Fatal attraction: tumor recruitment of myeloid-derived suppressor cells is mediated by IL-17-producing CD8+ T cells
The tumor microenvironment comprises immune cells, tumor cells, stromal cells, and the extracellular matrix. This microenvironment is the key location for neoplastic progression, nurturing the proliferation, survival, and migration of tumor cells. Although a relationship between inflammation and cancer has been appreciated for several decades, researchers have not elucidated a complete picture for the complex networks in the tumor microenvironment. Recently, in an article in the journal Gastroenterology (1), Zhuang et al. provided compelling evidence that IL-17-producing CD8+ T cells play an important role in the pathogenesis of gastric cancer. They proposed a model of cross-talk between the host immune system and tumor cells leading to IL-17-producing CD8+ T cell development and myeloid-derived suppressor cell-mediated immunosuppression.
IL-17-producing CD8+ T cells were first characterized by Liu et al. (2) as a distinct subset of CD8+ T cells that are fundamentally different from canonical cytotoxic T lymphocytes (Tc). Because of their low levels of cytotoxicity, the IL-17-producing CD8+ T cell subset was initially designated as T noncytotoxic 17 (Tnc17) (2). Several independent research groups have also reported that IL-17-producing CD8+ T cells have negative or low cytolytic activity and markers (3-5). To maintain a comparative convention with cytotoxic T lymphocytes, Tc1 or Tc2, these IL-17-producing CD8+ T cells are now termed Tc17 cells. Tc1 cells are well known to primarily secrete IFN-γ and kill their tumor targets by either perforin- or Fas-mediated mechanisms, whereas Tc2 cells secrete IL-4, IL-5, IL-6, and IL-10 and kill their tumor targets predominantly through the perforin pathway (6). In contrast, Tc17 cells secrete IL-17 and have fewer cytotoxic effector functions due to diminished levels of the T-box transcription factor Eomesodermin, IFN-γ, and the cytolytic molecule granzyme B (4).
Tc17 cells have been detected in a variety of tumors (1,4,7-13), autoimmune diseases (14-16), and infections (3,17). Such cells are believed to be involved in enhancing protection against viral infection (3,17) and antitumor immunity (10,11). Direct evidence from a study using adoptive transfer to identify the role of Tc17 cells in a B16 melanoma model demonstrated that Tc17 cells exhibit antitumor immunity and reduce tumor growth (11,12). However, an increasing body of work also indicates that Tc17 cells accumulate with disease progression (7-10) or promote tumor progression due to the low expression of perforin, granzyme B, and IFN-γ (4,7).
Cytokines in the tumor microenvironment play a crucial role in tumor growth and survival. TGF-β, IL-6, and prostaglandin E2 levels have been shown to be elevated in the malignant effusions of cancer patients (18). TGF-β and IL-6 appear to be essential for the induction of IL-17-producing T cells (2,19). In addition, prostaglandin E2 has been shown to induce IL-23 production (20,21), and IL-23 is important for IL-17-producing T cell survival and expansion (19). A tumor microenvironment with these cytokines will favor the differentiation of IL-17-producing T cells. Notably, Zhuang et al. demonstrated that a set of key cytokines (IL-6, IL-1β, and IL-23) derived from tumor-associated monocytes plays an essential role in the induction of Tc17 cells (1). Consistent with the findings of Kuang et al. (8), tumor-activated monocytes also have been shown to secrete IL-6, IL-1β, and IL-23 to trigger the expansion of Tc17 cells in hepatocellular carcinoma (HCC). Altogether, these results suggest that cytokines present in the microenvironments of a variety of tumors encourage the development of IL-17-producing T cells.
Zhuang et al. found IL-17-producing CD8+ T cells to be enriched at tumor sites in gastric cancer patients (1). A comprehensive analysis indicated that the percentage of IL-17-producing CD8+ T cells increased significantly as the gastric cancer progressed, and this percentage was associated with overall survival time. In addition, these intratumoral IL-17-producing CD8+ T cells expressed less IFN-γ, IL-4, IL-10, and IL-9. These results suggest that the IL-17-producing CD8+ T subset in gastric cancer is neither the Tc1 nor the Tc2 subset and can be categorized as the Tc17 subset. Further characterization of Tc17 cells revealed that they express minimal amounts of perforin, granzyme B, FoxP3, or programmed death 1 receptor. These characteristics imply that the main role of Tc17 cells is likely to be the secretion of IL-17 rather than the direct execution of effector functions such as cytotoxicity or immunosuppression.
If Tc17 cells do not have a direct effector function, what is the role of the IL-17 secreted by Tc17 cells? The most important finding in the Zhuang et al. (1) study is the identification of a downstream mechanism for IL-17. In particular, IL-17 stimulates tumor cells to secrete CXCL12, which may recruit myeloid-derived suppressor cells to the tumor microenvironment and promote tumor progression in gastric cancer. In fact, the role of IL-17 in cancer development is still paradoxical (22). However, the study by Zhuang et al. strongly supports the view that the interaction of monocytes and tumor cells creates a favorable environment for Tc17 development (1); the IL-17 secreted by Tc17 then attracts myeloid-derived suppressor cells to foster immune privilege in gastric cancer. When the cytotoxic CD8+ T cell is no longer a killer cell but a traitor, the consequence is tumor growth.
Regulatory T cells play a significant role in suppressing anti-tumor immunity, and their interaction with Tc17 cannot be ignored. Tsai et al. demonstrated that the presence of regulatory T cells and the consumption of IL-2 maintains or promotes Tc17 cell differentiation (13). These results suggest a beneficial effect of regulatory T cells for Tc17. What is the possibility that Tc17 are ‘inflammatory’ regulatory cells? Kryczek et al. identified a population of IL-17+ regulatory T cells that express both IL-17 and FoxP3 in ulcerative colitis and colon cancer carcinoma (23). They suggested that this cell population plays a role in chronic inflammatory environments. Zhuang et al. (1) also examined whether such intratumoral Tc17 cells expressed the conventional markers of regulatory T cells and observed these Tc17 cells to lack markers including perforin, granzyme B, programmed death 1 receptor, and FoxP3. These results indicate that the main role of these cells is likely to be the production of IL-17 rather than a direct effector function.
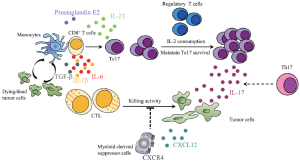
Taking these results together and synthesizing the recent findings from several research groups, we propose a model of a cancer immunoediting mechanism involving Tc17 cells, regulatory T cells, tumor cells, monocytes, myeloid-derived suppressor cells, cytotoxic T lymphocytes and their associated cytokines, and other mediators (Figure 1). Dead or dying tumor cells activate monocytes and create a unique microenvironment containing TGF-β, IL-6, and IL-1β, which induces naive CD8+ T cells to differentiate into Tc17 cells. Prostaglandin E2 and IL-23 thereby contribute to the survival and expansion of such Tc17 cells. Regulatory T cells, which consume IL-2, maintain the presence of the Tc17 cells. The IL-17 secreted by these Tc17 cells (Th17 may also contribute to the production of IL-17) stimulates tumor cells to release CXCL12. Consequently, CXCR4-bearing myeloid-derived suppressor cells migrate to the tumors. The recruited myeloid-derived suppressor cells exert their immunosuppressive function and inhibit cytotoxic T lymphocyte killing activity. As a consequence, tumor growth progresses unimpeded.
Acknowledgements
Disclosure: The authors declare that they have no conflicts of interest and acknowledge the financial support of grants from the National Health Research Institutes and Chang Gung Memorial Hospital (CMRPD180402 and BMRP440).
References
- Zhuang Y, Peng LS, Zhao YL, et al. CD8(+) T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology 2012;143:951-62.e8.
- Liu SJ, Tsai JP, Shen CR, et al. Induction of a distinct CD8 Tnc17 subset by transforming growth factor-beta and interleukin-6. J Leukoc Biol 2007;82:354-60. [PubMed]
- Hamada H, Garcia-Hernandez Mde L, Reome JB, et al. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol 2009;182:3469-81. [PubMed]
- Huber M, Heink S, Grothe H, et al. A Th17-like developmental process leads to CD8(+) Tc17 cells with reduced cytotoxic activity. Eur J Immunol 2009;39:1716-25. [PubMed]
- Kondo T, Takata H, Matsuki F, et al. Cutting edge: Phenotypic characterization and differentiation of human CD8+ T cells producing IL-17. J Immunol 2009;182:1794-8. [PubMed]
- Carter LL, Dutton RW. Relative perforin- and Fas-mediated lysis in T1 and T2 CD8 effector populations. J Immunol 1995;155:1028-31. [PubMed]
- Zhang JP, Yan J, Xu J, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol 2009;50:980-9. [PubMed]
- Kuang DM, Peng C, Zhao Q, et al. Tumor-activated monocytes promote expansion of IL-17-producing CD8+ T cells in hepatocellular carcinoma patients. J Immunol 2010;185:1544-9. [PubMed]
- Lee MH, Chang JT, Yu FW, et al. The Prevalence of IL-17+ Producing Cells in Patients with Nasopharyngeal Carcinoma. J Biol Lab Sci 2011;23:30-7.
- Hinrichs CS, Kaiser A, Paulos CM, et al. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood 2009;114:596-9. [PubMed]
- Yu Y, Cho HI, Wang D, et al. Adoptive transfer of Tc1 or Tc17 cells elicits antitumor immunity against established melanoma through distinct mechanisms. J Immunol 2013;190:1873-81. [PubMed]
- Yen HR, Harris TJ, Wada S, et al. Tc17 CD8 T cells: functional plasticity and subset diversity. J Immunol 2009;183:7161-8. [PubMed]
- Tsai JP, Lee MH, Hsu SC, et al. CD4+ T cells disarm or delete cytotoxic T lymphocytes under IL-17-polarizing conditions. J Immunol 2012;189:1671-9. [PubMed]
- Huber M, Heink S, Pagenstecher A, et al. IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J Clin Invest 2013;123:247-60. [PubMed]
- Ortega C, Fernández-A S, Carrillo JM, et al. IL-17-producing CD8+ T lymphocytes from psoriasis skin plaques are cytotoxic effector cells that secrete Th17-related cytokines. J Leukoc Biol 2009;86:435-43. [PubMed]
- Tzartos JS, Friese MA, Craner MJ, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol 2008;172:146-55. [PubMed]
- Yeh N, Glosson NL, Wang N, et al. Tc17 cells are capable of mediating immunity to vaccinia virus by acquisition of a cytotoxic phenotype. J Immunol 2010;185:2089-98. [PubMed]
- Tsai JP, Chen HW, Cheng ML, et al. Analysis of host versus tumor interaction in cancer patients: opposing role of transforming growth factor-beta1 and interleukin-6 in the development of in situ tumor immunity. Immunobiology 2005;210:661-71. [PubMed]
- Veldhoen M, Hocking RJ, Atkins CJ, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006;24:179-89. [PubMed]
- Sheibanie AF, Khayrullina T, Safadi FF, et al. Prostaglandin E2 exacerbates collagen-induced arthritis in mice through the inflammatory interleukin-23/interleukin-17 axis. Arthritis Rheum 2007;56:2608-19. [PubMed]
- Sheibanie AF, Tadmori I, Jing H, et al. Prostaglandin E2 induces IL-23 production in bone marrow-derived dendritic cells. FASEB J 2004;18:1318-20. [PubMed]
- Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. J Immunol 2009;183:4169-75. [PubMed]
- Kryczek I, Wu K, Zhao E, et al. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol 2011;186:4388-95. [PubMed]


