The bacteria-hypothesis of colorectal cancer: pathogenetic and therapeutic implications
Introduction
Back to 1863 Rudolf Virchow, a German pathologist, affirmed that cancer may be considered the end result of a chronic inflammatory process triggered by an adverse toxic environment, including infections. The concept that bacterial infections could lead to cancer was first proposed in the late 19th century, following the pioneering work of Robert Koch and Louis Pasteur, based on the discovery of bacteria at the sites of tumors. Nowadays up to 20% of malignancies worldwide can be attributed to infections with a global total of 1.2 million cases per year (1). The most convincing evidence, in this context, is the link between Helicobacter pylori and both gastric cancer and mucosa-associated lymphoid tissue (MALT) lymphoma. The hypothesis of the infectious origin of cancer is corroborated by the association of Salmonella typhi with gallbladder cancer, Chlamydia pneumoniae with lung cancer, and Streptococcus bovis (S. bovis) with colorectal cancer (CRC).
Based on these historical perspectives a growing body of evidence in the last years has raised up the putative causal role of gut microbiota in the carcinogenetic process (2). If the microbiota is involved in cancer development, being the colon the site where the microbiota reaches its highest concentration, it is expected to be its major site of action.
Colorectal cancer is the third most common cause of cancer-related death in woman and the fourth leading cause of cancer mortality in males. Over 140,000 new cases of CRC are estimated for the U.S. in 2012 with disease-specific mortality of up to 60,000 reported in 2011 (3). Colorectal cancer is classified as inherited (due to genetic instability), inflammatory (associated to inflammatory bowel disease) or sporadic, which accounts for more than 80% of all CRCs. Sporadic CRC, is the focus of both tremendous epidemiological research efforts, with the goal to determine potential causative and risk factors associated with the disease, and continuous basic research, aimed to clarify the pathogenetic mechanisms of the disease. Several potential risk factors have been identified, such as high-fat diet, red meat consumption, alcohol intake, and obesity, but the list continues to evolve, and in the past few decades has expanded to include infectious agents, and in particular alterations of the gut microecology.
Here, we will address the link between gut microbiota and CRC focusing on pathogenetic and therapeutic implications.
Gut microbiota and carcinogensis
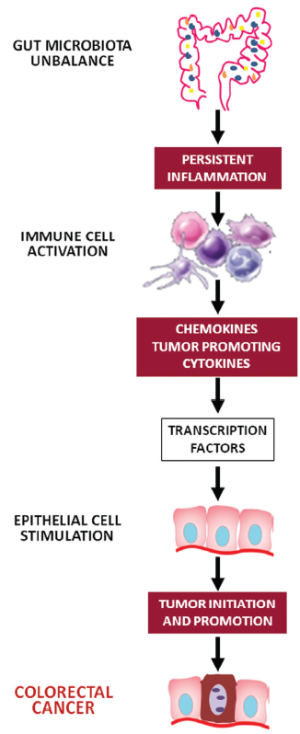
Our gut harbors the majority of mammalian-associated microbes. The fetal intestine is sterile but, following delivery, the colonization of the intestine by a variety of microorganisms begins. Gastrointestinal colonization involves a succession of bacterial populations varying as the diet changes and the host develops. This assemblage of bacteria inhabiting the gut is usually referred to as the commensal intestinal microbiota. Each human adult harbors approximately 1014 bacteria in the gut, which is about 10 times the number of cells making up the human body (4). There are at least 500 different bacterial species and these species can again be divided into different strains, highlighting the enormous complexity of this ecosystem. The bacteria in the gut interact with their human host and, although some bacteria are potentially pathogenic and can become a source of disease, this host-bacterial interaction is mainly symbiotic and health-conferring. The result of this interaction may lead to a “physiological inflammation” that regulates the presence of the resident gut microbiota or, to a “pathological inflammation”, the degree of which depends on the number and virulence of the invading pathogens (5). Physiological inflammation maintains a dynamic yet fragile homeostatic balance; however, persistent inflammation may be the link between gut bacteria and carcinogenesis process. Chronic inflammation can profoundly alter local immune response and lead to the release of reactive oxygen species (ROS) and nitric oxide (NO) that in turn may induce DNA damage and consequently alter tissue homeostasis (6). Nevertheless, cytokines and chemokines can act as tumor growth and survival factors and may induce tumor development by promoting angiogenesis and suppressing immune-surveillance. Cancer-promoting cytokines include tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, and IL-1. By contrast, IL-10 and transforming growth factor beta (TGF-β) inhibit carcinogenesis (6). In summary, chronic inflammation, immune evasion and immune suppression are the mechanisms by which bacteria may induce carcinogenesis.
The gut microbiota elicits both innate and adaptive immune mechanisms that cooperate to protect the host and maintain intestinal homeostasis. Activation of innate host defense depends on specific pattern recognition receptors (PRRs) that recognize highly conserved microbial signature molecules called “pathogen-associated molecular patterns” (PAMPs). The PRRs include the family of toll-like receptors (TLRs), which scan the extracellular space, and Nod-like receptors (NLRs), which guard the intracellular cytoplasmatic compartment (7). Different TLRs recognize different classes of PAMPs, characterizing different pathogens. After PAMP ligation, TLRs dimerize and transmit intracellular signals through four adaptor proteins: myeloid differentiation primary response gene 88 (MyD88), toll/interleukin-1-receptordomain-containing adaptor inducing interferon-β (TRIF), toll/interleukin-1-receptor-domain-containing adaptor protein (TIRAP), and TRIF-related adaptor molecule (TRAM), that have an important role in inflammation and tissue regeneration (8). Therefore, TLRs are likely candidates to mediate the effects of the innate immune response on tumorigenesis. Mice that lack either TLR4 or its MyD88 adaptor exhibit decreased epithelial cell proliferation and increased apoptosis in response to chemical-induced injury (9,10). Finally, the blockade of the TLR4 receptor in mice with CRC xenografts decreases the growth of colon tumors.
TLR4 has been associated with the process of tumor progression via the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathway resulting in the transcription of inflammatory cytokines, chemokines and antimicrobial genes. How NF-κB-induced inflammatory process drives carcinogenesis is unclear, although IL-6 seems to have a pivotal role. IL-6 induces the procarcinogenic signal transducer and activator of transcription (Stat)3 pathway and transcriptionally activates proliferative, antiapoptotic and proangiogenic genes involved in cancer growth, such as c-IAP-1 and c-IAP-2, Fas ligand, c-myc, p53, and cyclin D1 (Figure 1) (11).
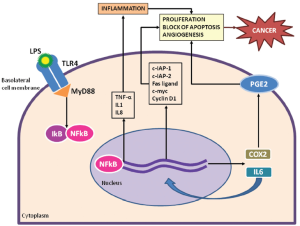
Findings from animal models of CRC are corroborated by human studies. The TLR4/MyD88 co-receptor complex is over-expressed in CRCs compared to the normal and adenomatous colonic epithelium, confirming that this signaling pathway is important in human sporadic CRC (12). Specific polymorphisms of toll receptors are also associated with an increased CRC risk and influence prognosis (13). In both murine models and human samples, TLR4 and IL-6 expression in the tumor microenvironment are associated with the presence of adenocarcinoma, and higher levels of TLR4 expression in the tumor stroma are noted with disease progression (14). TLR4 expression in the stroma of patients with stage 3 CRCs correlates with early relapse, suggesting the importance of this marker in predicting prognosis or as a therapeutic target (15).
The gut-mucosal arm of the adaptive immune system, localized predominantly in the small bowel, provides humoral and cell-mediated immunity against ingested antigens and luminal organisms. Effector lymphocytes are diffusely distributed in the lamina propria as isolated lymphoid follicles or are organized into structures termed “Peyer’s patches”. Locally recruited cells of the adaptive immune system may have either pro- or anti-tumorigenic roles. T cells, for instance, are required for inflammation, cancer development, and tumor progression (Figure 2), as well as for anticancer immunity (16). In sporadic CRC, there seems to be a well-defined balance between immunosurveillance (executed by CD8+ T cells, NK cells, and CD4+ T cells) and tumor-promoting inflammation (executed by innate immune cells, B cells, and various subtypes of T cells) (8).
Three effector pathways of T helper (Th) cell differentiation have been characterized: Th1, Th2 and Th17 responses. While the Th1 response is typically anticarcinogenic, the contribution of Th2 or Th17 responses to cancer remains to be defined (17). Microbiota-induced Th17 cytokines in the lamina propria are crucial for protection against intestinal pathogens but, they can also contribute to inflammation. Indeed, IL-23-responsive innate lymphoid cells in the lamina propria contribute to colitis in Rag-/- mice by producing IL-17 and interferon gamma (IFN-γ) (18). Whether the highly inflammatory nature of Th17 cells is sufficient to cause or contribute to carcinogenesis is still debated. Experimental evidence shows that Th17 cells progressively increase in the tumor microenvironment during tumor development and that IL-17 up-regulates the expression of pro-inflammatory cytokines and pro-angiogenic factors. On the other hand, a number of reports have described tumor-inhibitory effects of IL-23 and IL-17 in mouse models genetically engineered to overexpress IL-23 or IL-17. Therefore, the activation of the IL-23/IL-17 pathway may promote tumorigenesis by inducing local inflammatory response, or inhibit it by stimulating anti-tumor immunity (19). More recently, a T regulatory response (TReg), driven by IL-10 and TGF-β has been shown to counterbalance the pro-inflammatory effect of the Th17 response. The induction of TReg cells by commensal microorganisms and the occurrence of intestinal inflammation in their absence indicate that TReg cells regulate the equilibrium between non-inflammatory homeostasis and intestinal inflammation. However, experimental and clinical findings have demonstrated that TReg cells, by suppressing the innate and adaptive immune responses, are a major factor contributing to the immunosuppressive tumor microenvironment, thus fostering tumor progression (20). Strategies that deplete or inhibit Treg cells and promote a competent immune response in the tumor microenvironment could be the goal in future immunotherapeutic studies in cancer patients.
Gut microbiota and colorectal cancer
In 1975 Reddy et al., firstly linked the gut microbiota to CRC development. They found that only 20% of germ-free rats develop chemically induced CRC; in contrast, the tumor incidence in conventional rats was 93% and the neoplasms were multiple (21). This data has been recently confirmed by Vannucci et al. who found that germ-free rats, compared to conventionally reared animals, develop fewer and smaller tumors both spontaneously and after chemically-induced carcinogenesis (22). In addition, germ-free mice has also shown less oncogenic mutations and a decreased tumor formation in both colitis-associated cancer and Apc-related CRC (23).The absence of the physiological inflammation caused by the commensal microbiota may explain the capability of the germ-free rats to develop a more efficacious anti-cancer immune response.
Many bacterial species have been found in CRC samples and in tissue adjacent to tumors, namely, S. bovis, Bacteroides fragilis (B. fragilis), Escherichia coli (E. coli), etc (Table 1).

Full Table
The best known association is that between S. bovis bacteremia and CRC, recognized since 1951, when McCoy and Mason first reported a case of enterococcal endocarditis, likely from S. bovis, associated with a carcinoma of the cecum. Since then, the connection between S. bovis septicemia and colonic neoplasia has been confirmed by several other case reports and case-control studies. About 25-80% of patients with S. bovis bacteremia exhibit a CRC; in addition, a significantly higher fecal carriage of S. bovis has been reported in patients with CRC compared with control subjects (24). The mechanisms underlying this association are not known. Ellmerich et al. reported that S. bovis enhanced the expression of the proliferation markers and polyamines, and induced the formation of colonic adenoma in 50% of rats, as well as a higher number of aberrant colonic crypts. The authors also found that S. bovis and its wall antigens are able to increase the production of IL-8 in the colonic mucosa (25). IL-8 induces the formation of NO and ROS that contribute to the neoplastic process by altering cell DNA. On the basis of these data, several authors have suggested that all patients with S. bovis bacteremia should undergo a complete endoscopic evaluation of the colon.
B. fragilis strains comprise approximately 0.1% of the normal colonic flora and are found in the colonic flora in up to 80% of children and adults. The “enterotoxigenic B. fragilis” (ETBF), producing fragilisyn, has been associated with CRC. The toxin cleaves the extracellular domain of the E-cadherin, which is the principal structural component of the zonula adherens and is responsible for cell-to-cell adhesion (26). Treatment of HT29/C1 cells with B. fragilis toxin triggered the nuclear localization of β-catenin, which in turn, after binding with T-cell factor-dependent transcriptional activators, induced c-myc and cyclin D1 transcription and translation, resulting in persistent cellular proliferation (27). Activation of β-catenin signaling via mutations in one or more of the APC complex proteins, contributes to the development of inherited and sporadic forms of CRC and possibly other cancers. Toprak et al., by investigating the prevalence of ETBF in stool specimens from 73 CRC patients and 59 controls found the enterotoxin gene in 38% of the isolates from CRC patients compared with 12% of the isolates from the control group (26). More recently Wu et al. (27) showed that ETBF strongly induces CRC in multiple intestinal neoplasia (Min) mice, by activating Stat3 and a selective TH17 response. The authors also demonstrated that the antibody-mediated blockade of IL-17 as well as that of the receptor for IL-23, a key cytokine amplifying TH17 responses, inhibits ETBF-induced tumor formation (28).
E. coli is a normal inhabitant of the human gut. The colonic mucosa of patients with adenomas and carcinomas has shown an increased intracellular mucosal carriage of E. coli compared to healthy controls (29). Whether this increased carriage had a causal or incidental origin is currently not known. E. coli strains of the phylogenetic group B2 harbor a genomic island called “pks” that codes for the production of a polyketide-peptide genotoxin, colibactin. The in vivo infection with E. coli harboring the Pks Island, but not with a pks isogenic mutant, induced the formation of phosphorylated H2AX foci in mouse enterocytes, contributing to the development of sporadic CRC (30).
Until now the relation between gut microbiota and CRC was based on culture ex vivo methods. However, 60-80% of the gut bacteria are uncharacterized because they cannot be cultivated ex vivo. Recent advances in molecular methods, based on the highly conserved bacterial 16S ribosomal RNA (rRNA) gene have enhanced our ability to study and characterize both luminal and adherent bacteria communities in the gut. By using these approaches, only a few studies have investigated changes in the microbiota composition during CRC. Nevertheless, these studies indicate that the altered colonic environment in CRC could have implications for the composition of the microbiota in the lumen and on mucosal surfaces. Gueimonde et al., by qRT-PCR, analyzed samples of colonic mucosa from 34 patients (21 CRCs, 9 divertiulitis and 4 inflammatory bowel diseases) and found that patients with CRC had significantly lower levels of both Bifidobacterium longum and bifidum than patients with diveritulitis and inflammatory bowel disease (31). Similarly, Shen et al., by evaluating adherent bacteria in 21 adenoma and 23 non-adenoma subjects by a sophisticated molecular approach, sequenced and processed for phylogenetic and taxonomic analysis a total of 335 clones and found higher Proteobacteria and lower Bacteroidetes numbers in tumor cases compared with control subjects (32). Sobhani et al. using pyrosequencing of stool bacterial DNA and subsequent Principal Component Analysis (PCA) demonstrated a composition change in the microbiota of CRC patients; in particular Bacteroides/Prevotella species were more numerous in cancer patients (n.60) than in control subjects (n.119). In addition, IL-17 immunoreactive cells were expressed at significantly higher levels in cancer patients than in those with normal colonoscopy (33). Very recently Marchesi et al. compared differences in healthy and cancerous tissue within cancer patients and found that species of the genera Coriobacteridae, Roseburia, Fusobacterium and Faecalibacterium were over-represented in tumor tissue; these are generally regarded as gut commensals with probiotic features. Further, this study found decreased colonization by members of Enterobacteriaceae, such as Citrobacter, Shigella, Cronobacter, and Salmonella in CRC tissue from the investigated patients (34). Finally, Scanlan et al. investigated the diversity and presence of methanogens in healthy, polyp and cancer patients and found significant differences in bacterial stability over time. Specifically, the diversity of the Clostridium leptum and coccoides subgroups was increased compared to healthy controls. Importantly, metabonomic faecal water analysis was able to distinguish CRC and polyp groups from healthy controls, indicative of an altered metabolic activity of the intestinal microbiota in these patients (35).
Taken together, these data show that the gut microbiota may play a major role in CRC development at both quantitative and qualitative level.
Probiotics and colorectal cancer
The emerging relationship between the gut microbiota and cancer opens the door to new ways of thinking about cancer prevention. Probiotics are defined as viable microorganisms that, when administered in adequate amounts, confer a health benefit to the host. They may positively affect the gut microbiota and have a beneficial effect in the prevention and treatment of specific pathological conditions. There are many mechanisms by means of which probiotics positively affect the gut microbiota and liver health, i.e., inhibition of intestinal bacterial enzymes, stimulation of host immunity, competition for limited nutrients, inhibition of bacteria mucosal adherence and epithelial invasion, protection of intestinal permeability and control of bacterial translocation from the gut to the bloodstream. The biological activity of probiotics depends prevalently on delivering anti-inflammatory mediators that down-regulate pro-inflammatory cytokines, including IFN-γ and TNF-α, via the NF-κB pathway. The mechanisms through which probiotics may exert beneficial effects include macrophage activation, cytocrome P450 blocking, reduction of carcinogen generation, down-regulation of Ras-p21 expression, increase of cell differentiation, inhibition of COX-2 up-regulation, inhibition of NO synthase, increase of short chain fatty acid production, and reduction of intestinal pH with lessening of putrefactive bacteria (36,37).
The anticarcinogenic effects of probiotic microorganisms in vitro and in animal studies are well documented. In a very recent study, Bassaganya-Riera et al. investigated the ability of VSL#3 bacteria to modulate mucosal immune responses and thereby ameliorate colonic carcinogenesis in mouse models of inflammation driven CRC. In mice treated with VSL#3, adenoma and adenocarcinoma formation was diminished by both treatments (38). Chang et al. demonstrated that the oral administration of Lactobacillus acidophilus (L. acidophilus) KFRI342 to rats with 1,2-Dimethylhydrazine (DMH)-induced CRC inhibited the development of preneoplastic lesions and lowered the microbiota populations of both E. coli and aerobic bacteria, which have been associated with carcinogenesis (39). The possibility that probiotics modulates immunity may inhibit colon carcinogenesis has been also investigated. Foo et al. by evaluating the effect of long term (24 weeks) treatment with B. longum and Lactobacillus gasseri (L. gasseri) on the development of DMH-induced colonic precancerous lesions and tumors in 70 male mice showed that both probiotics significantly inhibited DMH-induced aberrant crypt foci formation, as well as decreased tumor multiplicity and the size (40). Several studies have shown that the intake of probiotics can influence enzyme activities and can be linked with the risk of colon carcinogenesis. Lactobacillus casei (L. casei) treatment of mucosa samples from duodenum, jejunum, ileum, cecum, and colon of 45 male Wistar rats was able to monitor the expression of selected cytochromes P450, testing the hypothesis that the L. casei probiotic might contribute to preventing CRC by decreasing levels of certain forms of xenobiotic-metabolizing enzymes (41). Finally probiotics may retard colon carcinogenesis by stimulating tumor cell apoptosis. Preinoculation with the probiotic L. acidophilus NCFM for 14 days in BALB/cByJ mice in which orthotopic CRCs were implanted, reduced the severity of colonic carcinogenesis caused by CT-26 cells (42), such as the level of colonic involvement and structural abnormality of epithelial/crypt damage (43). A significant down-regulation of the CXCR4 mRNA expression, associated with reduced apoptosis, was observed (44).
Data from human studies are still controversial. An epidemiological study performed in Finland demonstrated that, despite a high fat intake, CRC incidence is lower than in other countries because of the high consumption of milk, yoghurt and other dairy products (44). In two population-based case-control studies of CRC, an inverse association was observed for yoghurt and cultured milk, adjusted for potential confounding factors (45,46). An inverse relationship has been demonstrated between the frequency of consumption of yoghurt and other fermented milk products and breast cancer in women. On the other hand, two American prospective studies, the Nurses’ Health Study and the Health Professionals study, did not provide evidence that intake of dairy products is associated with a decreased risk of CRC (47). In a cohort study in the Netherlands, it was shown that the intake of fermented dairy products was not significantly associated with CRC risk in an elderly population with a relatively wide variation in dairy product consumption, although a weak non-significant inverse association with CRC was observed (48). The contrasting results may be related to study designs, population examined, follow-up, bacterial strains used, endpoints, dietary habit and so on. An intervention study in humans in which both probiotics and prebiotics were used was recently performed among 17 patients with FAP. In this single-center human study on patients with FAP, a 4-week intervention with (I) sulindac; (II) inulin/VSL#3; and (III) sulindac/inulin/VSL#3 was performed. Cell proliferation was lower after treatment with sulindac or VSL#3/inulin; the combination of sulindac/inulin/VSL#3 showed the opposite effect. Glutathione S-transferase activity increased after treatment with sulindac or VSL#3/inulin; the combination treatment showed the opposite effect (49). However, FAP is a rare disorder, so the main weakness of this study is the small number of patients included in a single-center fashion.
In 2006 Capurso et al. produced a systematic review of data from basic science (animal and in vitro models) and human (epidemiological and interventional) studies, addressing the risk of CRC and the use of probiotics (50). The in vitro studies, confirm the ability of probiotics to dialogue with intestinal cells. Overall, 26/29 animal model studies suggested that probiotics had a protective anticancer effect; however, given the different study designs and treatments, the results are difficult to compare. Finally, the epidemiological human studies are difficult to interpret given their extreme heterogeneity (50). Further experimental studies in animal models and clinical trials in humans are needed to quantify the effect and elucidate the mode of action of probiotics in prophylaxis and treatment of CRC.
Conclusions
Over the years, it has become apparent that the gut microbiota is not a bystander in the complex biological events regulating intestinal homeostasis, but it may lead to beneficial or detrimental effects to the host. Multiple lines of evidence support the notion that gut microbiota can contribute to colorectal carcinogenesis. Various bacteria have been linked with experimental carcinogenesis in animal models or correlated with CRC in human observational studies and multiple microbiota-based studies suggest differences in mucosa associated and luminal bacteria in subjects with CRC.
Therefore, a beneficial modulation of the composition and metabolic activity of the gut microbiota might represent an interesting approach to reducing the risk of CRC development. Even though the mechanisms by which probiotics may inhibit CRC are not fully elucidated, certain potential mechanisms have been disclosed, such as the alteration of the composition and the metabolic activities of the intestinal microbiota, the changing physicochemical conditions in the colon, the binding of dietary carcinogens, the production of short chain fatty acids, the protection of the colonic mucosa and enhancement the immune system. In the near future, high quality mechanicistic experimental studies and interventional human studies might provide the scientific premises for the clinical use of probiotic in the prevention of CRC.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- de Martel C, Franceschi S. Infections and cancer: established associations and new hypotheses. Crit Rev Oncol Hematol 2009;70:183-94. [PubMed]
- Compare D, Nardone G. Contribution of gut microbiota to colonic and extracolonic cancer development. Dig Dis 2011;29:554-61. [PubMed]
- Antonic V, Stojadinovic A, Kester KE, et al. Significance of infectious agents in colorectal cancer development. J Cancer 2013;4:227-40. [PubMed]
- Tlaskalová-Hogenová H, Stěpánková R, Kozáková H, et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol 2011;8:110-20. [PubMed]
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 2009;9:313-23. [PubMed]
- Terzić J, Grivennikov S, Karin E, et al. Inflammation and colon cancer. Gastroenterology 2010;138:2101-14.e5.
- Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology 2009;136:65-80. [PubMed]
- Ioannou S, Voulgarelis M. Toll-like receptors, tissue injury, and tumourigenesis. Mediators Inflamm 2010;2010. pii: 581837.
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004;118:229-41. [PubMed]
- Fukata M, Chen A, Klepper A, et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology 2006;131:862-77. [PubMed]
- Zhang G, Ghosh S. Toll-like receptor-mediated NF-kappaB activation: a phylogenetically conserved paradigm in innate immunity. J Clin Invest 2001;107:13-9. [PubMed]
- Wang EL, Qian ZR, Nakasono M, et al. High expression of Toll-like receptor 4/myeloid differentiation factor 88 signals correlates with poor prognosis in colorectal cancer. Br J Cancer 2010;102:908-15. [PubMed]
- Boraska Jelavić T, Barisić M, Drmic Hofman I, et al. Microsatelite GT polymorphism in the toll-like receptor 2 is associated with colorectal cancer. Clin Genet 2006;70:156-60. [PubMed]
- Santaolalla R, Sussman DA, Abreu MT. TLR signaling: a link between gut microflora, colorectal inflammation and tumorigenesis. Drug Discovery Today: Disease Mechanisms 2011;8:e57-62.
- Cammarota R, Bertolini V, Pennesi G, et al. The tumor microenvironment of colorectal cancer: stromal TLR-4 expression as a potential prognostic marker. J Transl Med 2010;8:112. [PubMed]
- Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol 2009;27:313-38. [PubMed]
- Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol 2006;6:836-48. [PubMed]
- Buonocore S, Ahern PP, Uhlig HH, et al. Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 2010;464:1371-5. [PubMed]
- Ji Y, Zhang W. Th17 cells: positive or negative role in tumor? Cancer Immunol Immunother 2010;59:979-87. [PubMed]
- Yang ZZ, Ansell SM. The Role of Treg Cells in the Cancer Immunological Response. Am J Immunol 2009;5:17-28.
- Reddy BS, Narisawa T, Wright P, et al. Colon carcinogenesis with azoxymethane and dimethylhydrazine in germ-free rats. Cancer Res 1975;35:287-90. [PubMed]
- Vannucci L, Stepankova R, Kozakova H, et al. Colorectal carcinogenesis in germ-free and conventionally reared rats: different intestinal environments affect the systemic immunity. Int J Oncol 2008;32:609-17. [PubMed]
- Rakoff-Nahoum S, Medzhitov R. Role of toll-like receptors in tissue repair and tumorigenesis. Biochemistry (Mosc) 2008;73:555-61. [PubMed]
- Gold JS, Bayar S, Salem RR. Association of Streptococcus bovis bacteremia with colonic neoplasia and extracolonic malignancy. Arch Surg 2004;139:760-5. [PubMed]
- Ellmerich S, Schöller M, Duranton B, et al. Promotion of intestinal carcinogenesis by Streptococcus bovis. Carcinogenesis 2000;21:753-6. [PubMed]
- Toprak NU, Yagci A, Gulluoglu BM, et al. A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin Microbiol Infect 2006;12:782-6. [PubMed]
- Wu S, Morin PJ, Maouyo D, et al. Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterology 2003;124:392-400. [PubMed]
- Wu S, Rhee KJ, Albesiano E, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med 2009;15:1016-22. [PubMed]
- Maddocks OD, Short AJ, Donnenberg MS, et al. Attaching and effacing Escherichia coli downregulate DNA mismatch repair protein in vitro and are associated with colorectal adenocarcinomas in humans. PLoS One 2009;4:e5517. [PubMed]
- Cuevas-Ramos G, Petit CR, Marcq I, et al. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci U S A 2010;107:11537-42. [PubMed]
- Gueimonde M, Ouwehand A, Huhtinen H, et al. Qualitative and quantitative analyses of the bifidobacterial microbiota in the colonic mucosa of patients with colorectal cancer, diverticulitis and inflammatory bowel disease. World J Gastroenterol 2007;13:3985-9. [PubMed]
- Shen XJ, Rawls JF, Randall T, et al. Molecular characterization of mucosal adherent bacteria and associations with colorectal adenomas. Gut Microbes 2010;1:138-47. [PubMed]
- Sobhani I, Tap J, Roudot-Thoraval F, et al. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS One 2011;6:e16393. [PubMed]
- Marchesi JR, Dutilh BE, Hall N, et al. Towards the human colorectal cancer microbiome. PLoS One 2011;6:e20447. [PubMed]
- Scanlan PD, Shanahan F, Clune Y, et al. Culture-independent analysis of the gut microbiota in colorectal cancer and polyposis. Environ Microbiol 2008;10:789-98. [PubMed]
- Liong MT. Roles of probiotics and prebiotics in colon cancer prevention: Postulated mechanisms and in-vivo evidence. Int J Mol Sci 2008;9:854-63. [PubMed]
- Kahouli I, Tomaro-Duchesneau C, Prakash S. Probiotics in colorectal cancer (CRC) with emphasis on mechanisms of actions and current prospectives. J Med Microbiol 2013;62:1107-23. [PubMed]
- Bassaganya-Riera J, Viladomiu M, Pedragosa M, et al. Immunoregulatory mechanisms underlying prevention of colitis-associated colorectal cancer by probiotic bacteria. PLoS One 2012;7:e34676. [PubMed]
- Chang JH, Shim YY, Cha SK, et al. Effect of Lactobacillus acidophilus KFRI342 on the development of chemically induced precancerous growths in the rat colon. J Med Microbiol 2012;61:361-8. [PubMed]
- Foo NP, Ou Yang H, Chiu HH, et al. Probiotics prevent the development of 1,2-dimethylhydrazine (DMH)-induced colonic tumorigenesis through suppressed colonic mucosa cellular proliferation and increased stimulation of macrophages. J Agric Food Chem 2011;59:13337-45. [PubMed]
- Matuskova Z, Siller M, Tunkova A, et al. Effects of Lactobacillus casei on the expression and the activity of cytochromes P450 and on the CYP mRNA level in the intestine and the liver of male rats. Neuro Endocrinol Lett 2011;32:8-14. [PubMed]
- Plotnikov A, Tichler T, Korenstein R, et al. Involvement of the immune response in the cure of metastatic murine CT-26 colon carcinoma by low electric field-enhanced chemotherapy. Int J Cancer 2005;117:816-24. [PubMed]
- Cho KH, Lee HS, Ku SK. Decrease in intestinal endocrine cells in Balb/c mice with CT-26 carcinoma cells. J Vet Sci 2008;9:9-14. [PubMed]
- Malhotra SL. Dietary factors in a study of cancer colon from Cancer Registry, with special reference to the role of saliva, milk and fermented milk products and vegetable fibre. Med Hypotheses 1977;3:122-6. [PubMed]
- Peters RK, Pike MC, Garabrant D, et al. Diet and colon cancer in Los Angeles County, California. Cancer Causes Control 1992;3:457-73. [PubMed]
- Young TB, Wolf DA. Case-control study of proximal and distal colon cancer and diet in Wisconsin. Int J Cancer 1988;42:167-75. [PubMed]
- Kampman E, Giovannucci E, van’t Veer P, et al. Calcium, vitamin D, dairy foods, and the occurrence of colorectal adenomas among men and women in two prospective studies. Am J Epidemiol 1994;139:16-29. [PubMed]
- Kampman E, Goldbohm RA, van den Brandt PA, et al. Fermented dairy products, calcium, and colorectal cancer in The Netherlands Cohort Study. Cancer Res 1994;54:3186-90. [PubMed]
- Friederich P, Verschuur J, van Heumen BW, et al. Effects of intervention with sulindac and inulin/VSL#3 on mucosal and luminal factors in the pouch of patients with familial adenomatous polyposis. Int J Colorectal Dis 2011;26:575-82. [PubMed]
- Capurso G, Marignani M, Delle Fave G. Probiotics and the incidence of colorectal cancer: when evidence is not evident. Dig Liver Dis 2006;38:S277-82. [PubMed]