [PubMed]
- Dineen SP, Lynn KD, Holloway SE, et al. Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res 2008;68:4340-6. [PubMed]
- Rogers TL, Holen I. Tumour macrophages as potential targets of bisphosphonates. J Transl Med 2011;9:177. [PubMed]
- Coscia M, Quaglino E, Iezzi M, et al. Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J Cell Mol Med 2010;14:2803-15. [PubMed]
- Duong T, Koopman P, Francois M. Tumor lymphangiogenesis as a potential therapeutic target. J Oncol 2012;2012:204946.
- Alitalo K. The lymphatic vasculature in disease. Nat Med 2011;17:1371-80. [PubMed]
- Kobayashi S, Kishimoto T, Kamata S, et al. Rapamycin, a specific inhibitor of the mammalian target of rapamycin, suppresses lymphangiogenesis and lymphatic metastasis. Cancer Sci 2007;98:726-33. [PubMed]
- Spirli C, Okolicsanyi S, Fiorotto R, et al. Mammalian target of rapamycin regulates vascular endothelial growth factor-dependent liver cyst growth in polycystin-2-defective mice. Hepatology 2010;51:1778-88. [PubMed]
- Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099-109. [PubMed]
- Lin B, Podar K, Gupta D, et al. The vascular endothelial growth factor receptor tyrosine kinase inhibitor PTK787/ZK222584 inhibits growth and migration of multiple myeloma cells in the bone marrow microenvironment. Cancer Res 2002;62:5019-26. [PubMed]
- Yau T, Chan P, Pang R, et al. Phase 1-2 trial of PTK787/ZK222584 combined with intravenous doxorubicin for treatment of patients with advanced hepatocellular carcinoma: implication for antiangiogenic approach to hepatocellular carcinoma. Cancer 2010;116:5022-9. [PubMed]
- de Jonge MJ, Dumez H, Kitzen JJ, et al. Phase I safety and pharmacokinetic study of SU-014813 in combination with docetaxel in patients with advanced solid tumours. Eur J Cancer 2011;47:1328-35. [PubMed]
- Konings IR, de Jonge MJ, Burger H, et al. Phase I and pharmacological study of the broad-spectrum tyrosine kinase inhibitor JNJ-26483327 in patients with advanced solid tumours. Br J Cancer 2010;103:987-92. [PubMed]
- Ruggeri B, Singh J, Gingrich D, et al. CEP-7055: a novel, orally active pan inhibitor of vascular endothelial growth factor receptor tyrosine kinases with potent antiangiogenic activity and antitumor efficacy in preclinical models. Cancer Res 2003;63:5978-91. [PubMed]


