FTO gene, obesity and colon cancer: from epidemiological evidence to laboratory studies
Introduction
Cancers are a severe co-morbidity of obesity, which is recognised as a major health issue worldwide. As obesity is difficult to treat, obesity-associated cancers, are a major concern for healthcare, requiring greater attention. It is estimated that obesity accounts for about 14% of cancer-caused death (1). Thus, it is important to study obesity-associated cancer for cancer prevention. Published laboratory studies have demonstrated that many cancer risk factors altered in obesity, such as insulin, insulin-like growth factor, estrogen, leptin, adiponection, IL-6, IL-17 and TNF-α, play key roles in obesity-associated cancers (2-5). Obesity has also been shown to be associated with poorer prognosis of many cancers which may be caused by the increase of drug resistance to chemotherapeutic agents (6,7). Multiple signalling pathways have been shown to be activated in obesity-associated cancers (8), which could be responsible for the increased cancer incidences and their poor prognosis.
It is not clear whether there is shared genetic basis between obesity and cancers. So far, more than 30 gene loci have been reported to be associated with obesity (9). Among them, fat mass and obesity associated (FTO) gene, a well known obesity-associated gene, is the first gene that has been confirmed to be related to the obesity (10). FTO protein is an AlkB-like 2-oxoglutarate—dependent nucleic acid demethylase with substrate specificity for 3-methylthymidine and 3-methyluracil in nuclear acids (11). It prefers single-stranded nuclear acids and thus methylated RNA rather than DNA (12,13). The molecular target of FTO has been identified to be N6-methyladenosine in mRNA (14). One of the physiological roles of the FTO has been identified to stimulate food intake as it is expressed highest in hypothalamic including arcuate (ARC), paraventricular (PVN), dorsomedial and ventromedial (VMN) nuclei (15-18), which are known to regulate satiety.
FTO genotype is strongly associated with fat mass in human bodies (19). Epidemiological studies have shown that FTO gene has a high frequency of gain-of-function mutations such as the mutation occurred at position rs9939609 (20-22). The FTO SNP rs9939609 is significantly associated with extremes of adiposity (23) in ascertained samples and body mass index (BMI) in general population (24). It has been demonstrated that homozygous “A” allele of FTO rs9939609 has 1.7 fold higher risk to be obese than that of “T” allele. Recently, Yang et al. reported that FTO affects not only the mean of BMI but also the variability of BMI (25). The association has been further demonstrated by laboratory studies. Over-expression of Fto in mice caused obesity (23) while knock out of Fto led to lean phenotype (26). In this review, we summarise the studies that investigated the role of FTO gene involved in obesity-associated colon cancer to discuss the possible molecular mechanisms of the colon carcinogenesis linking to the obesity.
Possible link between FTO and colon cancer
Epidemiological studies have shown that the genetic variants at the FTO gene locus are associated with several cancers including breast cancer, endometrial cancer, pancreatic cancer and colon cancer (27). Kaklamani et al. reported that FTO single nucleotide polymorphisms (SNPs) are associated with increased risk of breast cancer (28). In a case-control study with 354 breast cancer cases and 364 controls, the associations of SNPs of intron 1 of FTO including rs7206790, rs8047395, rs9939609 and rs1477196 with breast cancer risk were examined (27). It was found that all four SNPs examined were significantly associated with breast cancer risk with the SNP rs1477196 showing the strongest association. FTO is expressed both in normal and malignant breast tissue and FTO genotypes could be one of breast cancer risk factors.
Endometrial cancer is a cancer which is highly associated with obesity (29). Lurie et al. found that FTO rs9939609 is a susceptibility marker for white non-Hispanic women at higher risk of endometrial cancer (30). In a study with 832 cases of endometrial cancer patients and 2,049 controls, genetic variants at 7 obesity-related genes (SEC16B/RASAL, TMEM18, MSRA, SOX6, MTCH2, FTO, and MC4R) were found to be associated with endometrial cancer after adjustment of BMI (31), suggesting a potential role of these genes in endometrial cancer which is independent of obesity.
Genetic variants at FTO locus have also been associated with pancreatic cancer. In a meta-analysis study which includes 13 studies with 16,277 cases and 31,153 controls, FTO rs9939609 is also associated with pancreatic cancer (32). However, most of the participating studies do not take the BMI/obesity factor into account. Thus, it is unclear whether the association between FTO and pancreatic cancer is obesity-dependent or not. Tang et al. observed that FTO IVS1-2777AC/AA genotype is associated with pancreatic cancer but only limited in overweighted people (33).
Relatively fewer studies have reported that the FTO variant is associated with colon cancer. Nock et al. investigated the involvement of FTO in obesity-associated colorectal cancer in 759 Caucasians and 469 African-Americans and found that FTO variants are positively associated with colorectal cancer in African-Americans (34). However, Lim et al. examined the direct effect of polymorphisms of 15 loci (BDNF, FAIM2, FTO, GNPDA2, KCTD15, LYPLAL1, MC4R, MSRA, MTCH2, NEGR1, NRXN3, SEC16B, SH2B1, TFAP2B and TMEM18) in 2,033 colorectal cancer cases and 9,640 controls, and found that only 3 loci (KCTD15 rs29941 and MC4R rs17782313) were associated with colorectal cancer independent of obesity (35). The sample size used in this study may be not big enough to make solid conclusion.
Taken together, epidemiological studies provide evidence to support that genetic variants at the FTO locus are associated with several cancers including colon cancer. The mechanisms could be obesity dependent or independent (Figure 1).
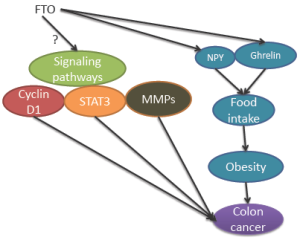
Mechanisms for FTO in obesity-associated colon cancer
FTO protein is highly expressed in hypothalamus, a food intake regulating organ, indicating its possible role in satiety (16,18). Genetic variants at FTO locus have been found to be associated with satiety (36). Further studies have demonstrated that FTO affects the food intake rather than the energy expenditure (18,37-41). For example, a particular allele of the FTO SNP rs9939609 has an effect to repress satiety and increase food intake (42-46). Church et al. reported that over-expression of Fto in mice increased food intake and obesity in both standard food feeding and high-fat diet (HFD) feeding (47). In contrast, deletion or missense mutations of FTO lead to leanness (48-51).
It has been shown that nutrition state affects the expression of FTO and other food intake-related genes including Etv5, Faim2 and Negr1 (52). Based on the studies on postpradial response of Fto in mice, fasting can increase expression of Fto and thus increase food intake (16,53,54). Starvation in Wistar rats resulted in an increase of both Fto mRNA and protein in neurons of paraventricular and ventromedial nucleus (53). This also provides evidence that FTO is involved in satiety regulation in normal conditions.
FTO can decrease monoamine such as noradrenaline, serotonin and dopamine, which repress food intake (55-59). In Fto null mice, catecholamine was increased (26). Hess demonstrated that FTO knock out resulted in disruption of dopamine pathway (60). On the other hand, FTO can also increase orexigenic hormones. FTO has been shown to increase these hormones including ghrelin, neuropeptide Y (NPY) and oxytocins (58,61,62). Olszewski et al. studied the effect of overexpression of FTO on mRNA expression of oxytocins, NPY and agouti-related peptide (AgPR). It was found that overexpression of FTO up-regulated oxytocins but not NPY and AgPR (61). Addition of oxytocins into cell culture of hypothalamic cells did not change FTO protein expression, suggesting the lack of feedback. Karra et al. showed that one allele of the FTO variant increased expression of ghrelin, which is known to increase food-intake (62).
The effect of FTO on BMI can account for the increased cancer incidence in patients with FTO SNPs. Obesity is known to play an important role in colon cancer via increased cancer risks in obesity including insulin, estrogen, adipokines and inflammatory factors (63). These factors can activate several signalling pathways such as PI3K/Akt, MAPK and STAT3 which are known to play key roles in carcinogenesis of colon cancer. Therefore, inhibition of these pathways by specific small molecule inhibitors or phytochemicals can be used for the prevention and treatment of obesity-associated colon cancer (8,64,65). Leptin is one of the major cancer risk factors in obesity. It can activate several signalling pathway to increase cancer incidence. FTO has been shown to increase blood levels of leptin (66). This could be a mediator for the link between FTO mutations and cancer risk. It has been demonstrated that FTO-increased leptin increase is via obesity (66).
The mediate effect of obesity in FTO-increased colon cancer incidence can be further evidenced by the association of FTO with other obesity-associated diseases. It has been shown that FTO is significantly associated with osteoarthritis, a disease highly related with obesity (67). In animal models, it has been demonstrated that obesity increases osteoarthritis incidence and accelerates its progression (68). Leptin is of critical role in obesity-associated osteoarthritis (68-70).
The role of FTO in multiple intracellular signalling pathways
Evidences have also shown that FTO is directly associated with cancer independent of obesity. Kikuchi et al. demonstrated that the FTO SNP rs9939609 is associated with pancreatic cancer risk in Japanese subjects, possibly through a mechanism that is independent of obesity (71). Iles et al. provided evidence that FTO variant increased melanoma independent of BMI [66]. The study showed a genetic variant in intron 8 of FTO, which is not linked with BMI, is associated with increased melanoma incidence. It is known that the BMI associated FTO variant is located in intron 1 (72).
Previous studies have shown that FTO can stimulate several intracellular signalling pathways important in carcinogenesis including signal transducer and activator of transcription 3 (STAT3), phosphoinositide 3-kinase/protein kinase B (PI3K/Akt), cyclin D1 and matrix metalloproteinases (MMPs) (73,74). The direct effect of FTO on these pathways is evidence that FTO can increase cancer incidence independent of obesity.
FTO and STAT3
STAT3 is a transcription factor that functions as a signal transducer and activator. STAT3 is also considered as an oncogene because it promotes cell survival/proliferation, motility and immune tolerance. In obesity-associated colon cancer, STAT3 is activated (2). Activated STAT3 can promote carcinogenesis by mediating the functions of the downstream proteins through its signaling pathway. FTO has been demonstrated to increase the activity of STAT3. Over-expression of Fto in rats increased STAT3 mRNA expression in the arcuate nucleus of the hypothalamus (58). The role of FTO in the STAT3 pathway in colon cells, however, is yet to be elucidated.
FTO and PI3K/Akt pathway
PI3K/Akt is an important survival pathway in many cancers including colon cancer (75-78). Its activation in obesity by various factors is responsible for the increased carcinogenesis and drug resistance. FTO has been shown to regulate Akt in both neurons and adipocytes. siRNA silencing of Fto resulted in increased pAkt while pAMPK, a negative regulator of pAkt was decreased (79). However, how FTO protein acts on the PI3K/Akt pathway is not clear. The possibility of FTO-regulated PI3K/Akt pathway may be involved in obesity-associated colon cancer and warrant further studies.
FTO and Cyclin D1
Increased cell proliferation is a major hall marker of carcinogenesis (80,81). A major regulator of cell proliferation is cyclin D1; increase of cyclin D1 can promote cell cycle and thus increase cell proliferation (82). Cyclin D1 has been shown to play a key role in the carcinogenesis associated with adenomatous polyposis coli (APC), a tumour suppressor gene (83). The loss of APC gene is closely associated with colon cancer. Loss of APC will lead to accumulation of beta-catenin, which in turn interact with TCF/LEF family to increase gene transcription including cyclin D1.
FTO has been shown to involve in the regulation of cell proliferation. Knockout of FTO resulted in decreased cyclin D1 and thus reduced cell cycle and cell proliferation in endometrial cells (84). This is evidence that FTO may involve in cell proliferation directly. However, the effect of FTO on cyclin D1 in colon cells has not been studied and warrants further studies.
FTO and MMPs
MMPs are enzymes that degrade the extra cellular matrix and thus play a key role in physiological activities such as extracellular matrix remodelling during growth (85,86). They have been demonstrated to play key roles in cancer metastasis through facilitating the detachment of cancer cells from original sites (85,86). MMPs can also change microenvironment of cancer cells to promote growth (87). MMPs can increase vascular endothelial growth factor (VEGF), a known factor in angiogenesis of tumour growth (86).
FTO has been shown to regulate MMPs to promote cell migration in endometrial cancer cell line Ishikawa. Treatment of Ishikawa cells with estrogen at concentration of 10-9 M up-regulated protein expression of FTO and MMPs via estrogen-receptors (84). Knockdown of FTO by siRNA resulted in decreased MMP2 and MMP9 and thus reduced cell migration in endometrial cells, indicating regulatory role of FTO in MMP production. Thus, it is proposed to target FTO for treatment of cancer.
FTO, estrogen and cancer risk
It has been shown that estrogen can stimulate FTO expression in both mRNA and protein levels via PI3K/Akt and MAPK pathways in endometrial cancer cells (84). Endometrial cancer can be estrogen-dependent or independent (88). This study showed that FTO was a target protein of estrogen in estrogen-dependent endometrial cancer (84). Estrogen can bind estrogen receptor-alpha to increase cell proliferation and decrease apoptosis. There are several down-stream pathways to mediate such an effect. FTO could be one of them. Increased expression of FTO in turn promotes cell proliferation via cyclin D1. In colon cancer the role of estrogen is complicated; it has different effects when it binds to estrogen receptor-alpha and estrogen receptor-beta (89). Estrogen receptor-beta has protective effect and estrogen receptor-alpha can promote carcinogenesis. It will be interesting to study the role of FTO in estrogen-mediated effects in colon cancer cells.
FTO in HFD fed mouse model of obesity-associated colon cancer
HFD is a common model for obesity and obesity-associated colon cancer studies. Several studies have demonstrated that HFD-induced obesity increased colon cancer incidence in mouse and rat models (90-92). Various mechanisms have been proposed. For example, leptin and adiponectin are the two major adipocytokines, which have been considered to be major factors in obesity-associated cancer (2,93). These changes could affect intracellular signalling pathways to increase colon cancer incidence (2).
FTO alterations have been found in HFD-feeding animals. Short-term HFD-feeding decreased FTO while long term HF-feeding increased FTO (58,94). It indicates FTO expression is involved in increased colon cancer incidence in this model. However, it is not known how important FTO is in the HFD-induced obesity-associated colon cancer compared to other cancer risk factors.
Possible co-operation between FTO and other obesity-associated genes
It is now recognised that many genes may involve in the regulation of fat accumulation in body. More than 30 genes have been identified to involve in BMI in European decent (9,95,96). Dorajoo et al. showed that Asians including Chinese, Indian and Malay share some of obesity-associated gene mutations (96). Each gene may only contribute a small percentage. The outcome of obesity and obesity-associated cancer may be due to the co-operation of several obesity-associated genes.
Except FTO, other genes important in obesity have been identified by GWAS. Kilpelainen et al. identified IRS1 and SPRY2 are associated with adipocyte physiology (95). Fox et al. studied subcutaneous adipose tissue and visceral adipose tissue in 5,560 women and 4,997 men (97). It was found that the association between visceral/subcutaneous adipose tissue (VAT/SAT) ratio and rs11118316 at LYPLAL1 gene is the most significant while SNP in the FTO gene was most associated with SAT. In addition, rs1659258 near THNSL2 gene is associated with VAT in women but not men (97). Okada et al. analysed 62,245 east Asian subjects and found several genes were associated with obesity including previously known SEC16B, BDNF, FTO, MC4R and GIPR loci as well as newly identified loci including CDKAL1 locus at 6p22 (rs2206734) and the KLF9 locus at 9q21 (rs11142387) (98).
GWAS has also identified several other genes which are associated with BMI and cancer such as HHEX gene rs1111875 mutation (99) and MC4R rs17782313 (30). MC4R rs17782313 is linked to increased endometrial cancer risk (30). The polymorphisms in HHEX have also linked with endometrial cancer risk although the effect was weak (100). A metastasis analysis showed that the association of FTO, MC4R, TMEM18, SDCCAG8, and TNKS/MSRA mutations with early-onset obesity were very strong (101). It could be an interesting topic to elucidate how these genes co-operate each other to affect cancer incidence.
Prevention
Although there are abundant evidences that FTO is associated with increased cancer incidence including colon cancer, there are no prevention approaches at present. It has been proposed to take some preventive approaches such as targeting FTO in the population with over-expression of FTO (47). A question is that FTO is also necessary for physiological function of human body. It has been shown that an inactivating mutation of FTO (resulting in the p.Arg316Gln alteration) in humans caused an autosomal recessive lethal syndrome (102). Mice lacking Fto gene or dominant mutation (resulting in p.Ile367Phe) had reduced fat mass and thus prevented from obesity (103,104). However, increased postnatal lethality and postnatal growth retardation were also found. Therefore it may be not practical to ablate FTO for the prevention of obesity. Inhibition of FTO downstream pathways may be a better choice. The advantage for inhibition of pathways is that it may also decrease the effect of other gene defects which may also activate the similar intracellular signalling pathways.
Phytochemicals could be used to disrupt the association between FTO and cancer. They are not expensive with low toxic and thus ideal compounds for disrupting FTO and obesity-induced activation of intracellular signalling pathways important for carcinogenesis (64). In addition, some phytochemicals such as beta-glucans can increase immune responses which can facilitate the early elimination of abnormal cells. Phytochemicals have now extensively studied for the prevention of cancer. Some of them may be applied to obesity-associated colon cancer such as EGCG, curcumin and genistein (64,89,105,106). How these phytochemicals act on FTO-signalling pathways has not been well studied and warrant further exploration.
Conclusions
Epidemiological studies indicate that FTO may be involved in obesity-associated colon cancer. Over-expression of FTO can increase food intake, leading to obesity, which can increase colon cancer risk and result in poorer prognosis and drug resistance. FTO protein may also act on different molecules in several survival signalling pathways such as cyclin D1, MMPs, STAT3 and PI3K/Akt, which play a crucial role in carcinogenesis. Over-expression of FTO protein has been shown to mediate other cancer risk factors such as estrogen and HFD to increase cell proliferation. Preventive approaches may be taken in those people with over-expression of FTO. Phytochemicals could be suitable for the prevention as they can inhibit FTO-induced signalling pathways.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625-38. [PubMed]
- Chen J. Multiple signal pathways in obesity-associated cancer. Obes Rev 2011;12:1063-70. [PubMed]
- Chen J. The Src/PI3K/Akt signal pathway may play a key role in decreased drug efficacy in obesity-associated cancer. J Cell Biochem 2010;110:279-80. [PubMed]
- Gislette T, Chen J. The possible role of IL-17 in obesity-associated cancer. ScientificWorldJournal 2010;10:2265-71. [PubMed]
- Huang XF, Chen JZ. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes Rev 2009;10:610-6. [PubMed]
- Chen J, Katsifis A, Hu C, et al. Insulin decreases therapeutic efficacy in colon cancer cell line HT29 via the activation of the PI3K/Akt pathway. Curr Drug Discov Technol 2011;8:119-25. [PubMed]
- Chen J, Huang XF, Qiao L, et al. Insulin caused drug resistance to oxaliplatin in colon cancer cell line HT29. J Gastrointest Oncol 2011;2:27-33. [PubMed]
- Chen J. Targeted therapy of obesity-associated colon cancer. Translational Gastrointestinal Cancer 2011;1:44-57.
- Speliotes EK, Willer CJ, Berndt SI, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010;42:937-48. [PubMed]
- Loos RJ, Bouchard C. FTO: the first gene contributing to common forms of human obesity. Obes Rev 2008;9:246-50. [PubMed]
- Gerken T, Girard CA, Tung YC, et al. The obesity-associated FTO gene encodes a 2-oxoglutarate-dependent nucleic acid demethylase. Science 2007;318:1469-72. [PubMed]
- Han Z, Niu T, Chang J, et al. Crystal structure of the FTO protein reveals basis for its substrate specificity. Nature 2010;464:1205-9. [PubMed]
- Micura R, Pils W, Höbartner C, et al. Methylation of the nucleobases in RNA oligonucleotides mediates duplex-hairpin conversion. Nucleic Acids Res 2001;29:3997-4005. [PubMed]
- Jia G, Fu Y, Zhao X, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 2011;7:885-7. [PubMed]
- Stratigopoulos G, Padilla SL, LeDuc CA, et al. Regulation of Fto/Ftm gene expression in mice and humans. Am J Physiol Regul Integr Comp Physiol 2008;294:R1185-96. [PubMed]
- Fredriksson R, Hägglund M, Olszewski PK, et al. The obesity gene, FTO, is of ancient origin, up-regulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain. Endocrinology 2008;149:2062-71. [PubMed]
- Qi L, Kang K, Zhang C, et al. Fat mass-and obesity-associated (FTO) gene variant is associated with obesity: longitudinal analyses in two cohort studies and functional test. Diabetes 2008;57:3145-51. [PubMed]
- Olszewski PK, Fredriksson R, Olszewska AM, et al. Hypothalamic FTO is associated with the regulation of energy intake not feeding reward. BMC Neurosci 2009;10:129. [PubMed]
- Sonestedt E, Gullberg B, Ericson U, et al. Association between fat intake, physical activity and mortality depending on genetic variation in FTO. Int J Obes (Lond) 2011;35:1041-9. [PubMed]
- Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007;316:889-94. [PubMed]
- Scuteri A, Sanna S, Chen WM, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet 2007;3:e115. [PubMed]
- Berulava T, Horsthemke B. The obesity-associated SNPs in intron 1 of the FTO gene affect primary transcript levels. Eur J Hum Genet 2010;18:1054-6. [PubMed]
- Davies RW, Lau P, Naing T, et al. A 680 kb duplication at the FTO locus in a kindred with obesity and a distinct body fat distribution. Eur J Hum Genet 2013. [Epub ahead of print]. [PubMed]
- Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007;316:889-94. [PubMed]
- Yang J, Loos RJ, Powell JE, et al. FTO genotype is associated with phenotypic variability of body mass index. Nature 2012;490:267-72. [PubMed]
- Fischer J, Koch L, Emmerling C, et al. Inactivation of the Fto gene protects from obesity. Nature 2009;458:894-8. [PubMed]
- Nock NL, Plummer SJ, Thompson CL, et al. FTO polymorphisms are associated with adult body mass index (BMI) and colorectal adenomas in African-Americans. Carcinogenesis 2011;32:748-56. [PubMed]
- Kaklamani V, Yi N, Sadim M, et al. The role of the fat mass and obesity associated gene (FTO) in breast cancer risk. BMC Med Genet 2011;12:52. [PubMed]
- Boeing H. Obesity and cancer--the update 2013. Best Pract Res Clin Endocrinol Metab 2013;27:219-27. [PubMed]
- Lurie G, Gaudet MM, Spurdle AB, et al. The obesity-associated polymorphisms FTO rs9939609 and MC4R rs17782313 and endometrial cancer risk in non-Hispanic white women. PLoS One 2011;6:e16756. [PubMed]
- Delahanty RJ, Beeghly-Fadiel A, Xiang YB, et al. Association of obesity-related genetic variants with endometrial cancer risk: a report from the Shanghai Endometrial Cancer Genetics Study. Am J Epidemiol 2011;174:1115-26. [PubMed]
- Li G, Chen Q, Wang L, et al. Association between FTO gene polymorphism and cancer risk: evidence from 16,277 cases and 31,153 controls. Tumour Biol 2012;33:1237-43. [PubMed]
- Tang H, Dong X, Hassan M, et al. Body mass index and obesity- and diabetes-associated genotypes and risk for pancreatic cancer. Cancer Epidemiol Biomarkers Prev 2011;20:779-92. [PubMed]
- Nock NL, Plummer SJ, Thompson CL, et al. FTO polymorphisms are associated with adult body mass index (BMI) and colorectal adenomas in African-Americans. Carcinogenesis 2011;32:748-56. [PubMed]
- Lim U, Wilkens LR, Monroe KR, et al. Susceptibility variants for obesity and colorectal cancer risk: the multiethnic cohort and PAGE studies. Int J Cancer 2012;131:E1038-43. [PubMed]
- Dougkas A, Yaqoob P, Givens DI, et al. The impact of obesity-related SNP on appetite and energy intake. Br J Nutr 2013;110:1151-6. [PubMed]
- Speakman JR. FTO effect on energy demand versus food intake. Nature 2010;464:E1; discussion E2.
- Cecil JE, Tavendale R, Watt P, et al. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med 2008;359:2558-66. [PubMed]
- Speakman JR, Rance KA, Johnstone AM. Polymorphisms of the FTO gene are associated with variation in energy intake, but not energy expenditure. Obesity (Silver Spring) 2008;16:1961-5. [PubMed]
- Wardle J, Llewellyn C, Sanderson S, et al. The FTO gene and measured food intake in children. Int J Obes (Lond) 2009;33:42-5. [PubMed]
- Timpson NJ, Emmett PM, Frayling TM, et al. The fat mass- and obesity-associated locus and dietary intake in children. Am J Clin Nutr 2008;88:971-8. [PubMed]
- Wardle J, Carnell S, Haworth CM, et al. Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab 2008;93:3640-3. [PubMed]
- Müller TD, Greene BH, Bellodi L, et al. Fat mass and obesity-associated gene (FTO) in eating disorders: evidence for association of the rs9939609 obesity risk allele with bulimia nervosa and anorexia nervosa. Obes Facts 2012;5:408-19. [PubMed]
- Lappalainen T, Lindström J, Paananen J, et al. Association of the fat mass and obesity-associated (FTO) gene variant (rs9939609) with dietary intake in the Finnish Diabetes Prevention Study. Br J Nutr 2012;108:1859-65. [PubMed]
- Rutters F, Nieuwenhuizen AG, Bouwman F, et al. Associations between a single nucleotide polymorphism of the FTO Gene (rs9939609) and obesity-related characteristics over time during puberty in a Dutch children cohort. J Clin Endocrinol Metab 2011;96:E939-42. [PubMed]
- Tanofsky-Kraff M, Han JC, Anandalingam K, et al. The FTO gene rs9939609 obesity-risk allele and loss of control over eating. Am J Clin Nutr 2009;90:1483-8. [PubMed]
- Church C, Moir L, McMurray F, et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet 2010;42:1086-92. [PubMed]
- Fisher E, Schulze MB, Stefan N, et al. Association of the FTO rs9939609 single nucleotide polymorphism with C-reactive protein levels. Obesity (Silver Spring) 2009;17:330-4. [PubMed]
- Gao X, Shin YH, Li M, et al. The fat mass and obesity associated gene FTO functions in the brain to regulate postnatal growth in mice. PLoS One 2010;5:e14005. [PubMed]
- McMurray F, Church CD, Larder R, et al. Adult onset global loss of the fto gene alters body composition and metabolism in the mouse. PLoS Genet 2013;9:e1003166. [PubMed]
- Church C, Lee S, Bagg EA, et al. A mouse model for the metabolic effects of the human fat mass and obesity associated FTO gene. PLoS Genet 2009;5:e1000599. [PubMed]
- Boender AJ, van Rozen AJ, Adan RA. Nutritional state affects the expression of the obesity-associated genes Etv5, Faim2, Fto, and Negr1. Obesity (Silver Spring) 2012;20:2420-5. [PubMed]
- Vujovic P, Stamenkovic S, Jasnic N, et al. Fasting induced cytoplasmic Fto expression in some neurons of rat hypothalamus. PLoS One 2013;8:e63694. [PubMed]
- den Hoed M, Westerterp-Plantenga MS, Bouwman FG, et al. Postprandial responses in hunger and satiety are associated with the rs9939609 single nucleotide polymorphism in FTO. Am J Clin Nutr 2009;90:1426-32. [PubMed]
- Ramos EJ, Meguid MM, Campos AC, et al. Neuropeptide Y, alpha-melanocyte-stimulating hormone, and monoamines in food intake regulation. Nutrition 2005;21:269-79. [PubMed]
- Fetissov SO, Meguid MM, Shafiroff M, et al. Dopamine in the VMN of the hypothalamus is important for diurnal distribution of eating in obese male Zucker rats. Nutrition 2000;16:65-6. [PubMed]
- Fetissov SO, Meguid MM, Chen C, et al. Synchronized release of dopamine and serotonin in the medial and lateral hypothalamus of rats. Neuroscience 2000;101:657-63. [PubMed]
- Pitman RT, Fong JT, Billman P, et al. Knockdown of the Fat Mass and Obesity Gene Disrupts Cellular Energy Balance in a cell-Type Specific Manner. PLoS One 2012;7:e38444. [PubMed]
- Larder R, Cheung MK, Tung YC, et al. Where to go with FTO? Trends Endocrinol Metab 2011;22:53-9. [PubMed]
- Hess ME, Hess S, Meyer KD, et al. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat Neurosci 2013;16:1042-8. [PubMed]
- Olszewski PK, Fredriksson R, Eriksson JD, et al. Fto colocalizes with a satiety mediator oxytocin in the brain and upregulates oxytocin gene expression. Biochem Biophys Res Commun 2011;408:422-6. [PubMed]
- Karra E, O’Daly OG, Choudhury AI, et al. A link between FTO, ghrelin, and impaired brain food-cue responsivity. J Clin Invest 2013;123:3539-51. [PubMed]
- Huang XF, Chen JZ. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes Rev 2009;10:610-6. [PubMed]
- Chen J. prevention of obesity-associated colon cancer by (-)-epigallocatechin-3 gallate and curcumin. Translational Gastrointestinal Cancer 2012;1:243-49.
- Chen J, Wang MB. The roles of miRNA-143 in colon cancer and therapeutic implications. Transl Gastrointest Cancer 2012;1:169-74.
- Labayen I, Ruiz JR, Ortega FB, et al. Association between the FTO rs9939609 polymorphism and leptin in European adolescents: a possible link with energy balance control. The HELENA study. Int J Obes (Lond) 2011;35:66-71. [PubMed]
- arcOGEN Consortium; arcOGEN Collaborators, Zeggini E, et al. Identification of new susceptibility loci for osteoarthritis (arcOGEN): a genome-wide association study. Lancet 2012;380:815-23.
- Wluka AE, Lombard CB, Cicuttini FM. Tackling obesity in knee osteoarthritis. Nat Rev Rheumatol 2013;9:225-35. [PubMed]
- Dumond H, Presle N, Terlain B, et al. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum 2003;48:3118-29. [PubMed]
- Griffin TM, Huebner JL, Kraus VB, et al. Extreme obesity due to impaired leptin signaling in mice does not cause knee osteoarthritis. Arthritis Rheum 2009;60:2935-44. [PubMed]
- Lin Y, Ueda J, Yagyu K, et al. Association between variations in the fat mass and obesity-associated gene and pancreatic cancer risk: a case-control study in Japan. BMC Cancer 2013;13:337. [PubMed]
- Iles MM, Law MH, Stacey SN, et al. A variant in FTO shows association with melanoma risk not due to BMI. Nat Genet 2013;45:428-32, 432e1.
- Chen J. Multiple signal pathways in obesity-associated skin cancer. Toxicol Appl Pharmacol 2010;247:166; author reply 167. [PubMed]
- Chen J. Multiple signal pathways in obesity-associated cancer. Obes Rev 2011;12:1063-70. [PubMed]
- Cho DC, Mier JW. Dual inhibition of PI3-kinase and mTOR in renal cell carcinoma. Curr Cancer Drug Targets 2013;13:126-42. [PubMed]
- Elfiky AA, Jiang Z. The PI3 kinase signaling pathway in prostate cancer. Curr Cancer Drug Targets 2013;13:157-64. [PubMed]
- Bu Z, Ji J. Therapeutic implications of mTOR inhibitors in the treatment of gastric cancer. Curr Cancer Drug Targets 2013;13:121-5. [PubMed]
- Chen J. Potential value and limitation of dual inhibitors of PI3K and mTOR in the treatment of cancer. Curr Cancer Drug Targets 2013;13:117-20. [PubMed]
- Pitman RT, Fong JT, Billman P, et al. Knockdown of the fat mass and obesity gene disrupts cellular energy balance in a cell-type specific manner. PLoS One 2012;7:e38444. [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [PubMed]
- Floor SL, Dumont JE, Maenhaut C, et al. Hallmarks of cancer: of all cancer cells, all the time? Trends Mol Med 2012;18:509-15. [PubMed]
- Bartkova J, Lukas J, Müller H, et al. Cyclin D1 protein expression and function in human breast cancer. Int J Cancer 1994;57:353-61. [PubMed]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999;398:422-6. [PubMed]
- Zhang Z, Zhou D, Lai Y, et al. Estrogen induces endometrial cancer cell proliferation and invasion by regulating the fat mass and obesity-associated gene via PI3K/AKT and MAPK signaling pathways. Cancer Lett 2012;319:89-97. [PubMed]
- Zucker S, Vacirca J. Role of matrix metalloproteinases (MMPs) in colorectal cancer. Cancer Metastasis Rev 2004;23:101-17. [PubMed]
- Bergers G, Brekken R, McMahon G, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2000;2:737-44. [PubMed]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010;141:52-67. [PubMed]
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors? J Clin Oncol 2013;31:2607-18. [PubMed]
- Chen J, Iverson D. Estrogen in obesity-associated colon cancer: friend or foe? Protecting postmenopausal women but promoting late-stage colon cancer. Cancer Causes Control 2012;23:1767-73. [PubMed]
- Liu Z, Uesaka T, Watanabe H, et al. High fat diet enhances colonic cell proliferation and carcinogenesis in rats by elevating serum leptin. Int J Oncol 2001;19:1009-14. [PubMed]
- Frezza EE, Wachtel MS, Chiriva-Internati M. Influence of obesity on the risk of developing colon cancer. Gut 2006;55:285-91. [PubMed]
- Fujisawa T, Endo H, Tomimoto A, et al. Adiponectin suppresses colorectal carcinogenesis under the high-fat diet condition. Gut 2008;57:1531-8. [PubMed]
- Sharma D, Saxena NK, Vertino PM, et al. Leptin promotes the proliferative response and invasiveness in human endometrial cancer cells by activating multiple signal-transduction pathways. Endocr Relat Cancer 2006;13:629-40. [PubMed]
- Cheung MK, Gulati P, O’Rahilly S, et al. FTO expression is regulated by availability of essential amino acids. Int J Obes (Lond) 2013;37:744-7. [PubMed]
- Kilpeläinen TO, Zillikens MC, Stančákova A, et al. Genetic variation near IRS1 associates with reduced adiposity and an impaired metabolic profile. Nat Genet 2011;43:753-60. [PubMed]
- Dorajoo R, Blakemore AI, Sim X, et al. Replication of 13 obesity loci among Singaporean Chinese, Malay and Asian-Indian populations. Int J Obes (Lond) 2012;36:159-63. [PubMed]
- Fox CS, Liu Y, White CC, et al. Genome-wide association for abdominal subcutaneous and visceral adipose reveals a novel locus for visceral fat in women. PLoS Genet 2012;8:e1002695. [PubMed]
- Okada Y, Kubo M, Ohmiya H, et al. Common variants at CDKAL1 and KLF9 are associated with body mass index in east Asian populations. Nat Genet 2012;44:302-6. [PubMed]
- Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 2007;316:1341-5. [PubMed]
- Gaudet MM, Yang HP, Bosquet JG, et al. No association between FTO or HHEX and endometrial cancer risk. Cancer Epidemiol Biomarkers Prev 2010;19:2106-9. [PubMed]
- Scherag A, Dina C, Hinney A, et al. Two new Loci for body-weight regulation identified in a joint analysis of genome-wide association studies for early-onset extreme obesity in French and german study groups. PLoS Genet 2010;6:e1000916. [PubMed]
- Boissel S, Reish O, Proulx K, et al. Loss-of-function mutation in the dioxygenase-encoding FTO gene causes severe growth retardation and multiple malformations. Am J Hum Genet 2009;85:106-11. [PubMed]
- Fischer J, Koch L, Emmerling C, et al. Inactivation of the Fto gene protects from obesity. Nature 2009;458:894-8. [PubMed]
- Church C, Lee S, Bagg EA, et al. A mouse model for the metabolic effects of the human fat mass and obesity associated FTO gene. PLoS Genet 2009;5:e1000599. [PubMed]
- Kubota M, Shimizu M, Sakai H, et al. Preventive effects of curcumin on the development of azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db obese mice. Nutr Cancer 2012;64:72-9. [PubMed]
- Fenton JI, McCaskey SJ. Curcumin and docosahexaenoic acid block insulin-induced colon carcinoma cell proliferation. Prostaglandins Leukot Essent Fatty Acids 2013;88:219-26. [PubMed]


