Circulating tumor cells in gastrointestinal malignancies
Introduction
Metastatic disease causes over 90% of cancer mortality (1). The first report of tumor cells found in the circulation of a patient with metastatic cancer was published nearly 150 years ago (2). These circulating tumor cells (CTCs) are thought to have potential metastatic activity or even originate from cancerous stem cells (3). Due to the prognostic and predictive potential, CTCs possess several techniques, cytometric (capturing individual cells) and nucleic-acid based [targeting messanger Ribonucleic Acid (mRNA) expression], have been developed in recent years to detect, isolate, enumerate, and/or characterize CTC in solid organ malignancies. These methods need to be sensitive, specific, reproducible, and overcome challenges of detecting rare tumor cells out of hundreds of thousands of peripheral blood cells. During the last two decades immunocytochemistry, flow cytometry, and reverse transcription- polymerase chain reaction (RT-PCR) have been used for these purposes (4,5).
During the past decade several new cytometric techniques have emerged, which rely on the different morphological and molecular characteristics of epithelial tumor cells. Common techniques for CTC enrichment (isolation) and detection (identification) include: (I) cellular density gradient separation of CTC and mononuclear cells (MN) [OncoQuick® (Greiner, Frickenhausen, Germany)] followed by immunolabeling; (II) Isolation by size of epithelial tumor cells (ISET) that involves direct filtration (mechanical separation through polycarbonate membrane with 8 µm pores) and immunolabeling; and (III) Immunomagnetic separation (IMS). The latter is the most commonly performed enrichment technique, using either classic separation methods: MACS® [Magnetic Activated Cell Separation (Miltenyi Biotec, Auburn, CA, USA)] followed by immunolabeling and genetic or molecular characterization by fluorescence in situ hybridization (FISH) or RT-PCR; Dynabeads® (Dynal Biotech, Invitrogen) with anti-EpCAM/Epithelial Specific Antigen (anti-BerEP4) antibodies (Abs) [IgG1 monoclonal Ab to Epithelial Cell Adhesion Molecule (EpCAM)]; or the semi-automated CellSearch® (Veridex, Raritan, NJ, USA) system that utilizes immunomagnetic beads coated with EpCAM Abs (capturing EpCAM-expressing epithelial tumor cells), followed by immunostaining for 4',6-diamidino-2-phenylindole (DAPI) (nucleated cell) and CK8+/-18+/-19 presence (epithelial cell) along with CD45 (leukocyte marker) absence (5-7). New innovative detection methods have been investigated, such as the CTC-chip (8), based on microfluidic platforms.
The prognostic implications of CTC detection and monitoring have been a matter of great controversy partially due to small patient populations and diverse detection techniques, protocols, and assays. Although the US Food and Drug Administration (FDA) approved the CellSearch® system in metastatic breast, prostate, and colorectal cancers (9) based on its prognostic value (10-12), use of the system has not been incorporated into clinical guidelines in metastatic colorectal cancer (mCRC) in terms of serial monitoring or clinical management.
Our objective is to review the usefulness, sensitivity, and specificity, as well as prognostic value, of CTC detection in both early and metastatic stages of the various gastrointestinal malignancies. The article outlines CTC status in colorectal, pancreatic, hepatobiliary, esophageal, gastric, and small intestine cancers, as well as neuroendocrine tumors.
Colorectal cancer
Multiple studies, mainly in the perioperative setting, examined CTC status in mCRC and non-metastatic colorectal cancer (nmCRC) via molecular and cytometric techniques.
Detection rates of CTC in mCRC are between 18.7% and 75% (6,13-22). Patients without detectable CTC post chemotherapy (regardless of their pre-chemotherapy status) survived longer (17). The two larger studies in mCRC showed that CTC status was an independent predictor of progression free survival (PFS) and overall survival (OS) among 430 (13) and 467 (14) patients. Those who became seronegative during treatment [after 3-5 weeks (10) versus 1-3 (14) weeks, respectively] had improved PFS and OS than those who remained positive. The discrepancy between the two studies was attributed to the use of bevacizumab in the latter study, yet it remains unclear when a repeat CTC count needs to be completed. Additional studies, with fewer patients, reported comparable results, indicating the importance of CTC detection. The CellSearch® system received the FDA approval in 2007 for mCRC monitoring with positive cutoff of ≥3 CTC/7.5 mL (10). Postoperative positive CTC are associated with decreased PFS and OS (22). CTC detection increases during intraoperative liver manipulation (22) and also after colonoscopy (23), without clear clinical significance. In any stage colorectal cancer (CRC) the cytometric detection rate was 32% (of 93 patients) (24) and associated with advanced disease (19,20) and peritoneal dissemination (21). Reduced CTCs counts following therapy correlated with response (in mCRC) (18) or remission (24), whereas elevated levels were associated with recurrence or disease progression (24), as well as reduced PFS and OS (18).
Wong et al. (9) used refined CK20-Dynabeads® protocol and detected CTC in 57% of 101 nmCRC patients pre-operatively, with correlation to disease stage and early recurrence. Gazzaniga et al. (25) reported decrease in CTC counts from median number of 2.7 to 0 post 3-4 months of chemotherapy and bevacizumab in 89% of 20 mCRC patients, yet 56% of them, developed disease progression. In the control group, treated with chemotherapy and cetuximab, 30% remained CTC positive (≥3) with disease progression in all of them. 93% of the remainder 14 patients with decreased CTC counts [0-2] were found to have stable disease or partial response. This phenomenon led the researchers to conclude that bevacizumab was likely associated with biological changes of CTC, perhaps endothelial mesenchymal transformation (EMT), thus undetectable by CellSearch®.
Detection of CTC was shown to depend on the IMS prior to CK20 RT-PCR, with intra-patient heterogeneity based on the beads-coated- EpCAM Abs used (26). A study that compared four different non-automated enrichment techniques in 38 patients with mCRC showed superiority of MACS® HEA MicroBeads over RosetteSep® (StemCell Technologies, Vancouver, Canada), OncoQuick®, and OncoQuick® Plus. Detectable CTC was associated with reduced PFS but similar OS (27).
Coumans et al. (28) explored 428 mCRC patients and predicted that 99% of patients will have at least 1 CTC in 5 L of blood at baseline and one month following chemotherapy, consistent with the low treatment success rates in metastatic cancer. They calculated survival decreased by 6.6 months for each 10-fold increase in CTC in metastatic cancer.
Several novel cytometric detection methods have been developed and studied in recent years. Fabbri et al. (29) used DEPArray (a di-electrophoresis-based platform), to detect CTC in 52.5% of 40 mCRC patients. Zhang et al. (30) developed an electrospun TiO2 nanofibers (TiNFs) platform and detected CTCs in 2 out of 3 patients with CRC. Sheng et al. (31) developed an aptamer-mediated, micropillar-based microfluidic device (as an alternative to Abs) for capturing CTC from whole blood. The device was able to isolate as few as 10 colorectal CTC from 1 mL of unprocessed whole blood, with about 93% captured cell viability. Pecot et al. (32) developed a microfluidic-based platform that is able to capture CTC in their post-EMT stage. The system includes a combination of Abs against epithelial cell surface antigens [i.e., EpCAM, HER2, MUC1, Epidermal growth factor receptor (EGFR)] and mesenchymal cell antigens (c-MET, N-cadherin, CD318, and mesenchymal stem cell antigen) and was shown to be more sensitive for CTC enumeration than CellSearch®. Desitter et al. (33) report the development of the ScreenCell®-single-use device which can filter, isolate, and sort CTC by size in a costly fashion and allows further biological analysis. Du et al. (34) tested a novel microfluidic capturing chip and detected CTC in 67.7% of 68 CRC patients: 22.2% of 9 patients with Dukes A, 41.6% of 24 with Dukes B, 95.5% of 22 with Dukes C, and in 100% of 13 with Dukes D (metastatic disease). CTC status correlated with clinical stage. Marrinucci et al. (35) used immunofluorescent staining with fiber-optic array scanning method to detect CTC in mCRC, and found significant intrapatient pleomorphism, yet with CTC correlation to the primary or secondary tumor samples. Yen et al. (36) developed KRAS membrane array and showed that mutation status in detected CTC can predict response to chemotherapy plus cetuximab in 76 mCRC patients and affect PFS and OS. The group (37) improved the gene expression array and developed weighted chemiluminescent membrane array for the detection of CTC harboring KRAS mutations. They reported increased sensitivity, specificity, and accuracy rates of 90.2%, 94.9%, and 93.5%, respectively.
Heitzer et al. (15) performed genomic profiling of CTC and showed mutation correlation between CTC, the primary tumor, and metastases, thus creating new possibilities for peripheral biopsy via CTC analysis. Fabbri et al. (29) showed KRAS mutational concordance between CTC and the primary tumor in 50% of mCRC patients. However, Gasch et al. (16) showed considerable intra- and interpatient molecular characterization heterogeneity in EGFR, KRAS, and PIK3CA gene mutations, as well as EGFR expression. CTC-genes in mCRC patients are associated with cell movement, adhesion, signaling, death and proliferation, and their expression correlates with clinical and prognostic factors (38).
On the other hand, cytometric methods produced very low detection rates in nmCRC (39). Using the CellSearch® system, CTC detection rate in nmCRC is between 0% and 25.7% (16,19-21,39).
Studies that explored CTC detection via molecular techniques, mainly RT-PCR, examined mostly carcinoembryonic Antigen (CEA) (40-47), CK19 (40,42,43), CK20 (40-45,48-51), and survivin (45,52) mRNA. No single method was proven to be superior to others. Molecular CTC detection with RT-PCR was reported in a meta-analysis that included seven studies in 4% to 57% of 1,260 patients with nmCRC (53) and is associated with reduced OS (48) and PFS(43,48,53,54). Uen et al. (55) explored post-operative CTC detection with a multipanel marker (CEA/hTERT/CK19/CK20 mRNA) in 194 patients with stage II CRC. They found 27-fold hazard ratio for relapse in patients with positive CTC status. A membrane array with the latter panel was tested in 157 patients with nmCRC showed reduced PFS and OS (56).
Multimarker CEA/CK/CD133 mRNA assay was not prognostic in 735 patients post curative surgery with Dukes A or B with predetermined favorable features (42). However, in Dukes B and C it is associated with reduced OS (40,42,57) and reduced PFS (40,42), suggesting the need for adjuvant therapy post curative surgery (42,54).
A recent meta-analysis (58) looked at CTC and disseminated tumor cells (DTC) in CRC with resectable liver metastases or widespread metastases and analyzed twelve studies (representing 1,329 patients). Positive CTC status had a 2.5-fold increased chance of death and a twofold increased chance of disease progression or recurrence (yet only PFS was statistically significant). In 2010, a meta-analysis (59) pooling 3,094 nmCRC patients from 36 studies, utilized different molecular and cytometric methods and showed reduced PFS and OS.
A PCR-based method, the transcription-reverse transcription concerted reaction (TRC) technique, was found to be comparable with the CellSearch® system in terms of CTC detection and correlation with OS in mCRC patients, yet the former was a quicker and cheaper alternative (60). Gervasoni et al. (61) compared the detection rates of CTC in CRC patients using three different methods. The detection rates were 75% in a multimarker RT-PCR assay, 20% in the CellSearch® system, and 14.3% in a dHPLC-based gene mutation analysis. None of the negative RT-PCR samples was found to be positive by the other two methods, indicating it provided the best CTC detection rate.
Bessa et al. found no significance to perioperative CTC detection via CEA mRNA RT-PCR in nmCRC (62,63). Positive CTC status was associated with disease recurrence (49) and reduced survival in colorectal liver metastasis patients undergoing radical liver resection, suggesting the need for adjuvant therapy (52). In any CRC stage, positive CTC is associated with clinical stage (44,45,50), reduced OS (44,64), and reduced PFS (50).
In conclusion, there is need to standardize and improve the sensitivity and specificity of the available CTC detection methods (65). Randomized trials are required to compare the standard monitoring versus CTC-guided management of patients with CRC. Currently only the CellSearch® system is FDA-approved. Prospective studies with tailored therapy based on CTC status are warranted to improve outcomes in subgroup of patients who require adjuvant therapy.
Pancreatic cancer
Several studies have evaluated the use of the CellSearch® system. In the setting of advanced or metastatic disease, detection rates of 40% to 80.5% has been reported in various studies using the CellSearch® system or other IMS methods followed by RT-PCR (66-68). ISET (69) had a CTC detection rate of 90% vs. 40% using the CellSearch® system in patients with inoperable or metastatic pancreatic cancer. High definition CTC assay (HD-CTC) is a new technique using different immunofluorescence staining and high resolution cell imaging. It detected more CTCs when compared to the CellSearch® system (5/15 vs. 4/15) in non-gastrointestinal cancers (70). CTC detection has been shown to negatively predict prognosis and response to chemotherapy (67). In a recent study (68) using the CellSearch® system and immunologic marker for MUC1 and EGFR in patients with metastatic pancreatic cancer treated with first line chemotherapy (n=40); ≥1 CTC per 7.5 mL peripheral blood were detected in 50%, 39% and 28% at baseline, 7 days and 6-10 weeks, respectively. Patients who have a positive CTC status at baseline had worse OS and PFS compared to CTC negative patients (125 vs. 298 days, P=0.12). De Albuquerque et al. (71) compared CTC detection between pancreatic cancer patients (n=34) and healthy controls using IMS with MUC1 and EpCAM Abs followed by RT-PCR. CTC was detected in approximately half of the cancer patients and none of the control group. PFS was 66 days for CTC positive patients compared to 138 days for CTC negative patients (P=0.01) showing that CTC detection is associated with poor prognosis.
CTC detection rates in non-metastatic resectable pancreatic cancer are currently limited. Intra-operative CTC detection by CEA mRNA via RT-PCR correlated with hematogenous metastasis after surgery (37.5% in CTC positive group vs. 11.4% in the negative group). The OS among stage I, II, and III patients was worse in the CTC positive group (P=0.03) (72).
CTC detection is shown to correlate with lower OS and PFS in pancreatic cancer. Further research is needed to increase the accuracy of CTC testing, thereby facilitating its use in early stage cancer, detection of micrometastasis and monitoring residual disease.
Hepatobiliary cancer
Studies utilizing cytometric methods for CTC detection in hepatocellular carcinoma (HCC) are scarce. Vona et al. (73) evaluated the clinical significance of ISET for CTC detection in patients with non-metastatic HCC compared to a control group comprising of chronic hepatitis, cirrhosis and healthy subjects. CTC was detected in 23 out of 44 patients with HCC and none in the control groups. CTC detection correlated with tumor invasion, portal tumor thrombosis, and reduced survival. Sabile et al. (74) mixed blood of human volunteers with HepG2 liver tumor cells and analyzed the efficiency of various isolation techniques. They showed that density gradient separation and ber-Ep4 immuno-capture are the most sensitive techniques for capturing liver CTC in vivo. When Ber Ep4 immunobeads are used after density gradient centrifugation followed by RT-PCR, ≥10 HCC CTC/5 mL peripheral blood were detected (75). Waguri et al. (76) used telomerase reverse transcriptase (hTERT) mRNA to detect 100-1 CTC in 2 mL blood of HCC patients after IMS. Fifty-three percent of cases were CTC positive compared to none in the control group. Zee et al. (77) evaluated the CellSearch® system for CTC detection in 20 patients with locally advanced or metastatic HCC patients. Nine patients had detectable CTC (≥2 CTC per 7.5 mL of blood). Schulze et al. (78) studied the CellSearch® system in 59 HCC patients and 19 control patients (with cirrhosis and benign hepatic lesions). Detection of ≥1 CTC was found in 18 HCC patient versus a single control patient (P=0.026). Identification of CTC correlated with worse OS (460 vs. 746 days, P=0.017). Further studies are needed to validate the system in HCC.
Molecular detection methods have utilized mainly alpha fetoprotein (AFP) mRNA (76,79-81), along with hTERT (82) and CK19 (83) mRNA. AFP mRNA based CTC detection ranges between 10% to 54% and can positively predict recurrence and extra- hepatic metastasis (4). Use of AFP mRNA as a marker for HCC is limited because of high false positive rates due to detection in patients with chronic liver disease (12.5%) (84). In a recent study (82), pre-operative peripheral blood hTERT mRNA did not predict recurrence in patients with HCC following resection or transplant.
CTC could be a promising method for surveillance of HCC and assessing eligibility for liver transplantation. Specific hepatic CTC markers are currently lacking. Further research is warranted to find a marker that has good efficacy in detecting liver derived CTC.
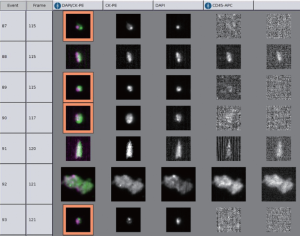
Moreover, the data regarding CTC detection in biliary cancer are also minimal. We (85) recently reported the use of CellSearch® system to detect CTC from 16 patients with cholangiocarcinoma and gallbladder cancer (Figure 1). Using a cutoff of >1 CTC/7.5 mL blood, four patients were found to have positive CTC status, all in stage III or IV. At 12 months of follow up, 25% of patients with positive CTC compared to 50% of patients with negative CTC were alive. Although it was not a statistically significant result, it served as a proof of concept in this rare disease. Prospective validation is currently ongoing for this matter.
On the other hand, molecular techniques utilizing CK19 or hTERT mRNA were used to detect CTC in 40 biliary cancer patients. CTCs were found in 45% of the patients and were associated with worse OS (86). A cohort of pancreaticobiliary cancer patients was studied after curative resection (n=53) using RT-PCR to detect CEA mRNA. Positive CTC status was found in 75% of relapsed patients but only in 5.4% in disease-free patients, suggesting that it might indicate early relapse (87). Using the same technique in biliary cancer patients, intraoperative detection of CEA mRNA was associated with recurrence and worse survival (72).
Esophageal and gastric cancer
Cytometric detection has been rarely reported in esophageal cancer (21,88). Hiraiwa et al. (21) detected CTC with the CellSearch® system in 38 patients. CTC detection did not correlate with tumor stage, however, in metastatic disease, it correlated with dissemination and decreased survival. De Albuquerque et al. (89) used IMS with MUC1 and EpCAM Abs followed by RT-PCR analysis of a multi-marker gene panel (KRT19, MUC1, EpCAM, CEACAM5, BIRCS, SCGB2A2, and ERBB2). Nakamora et al. (88) enriched blood samples of 47 squamous cell carcinoma (SCC) patients via Dynabeads® following immunostaining with anti-CK Abs. Eighteen patients (38%) were CTC positive. Four of 7 (57%) positive patients who underwent surgery and 2 out of 26 (7.7%) negative patients suffered from recurrence. Those who remained positive post chemotherapy (with or without radiation) had decreased survival rates than those who became CTC negative.
Most esophageal CTC detection studies utilized molecular techniques, mainly RT-PCR for the detection of tumor-specific mRNA. Researchers often examined CEA (47,90-97), but also SCC (90,98), cytokeratin (CK)7 (91), CK19, CK20, and survivin mRNA (92,99,100). CTC detection was also reported in primary malignant melanoma of the esophagus by RT-PCR (101). CEA or SCC mRNA were detected in 13.9% of 244 patients prior to esophagectomy and 16.8% post-surgery, possibly due to cell dissemination during surgery. CTC status post-surgery was an independent prognostic factor of PFS and was a predictor for local and systemic recurrence (90). CEA, CK19 and Survivin mRNA were detected in 54.2% and 38.9% of 72 patients with SCC by nested RT-PCR prior and post radiotherapy, respectively. Positive CTC post-radiotherapy was an independent poor prognostic factor (92). Detection rates are heterogeneous between studies, even for identical markers. CEA mRNA was reported in 61.6% (of 125 SCC perioperative patients) (95); 28.3%, 60.4%, and 42.9% (before, immediately after, and three days following surgery in 53 patients) (94); 25% (of 28 patients) (96); and 57.4% (of 54 SCC patients) (97). SCC mRNA was reported in 33% and 45.8% of 70 patients with SCC prior to curative esophagectomy and intraoperative, respectively (98). Nearly all of these studies correlated positive CTC status with poor prognosis, including reduced OS (99,100), post adjuvant therapy relapse (100), advanced disease (93,95,97), recurrence and shortened DFS (95).
Cytometric detection again has been rarely reported in gastric cancer (21,102). Hiraiwa et al. (21) demonstrated with the CellSearch® system that positive CTC status correlated with advanced stage, peritoneal dissemination, and decreased survival. The group also showed that CTC counts were higher in metastatic disease (55%) versus non-metastatic disease (14%). Matsusaka et al. (102) prospectively examined CTC with the CellSearch® system in 52 patients with advanced cancer prior to and following chemotherapy, showing decreased OS and PFS in CTC positive patients. De Albuquerque et al. (89) used IMS with MUC1 and EpCAM Abs followed by RT-PCR of a multi-marker gene panel, showing detection rate of 62.2%. Hosokawa et al. (103) developed a size-selective microcavity array followed by image-based immunophenotypic analysis using a fluorescence microscope, with high detection efficiency of over 80%. The system was found beneficial for detection of EpCAM-negative CTC. As mentioned (under CRC), Zhang et al. (30) developed an electrospun TiO (2) nanofibers (TiNFs) platform with anti-EpCAM assay and detected CTC in 7 out of 7 patients.
Again, most CTC detection studies utilized molecular techniques, mainly RT-PCR for mRNA expression. Most researchers examined CEA (47,104-114), CK7 (114), CK18 (110), CK19 (108-111,113-115), CK20 (51,109,110), hTERT (108,109,116), MUC1 (108,116,117), and survivin (111,118) mRNA, without significant evidence to support one method over the other. CEA mRNA was reported in 24.1% at diagnosis and 34.4% at 10.6 months follow-up in 29 patients with gastric adenocarcinoma (104). It has been reported in 18.8% of 16 resectable patients and in 100% of 10 metastatic patients (47). Other studies reported a range of detection between 7.7% and 78.6% (105-107,109,112-114). CTC detection has been associated with outcome and poor survival (108,110,112,118,119). Positive CTC status is associated with tumor invasion depth (105,108,117), major vascular invasion (113), metastatic disease (105,114), recurrence (107,110,112,120), advanced disease (112,117-119), and post-operative metastases (108,109,117). Conversion to a positive CTC status is associated with recurrence post curative resection (107). A multi-marker panel was an independent predictor for postoperative metastasis or recurrence with a sensitivity and specificity of 89.1% and 91.3%, respectively (108). Positive status was shown to correlate with shorter survival among chemotherapy-non-responders (115). In a blinded prospective study in 810 Japanese gastric cancer patients, positive CTC status along with increased VEGFR-1 expression were associated with metastatic disease (114).
Gastroenteropancreatic neuroendocrine tumors
This subset of neuroendocrine tumors (29) is a diverse group of cancers that secret various amines and peptides, has heterogeneous clinical manifestations, and strongly expresses EpCAM. Khan et al. (121) utilized the CellSearch® system to explore CTC status in 42 midgut neuroendocrine tumors (NET) (including 26 ileal and 7 appendiceal), 3 gastric, and 19 pancreatic NET patients, divided into progressive and non-progressive disease per imaging studies. CTC was positive in 43% and 21% of metastatic midgut and pancreatic NET, respectively. CTCs was found to be associated with disease progression. Also, an increase in more than 33% in the number of CTC following one month of therapy was associated with poor prognosis (122). Later the group conducted a single-center prospective study and reported prevalence of CTC (≥1) in 60.3% of 146 patients with metastatic NET (85.7% of 42 pancreatic NET, 50.5% of 101 midgut NET, and 33.3% of 3 hindgut NET). Detection of CTC is associated with advanced stage, high serum chromogranin A level, high tumor burden, as well as worse OS and PFS (123).
Small intestine malignancy
The data on the use of CTC in small intestinal carcinoma are limited. De Albuquerque et al. (89) used IMS with MUC1 and EpCAM Abs followed by RT-PCR analysis of the abovementioned multi-marker panel and showed detection rate of 33.3% of small intestine adenocarcinomas.
Limitations
The field of CTCs is rapidly evolving yet still faces many challenges on the path to finding the optimal method of detection for prognostication purposes (Table 1). Some of the challenges arise from the heterogeneity of different tumors biology, therefore different detection cutoff definition has been used even for a single technique (94). Another topic that was discussed above is the notion of EMT, where some CTCs do not express epithelial markers such as EpCAM and consequently could not be recognized by the current methods (21). This could be surpassed by using improved techniques or targeting different antigens other than CK or EpCAM (32). These issues in addition to differences in sample handling and processing has led to inconsistent research results. Also, the current methods are able to detect a “cancer cell” yet current studies are still ongoing to phenotypically or genetically characterize thoses CTCs (124). Each of the available methods has advantages and disadvantages. Where cellular methods such as CellSearch® provide high specificity in semi-automated fashion, RT-PCR molecular techniques provide better rates of detection, yet lacking the reproducibility or FDA approval and the ability to further test the identified cells (53).

Full Table
In conclusion, detection of CTCs may provide prognostic and predictive tool in gastrointestinal malignancies. Large clinical trials are warranted similarly to those conducted in CRC to clearly define the utility of these techniques in clinical practice.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Weiss L. Metastasis of cancer: a conceptual history from antiquity to the 1990s. Cancer Metastasis Rev 2000;19:I-XI, 193-383. [PubMed]
- Ashworth TR. A case of cancer in which cells similar to those in the tumors were seen in the blood after death. Aust Med J 1869;14:146-9.
- Maheswaran S, Haber DA. Circulating tumor cells: a window into cancer biology and metastasis. Curr Opin Genet Dev 2010;20:96-9. [PubMed]
- Bidard FC, Ferrand FR, Huguet F, et al. Disseminated and circulating tumor cells in gastrointestinal oncology. Crit Rev Oncol Hematol 2012;82:103-15. [PubMed]
- Lurje G, Schiesser M, Claudius A, et al. Circulating tumor cells in gastrointestinal malignancies: current techniques and clinical implications. J Oncol 2010;2010:392652.
- Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004;10:6897-904. [PubMed]
- Miron N, Susman S, Balacescu O, et al. Novel cellular and molecular approaches to stratification and treatment of colorectal cancer. J Gastrointestin Liver Dis 2012;21:413-21. [PubMed]
- Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007;450:1235-9. [PubMed]
- Wong SC, Chan CM, Ma BB, et al. Clinical significance of cytokeratin 20-positive circulating tumor cells detected by a refined immunomagnetic enrichment assay in colorectal cancer patients. Clin Cancer Res 2009;15:1005-12. [PubMed]
- Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213-21. [PubMed]
- De Mattos-Arruda L, Olmos D, Tabernero J. Prognostic and predictive roles for circulating biomarkers in gastrointestinal cancer. Future Oncol 2011;7:1385-97. [PubMed]
- Sun YF, Yang XR, Zhou J, et al. Circulating tumor cells: advances in detection methods, biological issues, and clinical relevance. J Cancer Res Clin Oncol 2011;137:1151-73. [PubMed]
- Cohen SJ, Punt CJ, Iannotti N, et al. Prognostic significance of circulating tumor cells in patients with metastatic colorectal cancer. Ann Oncol 2009;20:1223-9. [PubMed]
- Tol J, Koopman M, Miller MC, et al. Circulating tumour cells early predict progression-free and overall survival in advanced colorectal cancer patients treated with chemotherapy and targeted agents. Ann Oncol 2010;21:1006-12. [PubMed]
- Heitzer E, Auer M, Gasch C, et al. Complex Tumor Genomes Inferred from Single Circulating Tumor Cells by Array-CGH and Next-Generation Sequencing. Cancer Res 2013;73:2965-75. [PubMed]
- Gasch C, Bauernhofer T, Pichler M, et al. Heterogeneity of epidermal growth factor receptor status and mutations of KRAS/PIK3CA in circulating tumor cells of patients with colorectal cancer. Clin Chem 2013;59:252-60. [PubMed]
- Kawahara H, Watanabe K, Toyama Y, et al. Determination of circulating tumor cells for prediction of recurrent colorectal cancer progression. Hepatogastroenterology 2012;59:2115-8. [PubMed]
- Matsusaka S, Suenaga M, Mishima Y, et al. Circulating tumor cells as a surrogate marker for determining response to chemotherapy in Japanese patients with metastatic colorectal cancer. Cancer Sci 2011;102:1188-92. [PubMed]
- Maestro LM, Sastre J, Rafael SB, et al. Circulating tumor cells in solid tumor in metastatic and localized stages. Anticancer Res 2009;29:4839-43. [PubMed]
- Sastre J, Maestro ML, Puente J, et al. Circulating tumor cells in colorectal cancer: correlation with clinical and pathological variables. Ann Oncol 2008;19:935-8. [PubMed]
- Hiraiwa K, Takeuchi H, Hasegawa H, et al. Clinical significance of circulating tumor cells in blood from patients with gastrointestinal cancers. Ann Surg Oncol 2008;15:3092-100. [PubMed]
- Papavasiliou P, Fisher T, Kuhn J, et al. Circulating tumor cells in patients undergoing surgery for hepatic metastases from colorectal cancer. Proc (Bayl Univ Med Cent) 2010;23:11-4. [PubMed]
- Koch M, Kienle P, Sauer P, et al. Hematogenous tumor cell dissemination during colonoscopy for colorectal cancer. Surg Endosc 2004;18:587-91. [PubMed]
- Yalcin S, Kilickap S, Portakal O, et al. Determination of circulating tumor cells for detection of colorectal cancer progression or recurrence. Hepatogastroenterology 2010;57:1395-8. [PubMed]
- Gazzaniga P, Raimondi C, Gradilone A, et al. Circulating tumor cells, colon cancer and bevacizumab: the meaning of zero. Ann Oncol 2011;22:1929-30. [PubMed]
- Antolovic D, Galindo L, Carstens A, et al. Heterogeneous detection of circulating tumor cells in patients with colorectal cancer by immunomagnetic enrichment using different EpCAM-specific antibodies. BMC Biotechnol 2010;10:35. [PubMed]
- Königsberg R, Gneist M, Jahn-Kuch D, et al. Circulating tumor cells in metastatic colorectal cancer: efficacy and feasibility of different enrichment methods. Cancer Lett 2010;293:117-23. [PubMed]
- Coumans FA, Ligthart ST, Uhr JW, et al. Challenges in the enumeration and phenotyping of CTC. Clin Cancer Res 2012;18:5711-8. [PubMed]
- Fabbri F, Carloni S, Zoli W, et al. Detection and recovery of circulating colon cancer cells using a dielectrophoresis-based device: KRAS mutation status in pure CTCs. Cancer Lett 2013;335:225-31. [PubMed]
- Zhang N, Deng Y, Tai Q, et al. Electrospun TiO2 nanofiber-based cell capture assay for detecting circulating tumor cells from colorectal and gastric cancer patients. Adv Mater 2012;24:2756-60. [PubMed]
- Sheng W, Chen T, Kamath R, et al. Aptamer-enabled efficient isolation of cancer cells from whole blood using a microfluidic device. Anal Chem 2012;84:4199-206. [PubMed]
- Pecot CV, Bischoff FZ, Mayer JA, et al. A novel platform for detection of CK+ and CK- CTCs. Cancer Discov 2011;1:580-6. [PubMed]
- Desitter I, Guerrouahen BS, Benali-Furet N, et al. A new device for rapid isolation by size and characterization of rare circulating tumor cells. Anticancer Res 2011;31:427-41. [PubMed]
- Du HX, Zhang ZG, Yang ZL, et al. Separation of circulating cancer cells by unique microfluidic chip in colorectal cancer. Oncol Res 2011;19:487-500. [PubMed]
- Marrinucci D, Bethel K, Lazar D, et al. Cytomorphology of circulating colorectal tumor cells:a small case series. J Oncol 2010;2010:861341.
- Yen LC, Yeh YS, Chen CW, et al. Detection of KRAS oncogene in peripheral blood as a predictor of the response to cetuximab plus chemotherapy in patients with metastatic colorectal cancer. Clin Cancer Res 2009;15:4508-13. [PubMed]
- Yang MJ, Chiu HH, Wang HM, et al. Enhancing detection of circulating tumor cells with activating KRAS oncogene in patients with colorectal cancer by weighted chemiluminescent membrane array method. Ann Surg Oncol 2010;17:624-33. [PubMed]
- Barbazán J, Alonso-Alconada L, Muinelo-Romay L, et al. Molecular characterization of circulating tumor cells in human metastatic colorectal cancer. PLoS One 2012;7:e40476. [PubMed]
- Thorsteinsson M, Söletormos G, Jess P. Low number of detectable circulating tumor cells in non-metastatic colon cancer. Anticancer Res 2011;31:613-7. [PubMed]
- Shimada R, Iinuma H, Akahane T, et al. Prognostic significance of CTCs and CSCs of tumor drainage vein blood in Dukes’ stage B and C colorectal cancer patients. Oncol Rep 2012;27:947-53. [PubMed]
- Park SY, Choi GS, Park JS, et al. Influence of surgical manipulation and surgical modality on the molecular detection of circulating tumor cells from colorectal cancer. J Korean Surg Soc 2012;82:356-64. [PubMed]
- Iinuma H, Watanabe T, Mimori K, et al. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes’ stage B and C colorectal cancer. J Clin Oncol 2011;29:1547-55. [PubMed]
- Uen YH, Lu CY, Tsai HL, et al. Persistent presence of postoperative circulating tumor cells is a poor prognostic factor for patients with stage I-III colorectal cancer after curative resection. Ann Surg Oncol 2008;15:2120-8. [PubMed]
- Tsouma A, Aggeli C, Lembessis P, et al. Multiplex RT-PCR-based detections of CEA, CK20 and EGFR in colorectal cancer patients. World J Gastroenterol 2010;16:5965-74. [PubMed]
- Shen C, Hu L, Xia L, et al. Quantitative real-time RT-PCR detection for survivin, CK20 and CEA in peripheral blood of colorectal cancer patients. Jpn J Clin Oncol 2008;38:770-6. [PubMed]
- Guo J, Xiao B, Zhang X, et al. Combined use of positive and negative immunomagnetic isolation followed by real-time RT-PCR for detection of the circulating tumor cells in patients with colorectal cancers. J Mol Med (Berl) 2004;82:768-74. [PubMed]
- Noh YH, Im G, Ku JH, et al. Detection of tumor cell contamination in peripheral blood by RT-PCR in gastrointestinal cancer patients. J Korean Med Sci 1999;14:623-8. [PubMed]
- Liu Y, Qian J, Feng JG, et al. Detection of circulating tumor cells in peripheral blood of colorectal cancer patients without distant organ metastases. Cell Oncol (Dordr) 2013;36:43-53. [PubMed]
- Rahbari NN, Reissfelder C, Mühlbayer M, et al. Correlation of circulating angiogenic factors with circulating tumor cells and disease recurrence in patients undergoing curative resection for colorectal liver metastases. Ann Surg Oncol 2011;18:2182-91. [PubMed]
- Koyanagi K, Bilchik AJ, Saha S, et al. Prognostic relevance of occult nodal micrometastases and circulating tumor cells in colorectal cancer in a prospective multicenter trial. Clin Cancer Res 2008;14:7391-6. [PubMed]
- Lukyanchuk VV, Friess H, Kleeff J, et al. Detection of circulating tumor cells by cytokeratin 20 and prostate stem cell antigen RT-PCR in blood of patients with gastrointestinal cancers. Anticancer Res 2003;23:2711-6. [PubMed]
- Pilati P, Mocellin S, Bertazza L, et al. Prognostic value of putative circulating cancer stem cells in patients undergoing hepatic resection for colorectal liver metastasis. Ann Surg Oncol 2012;19:402-8. [PubMed]
- Thorsteinsson M, Jess P. The clinical significance of circulating tumor cells in non-metastatic colorectal cancer--a review. Eur J Surg Oncol 2011;37:459-65. [PubMed]
- Koch M, Kienle P, Kastrati D, et al. Prognostic impact of hematogenous tumor cell dissemination in patients with stage II colorectal cancer. Int J Cancer 2006;118:3072-7. [PubMed]
- Uen YH, Lin SR, Wu DC, et al. Prognostic significance of multiple molecular markers for patients with stage II colorectal cancer undergoing curative resection. Ann Surg 2007;246:1040-6. [PubMed]
- Wang JY, Lin SR, Wu DC, et al. Multiple molecular markers as predictors of colorectal cancer in patients with normal perioperative serum carcinoembryonic antigen levels. Clin Cancer Res 2007;13:2406-13. [PubMed]
- Yokobori T, Iinuma H, Shimamura T, et al. Plastin3 is a novel marker for circulating tumor cells undergoing the epithelial-mesenchymal transition and is associated with colorectal cancer prognosis. Cancer Res 2013;73:2059-69. [PubMed]
- Groot Koerkamp B, Rahbari NN, Büchler MW, et al. Circulating Tumor Cells and Prognosis of Patients with Resectable Colorectal Liver Metastases or Widespread Metastatic Colorectal Cancer: a meta-analysis. Ann Surg Oncol 2013. [Epub ahead of print]. [PubMed]
- Rahbari NN, Aigner M, Thorlund K, et al. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterology 2010;138:1714-26. [PubMed]
- Sato N, Hayashi N, Imamura Y, et al. Usefulness of transcription-reverse transcription concerted reaction method for detecting circulating tumor cells in patients with colorectal cancer. Ann Surg Oncol 2012;19:2060-5. [PubMed]
- Gervasoni A, Sandri MT, Nascimbeni R, et al. Comparison of three distinct methods for the detection of circulating tumor cells in colorectal cancer patients. Oncol Rep 2011;25:1669-703. [PubMed]
- Bessa X, Piñol V, Castellví-Bel S, et al. Prognostic value of postoperative detection of blood circulating tumor cells in patients with colorectal cancer operated on for cure. Ann Surg 2003;237:368-75. [PubMed]
- Bessa X, Elizalde JI, Boix L, et al. Lack of prognostic influence of circulating tumor cells in peripheral blood of patients with colorectal cancer. Gastroenterology 2001;120:1084-92. [PubMed]
- Taniguchi T, Makino M, Suzuki K, et al. Prognostic significance of reverse transcriptase-polymerase chain reaction measurement of carcinoembryonic antigen mRNA levels in tumor drainage blood and peripheral blood of patients with colorectal carcinoma. Cancer 2000;89:970-6. [PubMed]
- Torino F, Bonmassar E, Bonmassar L, et al. Circulating tumor cells in colorectal cancer patients. Cancer Treat Rev 2013. [Epub ahead of print]. [PubMed]
- Kurihara T, Itoi T, Sofuni A, et al. Detection of circulating tumor cells in patients with pancreatic cancer: a preliminary result. J Hepatobiliary Pancreat Surg 2008;15:189-95. [PubMed]
- Ren C, Han C, Zhang J, et al. Detection of apoptotic circulating tumor cells in advanced pancreatic cancer following 5-fluorouracil chemotherapy. Cancer Biol Ther 2011;12:700-6. [PubMed]
- Negin BP, Meropol NJ, Alpaugh RK, et al. Characterization and prognostic significance of circulating tumor cells in the peripheral blood of patients with metastatic pancreatic cancer. J Clin Oncol 2010;28:4127.
- Khoja L, Backen A, Sloane R, et al. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer 2012;106:508-16. [PubMed]
- Marrinucci D, Bethel K, Kolatkar A, et al. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys Biol 2012;9:016003. [PubMed]
- de Albuquerque A, Kubisch I, Breier G, et al. Multimarker gene analysis of circulating tumor cells in pancreatic cancer patients: a feasibility study. Oncology 2012;82:3-10. [PubMed]
- Uchikura K, Takao S, Nakajo A, et al. Intraoperative molecular detection of circulating tumor cells by reverse transcription-polymerase chain reaction in patients with biliary-pancreatic cancer is associated with hematogenous metastasis. Ann Surg Oncol 2002;9:364-70. [PubMed]
- Vona G, Estepa L, Béroud C, et al. Impact of cytomorphological detection of circulating tumor cells in patients with liver cancer. Hepatology 2004;39:792-7. [PubMed]
- Sabile A, Louha M, Bonte E, et al. Efficiency of Ber-EP4 antibody for isolating circulating epithelial tumor cells before RT-PCR detection. Am J Clin Pathol 1999;112:171-8. [PubMed]
- Guo J, Yao F, Lou Y, et al. Detecting carcinoma cells in peripheral blood of patients with hepatocellular carcinoma by immunomagnetic beads and rt-PCR. J Clin Gastroenterol 2007;41:783-8. [PubMed]
- Waguri N, Suda T, Nomoto M, et al. Sensitive and specific detection of circulating cancer cells in patients with hepatocellular carcinoma; detection of human telomerase reverse transcriptase messenger RNA after immunomagnetic separation. Clin cancer Res 2003;9:3004-11. [PubMed]
- Zee BC, Wang C, Kuhn T, et al. Detection of circulating tumor cells (CTCs) in patients with hepatocellular carcinoma (HCC). J Clin Oncol 2007;25:15037.
- Schulze K, Gasch C, Staufer K, et al. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. Int J Cancer 2013. [Epub ahead of print]. [PubMed]
- Morimoto O, Nagano H, Miyamoto A, et al. Association between recurrence of hepatocellular carcinoma and alpha-fetoprotein messenger RNA levels in peripheral blood. Surg Today 2005;35:1033-41. [PubMed]
- Komeda T, Fukuda Y, Sando T, et al. Sensitive detection of circulating hepatocellular carcinoma cells in peripheral venous blood. Cancer 1995;75:2214-9. [PubMed]
- Ijichi M, Takayama T, Matsumura M, et al. alpha-Fetoprotein mRNA in the circulation as a predictor of postsurgical recurrence of hepatocellular carcinoma: a prospective study. Hepatology 2002;35:853-60. [PubMed]
- Kim YD, Hwang S, Lee YJ, et al. Preoperative peripheral blood human telomerase reverse transcriptase mRNA concentration is not a prognostic factor for resection of hepatocellular carcinoma. Hepatogastroenterology 2012;59:1512-5. [PubMed]
- Kim H, Choi GH, Na DC, et al. Human hepatocellular carcinomas with “Stemness”-related marker expression: keratin 19 expression and a poor prognosis. Hepatology 2011;54:1707-17. [PubMed]
- Qu KZ, Zhang K, Li H, et al. Circulating microRNAs as biomarkers for hepatocellular carcinoma. J Clin Gastroenterol 2011;45:355-60. [PubMed]
- Al Ustwani O, Iancu D, Yacoub R, et al. Detection of circulating tumor cells in cancers of biliary origin. J Gastrointest Oncol 2012;3:97-104. [PubMed]
- Leelawat K, Narong S, Udomchaiprasertkul W, et al. Prognostic relevance of circulating CK19 mRNA in advanced malignant biliary tract diseases. World J Gastroenterol 2012;18:175-81. [PubMed]
- Mataki Y, Takao S, Maemura K, et al. Carcinoembryonic antigen messenger RNA expression using nested reverse transcription-PCR in the peripheral blood during follow-up period of patients who underwent curative surgery for biliary-pancreatic cancer: longitudinal analyses. Clin Cancer Res 2004;10:3807-14. [PubMed]
- Nakamura T, Yasumura T, Hayashi K, et al. Immunocytochemical detection of circulating esophageal carcinoma cells by immunomagnetic separation. Anticancer Res 2000;20:4739-44. [PubMed]
- de Albuquerque A, Kubisch I, Ernst D, et al. Development of a molecular multimarker assay for the analysis of circulating tumor cells in adenocarcinoma patients. Clin Lab 2012;58:373-84. [PubMed]
- Tanaka K, Yano M, Motoori M, et al. CEA-antigen and SCC-antigen mRNA expression in peripheral blood predict hematogenous recurrence after resection in patients with esophageal cancer. Ann Surg Oncol 2010;17:2779-86. [PubMed]
- Xi L, Nicastri DG, El-Hefnawy T, et al. Optimal markers for real-time quantitative reverse transcription PCR detection of circulating tumor cells from melanoma, breast, colon, esophageal, head and neck, and lung cancers. Clin Chem 2007;53:1206-15. [PubMed]
- Yin XD, Yuan X, Xue JJ, et al. Clinical significance of carcinoembryonic antigen-, cytokeratin 19-, or survivin-positive circulating tumor cells in the peripheral blood of esophageal squamous cell carcinoma patients treated with radiotherapy. Dis Esophagus 2012;25:750-6. [PubMed]
- Hashimoto T, Kajiyama Y, Tsutsumi-Ishii Y, et al. Circulating micrometastases of esophageal cancer detected by carcinoembryonic antigen mRNA reverse transcriptase-polymerase chain reaction: clinical implications. Dis Esophagus 2008;21:690-6. [PubMed]
- Liu Z, Jiang M, Zhao J, et al. Circulating tumor cells in perioperative esophageal cancer patients: quantitative assay system and potential clinical utility. Clin Cancer Res 2007;13:2992-7. [PubMed]
- Setoyama T, Natsugoe S, Okumura H, et al. Isolated tumour cells in blood and E-cadherin expression in oesophageal squamous cell cancer. Br J Surg 2007;94:984-91. [PubMed]
- Ito H, Kanda T, Nishimaki T, et al. Detection and quantification of circulating tumor cells in patients with esophageal cancer by real-time polymerase chain reaction. J Exp Clin Cancer Res 2004;23:455-64. [PubMed]
- Nakashima S, Natsugoe S, Matsumoto M, et al. Clinical significance of circulating tumor cells in blood by molecular detection and tumor markers in esophageal cancer. Surgery 2003;133:162-9. [PubMed]
- Kaganoi J, Shimada Y, Kano M, et al. Detection of circulating oesophageal squamous cancer cells in peripheral blood and its impact on prognosis. Br J Surg 2004;91:1055-60. [PubMed]
- Hoffmann AC, Vallböhmer D, Grimminger P, et al. Preoperative survivin mRNA detection in peripheral blood is an independent predictor of outcome in esophageal carcinoma. Pharmacogenomics 2010;11:341-7. [PubMed]
- Cao M, Yie SM, Wu SM, et al. Detection of survivin-expressing circulating cancer cells in the peripheral blood of patients with esophageal squamous cell carcinoma and its clinical significance. Clin Exp Metastasis 2009;26:751-8. [PubMed]
- Fujii K, Goto A, Matsunaga Y, et al. Primary malignant melanoma of the esophagus--detection of circulating tumor cells. Gan To Kagaku Ryoho 2010;37:1539-43. [PubMed]
- Matsusaka S, Chìn K, Ogura M, et al. Circulating tumor cells as a surrogate marker for determining response to chemotherapy in patients with advanced gastric cancer. Cancer Sci 2010;101:1067-71. [PubMed]
- Hosokawa M, Hayata T, Fukuda Y, et al. Size-selective microcavity array for rapid and efficient detection of circulating tumor cells. Anal Chem 2010;82:6629-35. [PubMed]
- Noh YH, Kim JA, Lim GR, et al. Detection of circulating tumor cells in patients with gastrointestinal tract cancer using RT-PCR and its clinical implications. Exp Mol Med 2001;33:8-14. [PubMed]
- Miyazono F, Natsugoe S, Takao S, et al. Surgical maneuvers enhance molecular detection of circulating tumor cells during gastric cancer surgery. Ann Surg 2001;233:189-94. [PubMed]
- Ikeguchi M, Kaibara N. Detection of circulating cancer cells after a gastrectomy for gastric cancer. Surg Today 2005;35:436-41. [PubMed]
- Seo JH, Choi CW, Kim BS, et al. Follow-up study of peripheral blood carcinoembryonic antigen mRNA using reverse transcription-polymerase chain reaction as an early marker of clinical recurrence in patients with curatively resected gastric cancer. Am J Clin Oncol 2005;28:24-9. [PubMed]
- Wu CH, Lin SR, Yu FJ, et al. Development of a high-throughput membrane-array method for molecular diagnosis of circulating tumor cells in patients with gastric cancers. Int J Cancer 2006;119:373-9. [PubMed]
- Wu CH, Lin SR, Hsieh JS, et al. Molecular detection of disseminated tumor cells in the peripheral blood of patients with gastric cancer: evaluation of their prognostic significance. Dis Markers 2006;22:103-9. [PubMed]
- Koga T, Tokunaga E, Sumiyoshi Y, et al. Detection of circulating gastric cancer cells in peripheral blood using real time quantitative RT-PCR. Hepatogastroenterology 2008;55:1131-5. [PubMed]
- Bertazza L, Mocellin S, Marchet A, et al. Survivin gene levels in the peripheral blood of patients with gastric cancer independently predict survival. J Transl Med 2009;7:111. [PubMed]
- Qiu MZ, Li ZH, Zhou ZW, et al. Detection of carcinoembryonic antigen messenger RNA in blood using quantitative real-time reverse transcriptase-polymerase chain reaction to predict recurrence of gastric adenocarcinoma. J Transl Med 2010;8:107. [PubMed]
- Kutun S, Celik A, Cem Kockar M, et al. Expression of CK-19 and CEA mRNA in peripheral blood of gastric cancer patients. Exp Oncol 2010;32:263-8. [PubMed]
- Mimori K, Fukagawa T, Kosaka Y, et al. Hematogenous metastasis in gastric cancer requires isolated tumor cells and expression of vascular endothelial growth factor receptor-1. Clin Cancer Res 2008;14:2609-16. [PubMed]
- Yeh KH, Chen YC, Yeh SH, et al. Detection of circulating cancer cells by nested reverse transcription-polymerase chain reaction of cytokeratin-19 (K19)--possible clinical significance in advanced gastric cancer. Anticancer Res 1998;18:1283-6. [PubMed]
- Shin JH, Chung J, Kim HO, et al. Detection of cancer cells in peripheral blood of stomach cancer patients using RT-PCR amplification of tumour-specific mRNAs. Aliment Pharmacol Ther 2002;16:137-44. [PubMed]
- Uen YH, Lin SR, Wu CH, et al. Clinical significance of MUC1 and c-Met RT-PCR detection of circulating tumor cells in patients with gastric carcinoma. Clin Chim Acta 2006;367:55-61. [PubMed]
- Cao W, Yang W, Li H, et al. Using detection of survivin-expressing circulating tumor cells in peripheral blood to predict tumor recurrence following curative resection of gastric cancer. J Surg Oncol 2011;103:110-5. [PubMed]
- Arigami T, Uenosono Y, Hirata M, et al. B7-H3 expression in gastric cancer: a novel molecular blood marker for detecting circulating tumor cells. Cancer Sci 2011;102:1019-24. [PubMed]
- Shimizu F, Nakayama J, Ishizone S, et al. Usefulness of the real-time reverse transcription-polymerase chain reaction assay targeted to alpha1,4-N-acetylglucosaminyltransferase for the detection of gastric cancer. Lab Invest 2003;83:187-97. [PubMed]
- Khan MS, Tsigani T, Rashid M, et al. Circulating tumor cells and EpCAM expression in neuroendocrine tumors. Clin Cancer Res 2011;17:337-45. [PubMed]
- Oberstein PE, Saif MW. Update on prognostic and predictive biomarkers for pancreatic neuroendocrine tumors. JOP 2012;13:368-71. [PubMed]
- Khan MS, Kirkwood A, Tsigani T, et al. Circulating tumor cells as prognostic markers in neuroendocrine tumors. J Clin Oncol 2013;31:365-72. [PubMed]
- Wicha MS, Hayes DF. Circulating tumor cells: not all detected cells are bad and not all bad cells are detected. J Clin Oncol 2011;29:1508-11. [PubMed]


