Neuroendocrine tumors of the pancreas: molecular pathogenesis and current surgical management
Introduction/background
Neuroendocrine tumors (NET) of the pancreas are infrequently occurring neoplasms that are associated with unique and variable clinical features. NET cause symptoms either related to the local progression of the tumor mass, or from excess secretion of any of a number of hormone products that can result in dramatic and unusual constellations of symptoms and signs. These tumors can be functional, but up to 60% are non-functional, and there is some data that nonfunctional NET may have a poorer prognosis (1), likely because they are clinically silent and therefore likely to be discovered late after the development of symptoms from a large mass and more frequent metastatic disease at diagnosis. Pancreatic NET look very similar or identical histologically when compared to carcinoid tumors of the gastrointestinal tract, but differences in hormone products and overall biology, as well as likely differences in the response to therapeutic agents indicates that they should be considered and treated as separate pathologic entities.
Pancreatic NET are much more uncommon neoplasms than the exocrine tumors that comprise the majority of pancreatic cancers. They account for approximately 3-5% of all pancreatic tumors with only about 1,000 new cases per year in the United States. Importantly, foregut NET may be associated with a number of hereditary cancer syndromes, notably multiple endocrine neoplasia type 1 (duodenal and pancreatic NET as well as bronchial and thymic carcinoids), and von Hippel-Lindau syndrome (pancreatic NET).
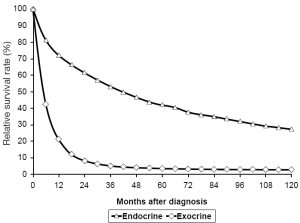
Overall, these cancers have a more favorable biologic behavior in the majority of affected patients, when compared with other more common primary pancreatic malignancies such as adenocarcinoma and cystic pancreatic neoplasms (Figure 1). Due to the relatively slow progression of NET in most patients, complete surgical resection of pancreatic endocrine tumors including targeting of all evident metastatic disease with curative intent is the preferred initial treatment whenever feasible and patients are an acceptable operative risk. This fact allows treatments that have the goal of reducing tumor burden or removing all grossly evident disease (cytoreduction) to be a consideration even in patients with metastatic disease at the time of diagnosis. The liver is the principal site for metastatic involvement of NET of the pancreas, and reduction of tumor volume can effectively control symptoms from functional tumors in addition to desired oncologic control of tumor progression. In addition to aggressive surgical resection combined with extensive regional lymphadenectomies and hepatic metastasectomy there are a number of innovative technologies that may be employed for metastatic disease, including radiofrequency ablation (RFA), transarterial chemoembolization (TACE), and radioembolization with Yttrium-90 microspheres. Unfortunately, pancreatic NET in general respond relatively poorly to traditional cytotoxic chemotherapy, and there have been no comprehensive well-controlled studies demonstrating benefit of adjuvant therapy after complete tumor resection. The current data is either lacking or conflicting for a survival benefit from systemic chemotherapy for pancreatic NET. For this reason, chemotherapy is often not recommended for patients with stable or slowly progressive metastatic disease in the absence of symptoms. Somatostatin analogues have been employed as medical therapy to alleviate symptoms of hormone overproduction and for presumed anti-tumor growth properties (3). Radiolabeled somatostatin analogues have been utilized for therapeutic targeting of endocrine tumor cells with somatostatin type 2 receptors on the cell surface, albeit with variable effectiveness. Newer targeted molecular therapies are being evaluated in clinical trials and everolimus (Afinitor, Novartis), a mammalian target of rapamycin or mTOR inhibitor has been approved for the treatment of patients with advanced unresectable metastatic NET of the pancreas. The safety and efficacy of this drug has not been established however for patients with metastatic gastrointestinal carcinoid tumors. There is a need for increased understanding of the molecular pathogenesis of these tumors with the goal of developing more effective systemic therapies aimed at specific molecular targets (4).
Epidemiology
While pancreatic cancer as a whole represents approximately 45,000 new cancer cases per year in the United States and 38,500 deaths, NET of the pancreas are only a small subset, comprising between 500 and 1,000 of those cases. This represents between 1-2% of all new pancreatic cancer cases yearly (2). Pancreatic NET may present at any age of life, but epidemiologic data suggests that they occur more in older populations, peaking between the 6th and 7th decades of life (5,6). Population studies from both Europe and Asia have quantified the incidence of pancreatic NET as less than 1 per 100,000 population (5,7-12). This rare group of tumors can present as a result of functionality or can go unnoticed, if non-functional, until later stages or mass effect present. This may explain the findings of post-mortem studies with prevalence as high as 10% (13-15).
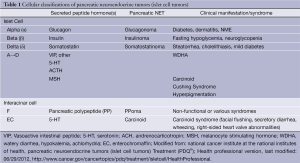
These tumors are often classified using several clinical and biochemical criteria: functionality versus non-functionality, specific predominant hormone production from tumor cells, and sporadic tumors versus those occurring in association with a hereditary syndrome. A general classification according to predominant peptide hormone product secreted and cell type is depicted in Table 1.
Full Table
Functional tumors are classified by the predominant hormone secreted that exerts its clinical effects. Insulinomas are very rare with an estimated incidence of 0.4 per 100,000 people. The most complete data from the United States has been reported by the experience at the Mayo Clinic. Patients had a median age of 50 years (range, 17-86 years) at time of first operation for insulinoma, with a slight preponderance of women (57%) (16-18).
Gastrinomas result in autonomous hypergastrinemia (Zollinger-Ellison syndrome) and there is limited reliable statistical data regarding the true incidence of this rare neoplasm. It has been estimated that this syndrome represents between 0.1% and 1% of patients with peptic ulcer disease in the U.S. population (19). Diagnosis is most common between the 3rd and 5th decades of life, with a male predominance of approximately 2:1.
Somatostatinoma, VIPoma, and glucagonoma represent the rarest of functional pancreatic NET, with an annual incidence on the order of 1 in 10 million (Table 2). As with other functional pancreatic NET, the incidence peaks between the 3rd and 5th decades of life. Reports of glucagonoma incidence, while very rare, suggest a fairly even distribution among the sexes (20).

Full Table
Clinical presentation
The clinical presentation of pancreatic NET is extremely variable and depends on whether the tumors are functional, or whether they are causing local compressive or invasive symptoms. About 50-85% pancreatic NET are functional and result in hypersecretion of any of a number of active peptide hormones that cause specific signs and symptoms, whereas 15-50% are non-functional (21). Functional tumors are often classified, and clinically defined, by the predominant hormone secreted and resultant syndrome of hormone excess, with the five most common tumors being gastrinoma, insulinoma, glucagonoma, VIPoma and somatostatinoma.
Gastrinomas are typically located in the submucosa of the duodenal wall or the pancreatic head (gastrinoma triangle) (22) (Figure 2). They can occur as isolated, sporadic tumors, or as part of the multiple endocrine neoplasia type 1 (MEN 1) syndrome (23). Patients often present with symptoms related to excessive acid production, including abdominal pain, weight loss, dysphagia/reflux, and secretory diarrhea. Active peptic ulceration of the stomach or duodenum is less common in the current era due to the use of powerful proton pump inhibitors (PPIs). These tumors cause Zollinger-Ellison Syndrome (ZES), which should be suspected if peptic ulcers are recurrent, resistant to therapy, or familial in nature. The diagnosis is made by the finding of both elevated serum gastrin and gastric acid hyperacidity. Gastrin is typically elevated >100 pg/mL, or demonstrates an abnormal response to secretin stimulation (2 U/kg i.v.), with an increase in gastrin >200 pg/mL. Gastric acid analysis demonstrates elevated gastric basal acid output (BAO) >15 mEq/hr, or >5 mEq/hr in patients with prior gastric surgery. Elevated gastrin alone can occur in achlorhydric states such as atrophic gastritis or pernicious anemia, or in association with relative gastric outlet obstruction or high dose treatment with PPIs and should not be confused with an autonomously functioning gastrinoma. Approximately 60-70% of gastrinomas occur in the duodenal wall as submucosal tumors that may be only 3-5 mm in size. In previous studies, biochemical cure was rarely achieved long-term by surgical resection in patients with MEN 1 (24), and therefore controversy has existed regarding surgical management of ZES in the familial setting. However, more recent studies suggest that excellent results can be achieved biochemically in some MEN 1 patients with more aggressive operative procedures (25,26).
Insulinomas secrete excessive levels of insulin, which leads to fasting hypoglycemia that can cause marked and even life-threatening symptoms. Endogenous inappropriate hyperinsulinism leads to fasting hypoglycemia with associated neuroglycopenic symptoms, including confusion, visual disturbances, and bizarre behavior. The hypoglycemic state in turn causes catecholamine release, leading to anxiety, excessive perspiration, and tachyarrhythmias (27). Owing to the rarity of insulinoma and these unusual symptoms, patients are often thought to have psychiatric issues or drug use until the association of the symptoms with fasting, and relief of symptoms by eating, is recognized. Because patients often find that frequent consumption of high-carbohydrate foods relieves symptoms, patients will often carry sugar pills or high calorie candy and weight gain is a common secondary finding. The diagnosis of insulinoma is made biochemically by performance of a supervised 72-hour fast. Termination of the fast occurs when patients glucose falls <40 mg/dL with associated symptoms of neuroglycopenia. Fasting insulin, glucose, and C-peptide as well as oral hypoglycemic medication screen are obtained prior to administration of D50. Patients with endogenous hyperinsulinism will have inappropriately normal or elevated insulin levels with profound hypoglycemia. Elevated C-peptide confirms an endogenous source of the hyperinsulinism. Sporadic insulinomas occur with an incidence less than 1 per million population, and are usually small, solitary tumors about 1 to 1.5 cm that may occur with an even distribution within the pancreatic parenchyma.
Glucagonoma is a nearly uniform malignant pancreatic islet cell tumor producing excess glucagon. Approximately 64-90% of patients have a characteristic raised red pruritic rash called necrolytic migratory erythema (NME), which usually involves the pretibial, perioral and intertriginous areas. Other symptoms include hypoaminoacidemia, type 2 diabetes, weight loss and severe cachexia. Twenty-four percent of patients may develop DVTs and 11% have pulmonary embolism. Glucagonoma is diagnosed by a plasma glucagon level >500 pg/mL with decreased levels of amino acids. Most patients present with large (>5 cm) or locally advanced disease for which surgical resection is seldom curative. Medical management includes total parenteral nutrition (TPN) for cachexia, as well as octreotide which reduces glucagon levels and improves rash and cachexia.
The clinical manifestations of glucagonoma are related to glucose intolerance caused by excessive secretion of the counter-regulatory hormone glucagon. Symptoms include sore mouth, altered bowel habits, and venous thrombosis. NME is found in up to 90% of patients with glucagonoma and is pathognomonic for this tumor. NME must be accompanied by elevated levels of glucagon in the blood in order to confirm the diagnosis (27).
The production of excessive vasoactive intestinal peptide (VIP) occurs in patients with VIPomas. First described by Verner and Morrison in 1958 (also known as Verner-Morrison syndrome), it is a distinct condition caused by these tumors and is characterized by a profuse secretory diarrhea, dehydration, achlorhydria and hypokalemia; this condition is also known as watery diarrhea hypokalemia achlorhydria (WDHA) or pancreatic cholera (27,28). Approximately 85-90% of tumors are in the pancreas and elevated polypeptide (PP) levels help distinguish pancreatic from extrapancreatic duodenal VIPomas. The diagnosis is made by the presence of fasting plasma VIP level >500 pg/mL in association with secretory diarrhea. Octreotide reduces VIP levels and diarrhea in approximately 80% of patients. Most are malignant, however surgery may be curative.
Somatostatinomas are rare, malignant pancreatic NET found in either the pancreas (50%) or the duodenum (50%). Somatostatinoma syndrome includes steatorrhea, weight loss, cholelithiasis, glucose intolerance and hypochlorhydria due to secretory diarrhea (27). Many are diagnosed incidentally at the time of cholecystectomy. The mean age of presentation is 51-53 years. As with other functional pancreatic NET, the diagnosis of somatostatinoma is confirmed by elevated blood levels of somatostatin in association with either a pancreatic or duodenal mass. Non-functional tumors are typically clinically silent, until they grow large enough to produce symptoms related to mass-effect or invasion. Such symptoms include pain, bleeding, or obstruction. Most patients with somatostatinoma have unresectable metastatic disease at the time of diagnosis.
Diagnostic evaluation
Because pancreatic NET are rare and have variable clinical presentation, diagnosis is often delayed and requires extensive biochemical, radiologic and endoscopic evaluation. While functional tumors may result in marked signs and symptoms of hormone excess, the primary neoplasm may be very small and occult or difficult to localize by imaging tests.
Biochemical tests
Given that many pancreatic NET are hormone-secreting (40-60%), initial diagnostic evaluation should consist of serum measurement of appropriate hormones, or urinary evaluation of their metabolites. In addition to the hormone products of secretory tumors, there are certain general tumor markers that may prove useful in the diagnosis of non-functional pancreatic NET. These tumor markers include chromogranin A, pancreatic polypeptide (29), neurotensin, alpha subunit of human chorionic gonadotropin (alpha hCG), neuron-specific enolase, synaptophysin, and urinary 5-hydroxyindoleacetic acid (5-HIAA) excretion (30). In addition to this standard panel of markers, there are newer markers that may be potentially helpful for diagnosis; neuroendocrine secretory protein-55 is a member of the chromogranin family that is elevated in some patients with pancreatic NET (31).
Radiographic imaging
Common radiographic tools used in the localization of pancreatic NET include CT, MRI and ultrasonography. Biphasic, thin-slice CT with contrast has a sensitivity of approximately 95% for pancreatic NET greater than 3 cm in size, but is much less sensitive for tumors less than 1 cm (32). The sensitivity of CT also decreases for multiple tumors, or tumors located in the distal pancreas. For those tumors measuring <1 cm in diameter, MRI is a useful tool; similarly, MRI is better at detecting hepatic metastases than CT (33).
Advances in molecular imaging, have improved diagnosis in patients with pancreatic NET. NET express somatostatin receptors. Somatostatin receptor scintigraphy (SRS), utilizes octreotide (a synthetic form of somatostatin) that is chemically bound to a radioactive substance to detect tumor cells that are avid for octreotide (33). Additionally, SRS can be performed with positron emission tomography (PET) which offers higher resolution and more rapid imaging. 18Fluorodeoxyglucose (FDG) PET is also useful for imaging pancreatic NET. Such scans are performed by injecting radioactive glucose intravenously. Using this scan, the aggressiveness of the tumor can be visualized, given that more aggressive tumors utilized glucose more rapidly than their surrounding tissues (34). Patients suspected of having a pancreatic NET should initially undergo a high quality cross-sectional imaging test such as computed tomography or MRI; these initial radiographic tests may not identify a small, occult functioning tumor, but should be performed to rule out a very large primary pancreatic mass or hepatic metastases. Functional studies such as PET or octreotide scanning have somewhat lower sensitivity depending on tumor size and avidity, as well as the density of somatostatin receptors on the tumor cell surface for the sensitivity of SRS (35).
Endoscopic evaluation
Following a cross-sectional radiographic imaging study, endoscopic ultrasound (EUS) is an excellent and sensitive minimally invasive test that has proved to be useful for the localization of pancreatic NET, particularly with small tumors in the head of the pancreas that are unable to be detected by more conventional means (CT, MRI). This modality can also detect peri-pancreatic lymphadenopathy, thus aiding in the staging of tumors. Additionally, EUS combined with fine needle aspiration biopsy can provide definitive diagnosis of pancreatic NET (36). This study is often our imaging test of choice to evaluate the stomach, liver, left adrenal gland, duodenal wall, pancreas, and regional lymph nodes for diagnosis and staging of pancreatic NET (37,38). Recent developments even suggest that EUS may be used to deliver therapeutic agents for treatment of pancreatic NET.
Surgical management
The management of patients with pancreatic NET is multimodal, involving surgery, radiation and chemotherapy. While medical management of symptoms is often necessary preoperatively (such as proton pump inhibitors for gastrinomas, or somatostatin analogues for glucagonomas and VIPomas), to date, surgery remains the only potentially curative option for pancreatic NET.
Surgery
Major pancreatic procedures can be performed safely in most patients with pancreatic NET (39,40). Because many of these tumors have a more favorable biologic behavior than the common exocrine pancreatic malignancies, an aggressive surgical approach aimed at early intervention prior to malignant spread and major pancreatic resection where justified is indicated. The appropriate surgical procedure performed depends on multiple factors such as the specific type of pancreatic NET (gastrinoma vs. insulinoma), presence of metastatic disease, and comorbid conditions of the patient. Although pancreatic endocrine neoplasms are generally thought to pursue an indolent clinical course in the majority of patients, regional lymph node metastases and hepatic and distant metastases can occur and be life-limiting due to progression of the tumor. Some tumors may be clinically insignificant or follow a benign course, although a subset of tumors pursue a malignant, lethal natural history; the risk of operative management must be appropriate to the disease course (41).
Pancreatic NET that are associated with the multiple endocrine neoplasia type 1 (MEN 1) syndrome present unique diagnostic and therapeutic challenges. In MEN 1, involvement of the pancreas is characterized by a diffuse preneoplastic hyperplasia “field affect” that precedes the development of discrete tumor foci within the involved organ, the development of multiple tumors within a target tissue, and the potential for development of tumors in more than one target tissue. Furthermore, in the setting of a familial cancer syndrome, affected patients develop tumors at a much earlier age than patients with corresponding sporadic tumors. For example, in keeping with the two-hit model for a tumor suppressor gene (42,43), patients with multiple endocrine neoplasia type 1 (MEN 1) inherit one mutation in the germline, and require only one additional genetic event to inactivate the remaining wild-type allele and result in tumor formation. The result is a propensity to develop multiple endocrine tumors at a young age, when patients are otherwise healthy and active. The optimal surgical management of these tumors is complicated by a relative lack of sensitive and specific tumor markers for their early detection, difficulty in accurately localizing small tumors by preoperative imaging tests, and uncertainty about the malignant potential or expected natural history of small apparently benign tumors (41,44).
Because of the high probability that a small, solitary, grossly encapsulated NET of the pancreas will be benign, enucleation is usually appropriate (Figure 3). Localized resection preserves pancreatic endocrine and exocrine function, and prevents the need for division of the major pancreatic duct or need for construction of a surgical enteric-pancreatic duct anastomosis. Alternatively, larger potentially malignant NETs or tumors felt to carry a high risk of malignant progression may require major pancreatic resection (Figure 3). It is obviously desirable to intervene early to prevent malignant spread, while preserving pancreatic function and minimizing morbidity and mortality (from either cancer or surgery). In addition, pancreatic NET that occur in patients with MEN 1 are more likely to require pancreatic resection in young patients with normal, soft, non-fibrotic pancreatic parenchyma, and a usual absence of dilated pancreatic and biliary ducts; these findings make major pancreatic resection more morbid, with a significant risk of pancreatic fistula.
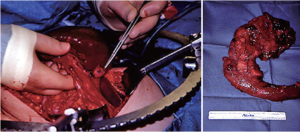
Surgical decision making in these patients should be based on the unique features of these uncommon neoplasms, the expected natural history of the tumor, and the most significant operative risks. The ideal surgical treatment of pancreatic NET relieves the patient of significant risk of malignant progression, while preserving pancreatic endocrine and exocrine function, and minimizing morbidity from either surgery or the underlying disease process.
For localized pancreatic NET, surgical excision with curative intent is the mainstay of treatment (45,46). This typically involves surgical resection of the tumor, as well as an extended regional lymphadenectomy. For tumors that are typically small and benign (as in the case of insulinomas), simple enucleation is often the procedure of choice; if located in the tail of the pancreas, a distal pancreatectomy may be more feasible. Sometimes a more extensive dissection may be required, as in the case of gastrinomas. These tumors are typically small (<1 cm), often multiple, and may be peri- or extra-pancreatic. As such, duodenal exploration and extended lymphadenectomy may be required (45,46).
For patients with metastatic disease, management is particularly challenging. In these cases, surgical intervention is rarely curative, but is often still necessary for palliation of symptoms, such as bleeding and obstruction, and prevention of further complications of the disease (47). When functional tumors present as advanced-stage disease, surgical debulking can often alleviate the severe, life-limiting symptoms of hormonal excess (14,47,48).
Radiotherapy
Radiofrequency ablation (RFA) has been shown to be a useful adjunctive procedure to surgery, when dealing with hepatic metastases. It’s effectiveness in colon cancer with liver metastases has long been established. More recently, RFA has been shown to aid in the relief of symptoms of pancreatic NET complicated by liver metastases, as well as for local control of such lesions (49). In addition to RFA, external beam radiation is another form of radiotherapy that has shown some promise in the management of metastatic pancreatic NET; this technique has been shown to improve symptoms of bone pain in patients with bone metastases (50).
Chemotherapy
To date, the role of chemotherapy in the management of metastatic pancreatic NET has been limited, primarily due to the fact that pancreatic NET usually run and indolent course. As such, they usually do not respond well to chemotherapeutic intervention. However, this treatment modality is still worth considering, particularly when tumors are non-resectable, poorly-differentiated, and in the presence of angiolymphatic invasion (51).
Hereditary syndromes associated with NET of the pancreas
While the vast majority of these tumors occur sporadically, there is a small percentage that present as part of a genetic syndrome. Multiple endocrine neoplasia type 1, von Hippel Lindau, neurofibromatosis type 1, and tuberous sclerosis represent hereditary cancer syndromes with a pancreatic NET predisposition. Lifetime prevalence of pancreatic NET varies greatly among patients with different hereditary endocrinopathies. Greater than 80% of MEN 1 patients will develop a pancreatic neuroendocrine tumor during their lifetime, while only up to 20% of von Hippel Lindau patients will. Neurofibromatosis type 1 patients and tuberous sclerosis patients have yet lower lifetime prevalence rates at 10% and 1%, respectively.
Molecular genetics and pathogenesis
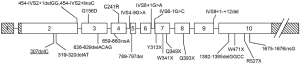
NET arise from embryonic tissues either derived from neuroectoderm (the neural crest) or endoderm and therefore may potentially occur in diverse organ sites, but primarily localize to the gastrointestinal tract and the pancreas. It has been described above that carcinoids of the GI tract and pancreatic NET tumors appear essentially indistinguishable by histopathologic examination, but separate tumor types are believed to have genetic and functional differences as well as different responses to therapeutic interventions. From an oncologic perspective, NET are classified generally into well-differentiated and poorly-differentiated tumors, irrespective of tissue of origin. Pancreatic NET are infrequently occurring tumors and most develop as sporadic neoplasms, with a smaller subset occurring in association with a hereditary cancer syndrome such as multiple endocrine neoplasia type 1 (MEN 1), or von Hippel Lindau (VHL) syndrome. Importantly, key genes that result in a rare hereditary cancer syndrome, when inherited as a germline mutation present in every cell in the body, are the same gene changes that may develop by chance in somatic cells, and be responsible for a subset of the more common sporadic tumors. For example, approximately 21% of sporadic pancreatic NET harbor mutations in the MEN1 tumor suppressor gene, with considerable variation in frequency depending on the specific tumor type. In this regard, only approximately 8% of insulinomas and nonfunctioning NET have MEN1 tumor suppressor gene mutations, but they occur more frequently in gastrinomas (37%), VIPomas (44%), and glucagonomas (67%) (52). The MEN1 tumor suppressor gene encodes a 610 amino acid nuclear protein product, termed menin, which is ubiquitously expressed and highly conserved evolutionarily down to Zebrafish with the murine Men1 gene 98% homologous. It is therefore likely to have a pivotal role in regulation of cell growth. The germline mutations in the menin gene include missense, nonsense, deletions and RNA splicing defects and can occur anywhere in the 9 coding exons, as wells as in the intron-exon junctions (53) (Figure 4).
Menin is predominately a nuclear protein (54) that binds to JunD, a member of the AP-1 transcription factor family, and represses JunD mediated transcription (55,56). In addition, menin has been shown to physically interact with a diverse variety of other proteins including transcription factors, DNA processing factors, DNA repair proteins, and cytoskeletal proteins (Smad3, NF-kappa-B, nm23, Pem, FANCD2, RPA2, ASK, and others) (57-63). The combination of findings from all current studies has not yielded a clear picture of the mechanisms of menin’s tumor suppressor activity or the specific role for menin in endocrine tumorigenesis, although its diverse interactions suggest possible roles in transcriptional regulation, DNA processing and repair, and cytoskeletal integrity. Knockout of both Men1 alleles in mice results in embryonic lethality (64), suggesting that menin may have a broader role in the regulation of cell growth that is not limited to the endocrine tissues affected in patients with MEN 1 syndrome. Heterozygous Men1+/- mice demonstrate somatic loss of the wild type Men1 allele in tumors (64) and develop a pattern of endocrine tumor formation that very closely reflects the endocrine abnormalities in the human MEN 1 syndrome.
DNA microarray analysis of global gene expression has been performed by our group, comparing 8 MEN 1-associated NET to normal islet cell preparations (65). This study demonstrated 45 up-regulated and 148 down-regulated genes in the tumor group, mostly representing genes involved in cell growth or signal transduction. Interesting, 19 apoptosis-related genes, including IER3, PHLDA2, IAPP, and SST, were down-regulated. Other groups have reported gene differential expression studies although the results are not entirely concordant (66,67).
Few previous molecular genetic studies have focused specifically on neuroendocrine cells. However in other in vitro systems, some of the effects of menin have been elucidated (68). Over expression of menin has been shown to diminish the tumorigenic phenotype of Ras-transformed NIH-3T3 cells, consistent with its putative tumor suppressor function (69). In addition, studies have suggested a possible role for menin in repressing telomerase activity in somatic cells, perhaps explaining in part its tumor suppressor properties (70). Menin has most recently been shown to regulate transcription in differentiated cells by associating with and modulating the histone methyltransferase activity of a nuclear protein complex to activate specific gene expression, including the cyclin-dependent kinase (CDK) inhibitors p27Kip1 and p18Ink4c (71-73), as well as other cell cycle regulators.
Among the important signaling pathways that have been elucidated in NET are phosphatidyl-inositol 3-kinase (PI3K)/Akt, mitogen activated protein kinases (MAPKs), and Notch1/Hairy Enhancer of Split-1 (HES-1)/achaete-scute complex like-1 (ASCL1). Most of the work on these pathways in NET have been studies of carcinoids (74-79).
ASCL1 is expressed at high levels in NET such as medullary thyroid cancer (MTC), pheochromocytomas, carcinoids, and small cell lung cancer. In vivo abolition of ASCL1 in transgenic knockout mice leads to the failed development of pulmonary neuroendocrine cells, a paucity of thyroid C-cells, and a 50% reduction in adrenal chromaffin cell population (80,81). These results suggest that ASCL1 is required for the development of diverse cell types of neuroendocrine lineage. Therefore, inhibition of ASCL1 expression may be an important way to suppress NET growth.
There is evidence that the Notch 1 signaling pathway has a negative effect on NET cell growth. However, a number of studies have shown that Notch1 signaling is very minimal or absent in NET (75,82-84). This finding could explain the high expression of ASCL1 protein in these tumors. Transient expression of active, Notch1 via adenoviral vector in carcinoid tumor cells in vitro results in growth suppression and significant reduction in NET markers such as serotonin, chromogranin A (CgA), synaptophysin, and ASCL1 supporting the tumor suppressor role of Notch1 signaling (83). Importantly, these NET cells lack Notch1 activation at baseline. Therefore, the identification of compound(s) that activate endogenous Notch1 in carcinoids is a line of investigation for potential clinical application in the treatment of patients with these tumors. Recently, Chen and colleagues have shown that histone deactylase (HDAC) inhibitors upregulate Notch1 in NETs and inhibit tumor growth (85,86). Clinical trials with these agents are currently ongoing.
Ras regulates multiple signaling pathways of which the best understood is the Ras/Raf/mitogen-activated extracellular protein kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway. The ras/raf signaling pathway has been recognized as a pivotal signaling pathway in cancer biology. Activation of raf-1 pathway in MTC and other NET by expression of estradiol inducible estrogen receptor fused with catalytic domain of raf-1 fusion protein leads to complete suppression of ASCL1 mRNA and protein (77,78,87,88), and decrease in the level of ASCL1 protein correlates with reduction in tumor markers calcitonin and CgA. Furthermore, raf-1 activation in MTC cells results in growth suppression.
Targeted therapies
Patients with localized disease, or limited regional lymph node or hepatic metastases, are best treated with an attempted complete surgical resection, which is the only potentially curative therapeutic option available for pancreatic NET. Although NET in general demonstrate limited response to treatment with conventional systemic cytotoxic chemotherapy, other pharmacologic treatment options are available with differing therapeutic targeting strategies. These include treatment with somatostatin analogs that have been shown to result in symptomatic improvement, reduction in biochemical tumor markers, and to a lesser extent tumor antiproliferative effects. Long-acting somatostatin analogs are frequently given to patients and these agents provide the best means of providing symptomatic relief for patients with marked hormone related symptoms. Interferon may have efficacy in reducing symptoms from hormone excess in a subset of patients, but is associated with risk of significant adverse side effects, and recent studies have not been able to consistently demonstrate an objective reduction in tumor growth and progression.
Other targeted treatment strategies include peptide receptor radionuclide therapy (PRRT) with radiolabeled somatostatin analogs which target tumor cell surface somatostatin receptors (SSTR), and result in the internalization of the peptide/receptor complex inside the tumor cell. Although previous trials show variable effectiveness, newer radionuclides have shown increased efficacy. At present, PRRT may have a role in treatment of advanced low-grade enteropancreatic NET, but clinical use is limited by variable target receptor density, anatomic limitations, and late toxicity.
It is of particular importance that recent data suggest newer targeted agents, particularly sunitinib and everolimus, have demonstrated antitumor activity in patients with advanced metastatic NET (89,90). Because NET are highly vascular, agents with anti-angiogenetic properties that have been studied include drugs targeting VEGF (bevacizumab), small molecules inhibiting the receptor tyrosine kinase domains of VEGFR and related receptors (sunitinib, sorafenib). Sunitinib malate is an oral tyrosine kinase inhibitor with multiple targets, including all receptors for platelet-derived growth factor (PDGF-Rs), vascular endothelial growth factor receptors (VEGFRs), RET, and c-KIT (CD117).
After initial favorable results, including evidence of objective response in phase I trials, larger phase II/III studies were undertaken in patients with advanced/metastatic well-differentiated pancreatic NET. Sunitinib was studied in a double-blind, placebo controlled, randomized phase III study comparing 37.5 mg sunitinib continuous daily dosing versus placebo in patients with progressive, well-differentiated unresectable pancreatic NET (91). Although objective responses were infrequent (<10%), progression free survival (PFS) was more than double in sunitinib-treated patients when compared with those patients receiving placebo (11.4 vs. 5.5 months, HR 0.42, P<0.001). There was also the suggestion of overall survival benefit with sunitinib versus placebo.
Everolimus (RAD001) is an oral mammalian target of rapamycin (mTOR) that has also been studied extensively in patients with advanced NET not amenable to curative surgical resection. The RADIANT-1, -2, and -3 trials have studied the efficacy of everolimus in patients with NET. The third largest study was a prospective, randomized phase III study (92) of patients with progressive advanced low- or intermediate-grade pancreatic NET in which patients were randomly assigned to either everolimus or placebo with a double-blind crossover study design. About half of the patients had received previous chemotherapy. Again, although objective responses were infrequent, the disease control rate (78% versus 53%) and PFS were significantly increased in patients treated with everolimus when compared with the placebo group (11.4 vs. 5.4 months, HR 0.34, P<0001). Furthermore, everolimus resulted in significantly greater sustained decreases in biochemical tumor markers including chromogranin A (CgA) and neuron-specific enolase (NSE). No impact on overall survival was observed, but 73% of patients on placebo crossed over to everolimus after experiencing disease progression. This study concluded that everolimus, as compared with placebo, resulted in significantly prolonged progression-free survival among patients with progressive advanced pancreatic NET, and was associated with a low rate of severe adverse events.
Summary
Pancreatic NET tumors are relatively uncommon, usually well-differentiated neoplasms that as a group tend to have a less aggressive biologic behavior when compared with the more common and highly malignant exocrine adenocarcinomas of the pancreas. They may have a variable presentation either due to the consequences of specific peptide hormone products produced by the tumor cells resulting in specific clinical signs and symptoms, or the mass effects of local tumor advancement. Complete surgical excision is the only curative treatment. In general, the response of these tumors to conventional cytotoxic chemotherapy is limited, and although these tumors are generally considered to be indolent, they frequently progress and are fatal when patients develop widespread disease that is not amenable to surgical resection. There are unique features and special considerations in the management of NET of the pancreas that occur in association with a hereditary endocrine neoplasia syndrome such as MEN 1 or VHL. The molecular pathogenesis of NET is being studied, with the resultant development of a few agents that have shown biologic activity and clinical benefit in patients with advanced disease. The agents that have shown benefit and have been most extensively studied to date include growth factor receptor angiogenesis inhibitors, and mTOR inhibitors. Importantly, these advances hold the promise of leading to the development of novel molecular targets.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Roland CL, Bian A, Mansour JC, et al. Survival impact of malignant pancreatic neuroendocrine and islet cell neoplasm phenotypes. J Surg Oncol 2012;105:595-600. [PubMed]
- Ries LAG, Harkins D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2003, National Cancer Institute. Bethesda, MD, Available online: , 2006.
- Toumpanakis C, Caplin ME. Update on the role of somatostatin analogs for the treatment of patients with gastroenteropancreatic neuroendocrine tumors. Semin Oncol 2013;40:56-68. [PubMed]
- Benavent M, de Miguel MJ, Garcia-Carbonero R. New targeted agents in gastroenteropancreatic neuroendocrine tumors. Target Oncol 2012;7:99-106. [PubMed]
- Lepage C, Bouvier AM, Phelip JM, et al. Incidence and management of malignant digestive endocrine tumours in a well defined French population. Gut 2004;53:549-53. [PubMed]
- Fitzgerald TL, Hickner ZJ, Schmitz M, et al. Changing incidence of pancreatic neoplasms: a 16-year review of statewide tumor registry. Pancreas 2008;37:134-8. [PubMed]
- Buchanan KD, Johnston CF, O’Hare MM, et al. Neuroendocrine tumors. A European view. Am J Med 1986;81:14-22. [PubMed]
- Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer 1995;75:171-90. [PubMed]
- Eriksson B, Oberg K, Skogseid B. Neuroendocrine pancreatic tumors. Clinical findings in a prospective study of 84 patients. Acta Oncol 1989;28:373-7. [PubMed]
- Lam KY, Lo CY. Pancreatic endocrine tumour: a 22-year clinico-pathological experience with morphological, immunohistochemical observation and a review of the literature. Eur J Surg Oncol 1997;23:36-42. [PubMed]
- Moldow RE, Connelly RR. Epidemiology of pancreatic cancer in Connecticut. Gastroenterology 1968;55:677-86. [PubMed]
- Watson RG, Johnston CF, O’Hare MM, et al. The frequency of gastrointestinal endocrine tumours in a well-defined population--Northern Ireland 1970-1985. Q J Med 1989;72:647-57. [PubMed]
- Grimelius L, Hultquist GT, Stenkvist B. Cytological differentiation of asymptomatic pancreatic islet cell tumours in autopsy material. Virchows Arch A Pathol Anat Histol 1975;365:275-88. [PubMed]
- Halfdanarson TR, Rabe KG, Rubin J, et al. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol 2008;19:1727-33. [PubMed]
- Kimura W, Kuroda A, Morioka Y. Clinical pathology of endocrine tumors of the pancreas. Analysis of autopsy cases. Dig Dis Sci 1991;36:933-42. [PubMed]
- Placzkowski KA, Vella A, Thompson GB, et al. Secular trends in the presentation and management of functioning insulinoma at the Mayo Clinic, 1987-2007. J Clin Endocrinol Metab 2009;94:1069-73. [PubMed]
- Service FJ, Dale AJ, Elveback LR, et al. Insulinoma: clinical and diagnostic features of 60 consecutive cases. Mayo Clin Proc 1976;51:417-29. [PubMed]
- Service FJ, McMahon MM, O’Brien PC, et al. Functioning insulinoma--incidence, recurrence, and long-term survival of patients: a 60-year study. Mayo Clin Proc 1991;66:711-9. [PubMed]
- Berna MJ, Hoffmann KM, Serrano J, et al. Serum gastrin in Zollinger-Ellison syndrome: I. Prospective study of fasting serum gastrin in 309 patients from the National Institutes of Health and comparison with 2229 cases from the literature. Medicine (Baltimore) 2006;85:295-330. [PubMed]
- Wermers RA, Fatourechi V, Wynne AG, et al. The glucagonoma syndrome. Clinical and pathologic features in 21 patients. Medicine (Baltimore) 1996;75:53-63. [PubMed]
- Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [PubMed]
- Stabile BE, Morrow DJ, Passaro EJ. The gastrinoma triangle: operative indications. Am J Surg 1984;147:25-31. [PubMed]
- Kulke MH, Anthony LB, Bushnell DL, et al. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas 2010;39:735-52. [PubMed]
- Norton JA, Fraker DL, Alexander HR, et al. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med 1999;341:635-44. [PubMed]
- Dickson PV, Rich TA, Xing Y, et al. Achieving eugastrinemia in MEN1 patients: both duodenal inspection and formal lymph node dissection are important. Surgery 2011;150:1143-52. [PubMed]
- Norton JA, Alexander HR, Fraker DL, et al. Comparison of surgical results in patients with advanced and limited disease with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome. Ann Surg 2001;234:495-505; discussion 505-6. [PubMed]
- Vaidakis D, Karoubalis J, Pappa T, et al. Pancreatic insulinoma: current issues and trends. Hepatobiliary Pancreat Dis Int 2010;9:234-41. [PubMed]
- Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumors. Gastroenterology 2008;135:1469-92. [PubMed]
- Mutch MG, Frisella MM, DeBenedetti MK, et al. Pancreatic polypeptide is a useful plasma marker for radiographically evident pancreatic islet cell tumors in patients with multiple endocrine neoplasia type 1. Surgery 1997;122:1012-9; discussion 1019-20. [PubMed]
- Ferolla P, Faggiano A, Mansueto G, et al. The biological characterization of neuroendocrine tumors: the role of neuroendocrine markers. J Endocrinol Invest 2008;31:277-86. [PubMed]
- Srivastava A, Padilla O, Fischer-Colbrie R, et al. Neuroendocrine secretory protein-55 (NESP-55) expression discriminates pancreatic endocrine tumors and pheochromocytomas from gastrointestinal and pulmonary carcinoids. Am J Surg Pathol 2004;28:1371-8. [PubMed]
- Reznek RH. CT/MRI of neuroendocrine tumours. Cancer Imaging 2006;6:S163-77. [PubMed]
- Dromain C, de Baere T, Lumbroso J, et al. Detection of liver metastases from endocrine tumors: a prospective comparison of somatostatin receptor scintigraphy, computed tomography, and magnetic resonance imaging. J Clin Oncol 2005;23:70-8. [PubMed]
- Hofman MS, Hicks RJ. Changing paradigms with molecular imaging of neuroendocrine tumors. Discov Med 2012;14:71-81. [PubMed]
- Yim JH, Siegel BA, DeBenedetti MK, et al. Prospective study of the utility of somatostatin-receptor scintigraphy in the evaluation of patients with multiple endocrine neoplasia type 1. Surgery 1998;124:1037-42. [PubMed]
- Chatzipantelis P, Salla C, Konstantinou P, et al. Endoscopic ultrasound-guided fine-needle aspiration cytology of pancreatic neuroendocrine tumors: a study of 48 cases. Cancer 2008;114:255-62. [PubMed]
- Anderson MA, Carpenter S, Thompson NW, et al. Endoscopic ultrasound is highly accurate and directs management in patients with neuroendocrine tumors of the pancreas. Am J Gastroenterol 2000;95:2271-7. [PubMed]
- Kim MK. Endoscopic ultrasound in gastroenteropancreatic neuroendocrine tumors. Gut Liver 2012;6:405-10. [PubMed]
- Krampitz GW, Norton JA, Poultsides GA, et al. Lymph nodes and survival in pancreatic neuroendocrine tumors. Arch Surg 2012;147:820-7. [PubMed]
- Norton JA, Kivlen M, Li M, et al. Morbidity and mortality of aggressive resection in patients with advanced neuroendocrine tumors. Arch Surg 2003;138:859-66. [PubMed]
- Lairmore TC, Chen VY, DeBenedetti MK, et al. Duodenopancreatic resections in patients with multiple endocrine neoplasia type 1. Ann Surg 2000;231:909-18. [PubMed]
- Knudson AG Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A 1971;68:820-3. [PubMed]
- Knudson AG Jr, Hethcote HW, Brown BW. Mutation and childhood cancer: a probabilistic model for the incidence of retinoblastoma. Proc Natl Acad Sci U S A 1975;72:5116-20. [PubMed]
- Lowney JK, Frisella MM, Lairmore TC, et al. Pancreatic islet cell tumor metastasis in multiple endocrine neoplasia type 1: correlation with primary tumor size. Surgery 1998;124:1043-8; discussion 1048-9. [PubMed]
- Dralle H, Krohn SL, Karges W, et al. Surgery of resectable nonfunctioning neuroendocrine pancreatic tumors. World J Surg 2004;28:1248-60. [PubMed]
- Kimura W, Tezuka K, Hirai I. Surgical management of pancreatic neuroendocrine tumors. Surg Today 2011;41:1332-43. [PubMed]
- Gomez-Rivera F, Stewart AE, Arnoletti JP, et al. Surgical treatment of pancreatic endocrine neoplasms. Am J Surg 2007;193:460-5. [PubMed]
- Panzuto F, Boninsegna L, Fazio N, et al. Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. J Clin Oncol 2011;29:2372-7. [PubMed]
- Steinmüller T, Kianmanesh R, Falconi M, et al. Consensus guidelines for the management of patients with liver metastases from digestive (neuro)endocrine tumors: foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2008;87:47-62. [PubMed]
- Ramage JK, Davies AH, Ardill J, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours. Gut 2005;54 Suppl 4:iv1-16. [PubMed]
- Oberg K, Astrup L, Eriksson B, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine tumours (including bronchopulmonary and thymic neoplasms). Part I-general overview. Acta Oncol 2004;43:617-25. [PubMed]
- Duerr EM, Chung DC. Molecular genetics of neuroendocrine tumors. Best Pract Res Clin Endocrinol Metab 2007;21:1-14. [PubMed]
- Mutch MG, Dilley WG, Sanjurjo F, et al. Germline mutations in the multiple endocrine neoplasia type 1 gene: evidence for frequent splicing defects. Hum Mutat 1999;13:175-85. [PubMed]
- Guru SC, Goldsmith PK, Burns AL, et al. Menin, the product of the MEN1 gene, is a nuclear protein. Proc Natl Acad Sci U S A 1998;95:1630-4. [PubMed]
- Agarwal SK, Guru SC, Heppner C, et al. Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription. Cell 1999;96:143-52. [PubMed]
- Gobl AE, Berg M, Lopez-Egido JR, et al. Menin represses JunD-activated transcription by a histone deacetylase-dependent mechanism. Biochim Biophys Acta 1999;1447:51-6. [PubMed]
- Heppner C, Bilimoria KY, Agarwal SK, et al. The tumor suppressor protein menin interacts with NF-kappaB proteins and inhibits NF-kappaB-mediated transactivation. Oncogene 2001;20:4917-25. [PubMed]
- Kaji H, Canaff L, Lebrun JJ, et al. Inactivation of menin, a Smad3-interacting protein, blocks transforming growth factor type beta signaling. Proc Natl Acad Sci U S A 2001;98:3837-42. [PubMed]
- Lemmens IH, Forsberg L, Pannett AA, et al. Menin interacts directly with the homeobox-containing protein Pem. Biochem Biophys Res Commun 2001;286:426-31. [PubMed]
- Ohkura N, Kishi M, Tsukada T, et al. Menin, a gene product responsible for multiple endocrine neoplasia type 1, interacts with the putative tumor metastasis suppressor nm23. Biochem Biophys Res Commun 2001;282:1206-10. [PubMed]
- Sukhodolets KE, Hickman AB, Agarwal SK, et al. The 32-kilodalton subunit of replication protein A interacts with menin, the product of the MEN1 tumor suppressor gene. Mol Cell Biol 2003;23:493-509. [PubMed]
- Agarwal SK, Kennedy PA, Scacheri PC, et al. Menin molecular interactions: insights into normal functions and tumorigenesis. Horm Metab Res 2005;37:369-74. [PubMed]
- Schnepp RW, Hou Z, Wang H, et al. Functional interaction between tumor suppressor menin and activator of S-phase kinase. Cancer Res 2004;64:6791-6. [PubMed]
- Crabtree JS, Scacheri PC, Ward JM, et al. A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors. Proc Natl Acad Sci U S A 2001;98:1118-23. [PubMed]
- Dilley WG, Kalyanaraman S, Verma S, et al. Global gene expression in neuroendocrine tumors from patients with the MEN1 syndrome. Mol Cancer 2005;4:9. [PubMed]
- Capurso G, Lattimore S, Crnogorac-Jurcevic T, et al. Gene expression profiles of progressive pancreatic endocrine tumours and their liver metastases reveal potential novel markers and therapeutic targets. Endocr Relat Cancer 2006;13:541-58. [PubMed]
- Hansel DE, Rahman A, House M, et al. Met proto-oncogene and insulin-like growth factor binding protein 3 overexpression correlates with metastatic ability in well-differentiated pancreatic endocrine neoplasms. Clin Cancer Res 2004;10:6152-8. [PubMed]
- Lairmore TC, Chen H. Role of menin in neuroendocrine tumorigenesis. Adv Exp Med Biol 2009;668:87-95. [PubMed]
- Kim YS, Burns AL, Goldsmith PK, et al. Stable overexpression of MEN1 suppresses tumorigenicity of RAS. Oncogene 1999;18:5936-42. [PubMed]
- Lin SY, Elledge SJ. Multiple tumor suppressor pathways negatively regulate telomerase. Cell 2003;113:881-9. [PubMed]
- Chen YX, Yan J, Keeshan K, et al. The tumor suppressor menin regulates hematopoiesis and myeloid transformation by influencing Hox gene expression. Proc Natl Acad Sci U S A 2006;103:1018-23. [PubMed]
- Yokoyama A, Somervaille TC, Smith KS, et al. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell 2005;123:207-18. [PubMed]
- Yokoyama A, Wang Z, Wysocka J, et al. Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Mol Cell Biol 2004;24:5639-49. [PubMed]
- Kunnimalaiyaan M, Chen H. The Raf-1 pathway: a molecular target for treatment of select neuroendocrine tumors? Anticancer Drugs 2006;17:139-42. [PubMed]
- Kunnimalaiyaan M, Yan S, Wong F, et al. Hairy Enhancer of Split-1 (HES-1), a Notch1 effector, inhibits the growth of carcinoid tumor cells. Surgery 2005;138:1137-42; discussion 1142.. [PubMed]
- Lal A, Chen H. Treatment of advanced carcinoid tumors. Curr Opin Oncol 2006;18:9-15. [PubMed]
- Sippel RS, Carpenter JE, Kunnimalaiyaan M, et al. The role of human achaete-scute homolog-1 in medullary thyroid cancer cells. Surgery 2003;134:866-71; discussion 871-3. [PubMed]
- Sippel RS, Carpenter JE, Kunnimalaiyaan M, et al. Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol 2003;285:G245-54. [PubMed]
- Van Gompel JJ, Sippel RS, Warner TF, et al. Gastrointestinal carcinoid tumors: factors that predict outcome. World J Surg 2004;28:387-92. [PubMed]
- Borges M, Linnoila RI, van de Velde HJ, et al. An achaete-scute homologue essential for neuroendocrine differentiation in the lung. Nature 1997;386:852-5. [PubMed]
- Lanigan TM, DeRaad SK, Russo AF. Requirement of the MASH-1 transcription factor for neuroendocrine differentiation of thyroid C cells. J Neurobiol 1998;34:126-34. [PubMed]
- Sriuranpong V, Borges MW, Ravi RK, et al. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res 2001;61:3200-5. [PubMed]
- Nakakura EK, Sriuranpong VR, Kunnimalaiyaan M, et al. Regulation of neuroendocrine differentiation in gastrointestinal carcinoid tumor cells by notch signaling. J Clin Endocrinol Metab 2005;90:4350-6. [PubMed]
- Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, et al. Overexpression of the NOTCH1 intracellular domain inhibits cell proliferation and alters the neuroendocrine phenotype of medullary thyroid cancer cells. J Biol Chem 2006;281:39819-30. [PubMed]
- Greenblatt DY, Vaccaro AM, Jaskula-Sztul R, et al. Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncologist 2007;12:942-51. [PubMed]
- Kunnimalaiyaan M, Vaccaro AM, Ndiaye MA, et al. Inactivation of glycogen synthase kinase-3beta, a downstream target of the raf-1 pathway, is associated with growth suppression in medullary thyroid cancer cells. Mol Cancer Ther 2007;6:1151-8. [PubMed]
- Chen H, Carson-Walter EB, Baylin SB, et al. Differentiation of medullary thyroid cancer by C-Raf-1 silences expression of the neural transcription factor human achaete-scute homolog-1. Surgery 1996;120:168-72; discussion 173. [PubMed]
- Chen H, Udelsman R, Zeiger M, et al. Human achaete-scute homolog-1 is highly expressed in a subset of neuroendocrine tumors. Oncol Rep 1997;4:775-8. [PubMed]
- Benavent M, de Miguel MJ, Garcia-Carbonero R. New targeted agents in gastroenteropancreatic neuroendocrine tumors. Target Oncol 2012;7:99-106. [PubMed]
- Fazio N, Cinieri S, Lorizzo K, et al. Biological targeted therapies in patients with advanced enteropancreatic neuroendocrine carcinomas. Cancer Treat Rev 2010;36 Suppl 3:S87-94. [PubMed]
- Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501-13. [PubMed]
- Yao JC, Shah MH, Ito T, Bohas CL, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011;364:514-23. [PubMed]


