Obesity and nonalcoholic fatty liver disease
Introduction
Obesity is now a global phenomenon. The economic consequences of this burgeoning trend are considerable, with the direct costs of obesity estimated at 0.7% to 2.8% of a nation’s total healthcare expenditure (1). The direct costs of overweight/obesity were $21 billion for Australia alone (2). Much of the healthcare costs are related to its known associations with cardiovascular disease, metabolic syndrome and cancers. Until recently, the hepatic consequences of obesity received little attention. This perception has changed with the recognition of nonalcoholic fatty liver disease (NAFLD), a common accompaniment and not always an innocent bystander. Obesity is also a co-factor in the outcome of several other liver diseases including chronic hepatitis C and alcoholic liver disease (3). This is a review focuses on selected aspects of NAFLD relating to clinical evaluation and management.
Nonalcoholic fatty liver disease (NAFLD)
Over 30 years ago, Ludwig and colleagues described a group of 20 women with obesity, type 2 diabetes (T2D) and other metabolic disorders whose liver biopsies showed large fat droplets within hepatocytes and other changes resembling those seen with alcoholic hepatitis (4). None of these individuals had a history of significant alcohol use. Although the link between obesity and hepatic fat was previously known, many considered such individuals to be “closet” alcoholics. Subsequent studies have confirmed the existence of NAFLD as a distinct disorder with key links to the metabolic syndrome (5).
NAFLD includes a histologic spectrum from simple steatosis (SS) and nonalcoholic steatohepatitis (NASH) through to cirrhosis (4). At one end of the spectrum is SS, characterized by large fat droplet accumulation within hepatocytes. NASH is distinguished by steatosis together with lobular inflammation, hepatocyte ballooning and variable degrees of fibrosis. Mallory-Denk bodies are often present but are not necessary for the diagnosis. Unlike chronic viral hepatitis C, fibrosis is initially seen in the vicinity of the central vein and lining the hepatic sinusoids (perisinusoidal) or surrounding individual hepatocytes (‘pericellular’). Subsequent fibrosis stages involve periportal areas, portal-central (bridging fibrosis) and cirrhosis (6). Paediatric cases may present with hepatic steatosis and portal fibrosis alone (7). Cirrhosis is at the other end of the histologic spectrum. The parenchyma tissue surrounding the cirrhotic nodules may show steatosis or NASH but often in advanced cases, these are absent, leading to a diagnosis of “cryptogenic cirrhosis”. The true nature of the cirrhosis is revealed by the NAFLD-type metabolic profile (obesity, T2D) in these cases (8). The absence of steatosis/NASH changes in these cases may be related to altered levels of adiponectin, an adipocytokine with anti-steatotic and anti-inflammatory properties (9).
Prevalence and risk factors
NAFLD is the most common chronic liver disorder worldwide, with a population prevalence of 20% to 30% for NAFLD and 2% to 3% for NASH (4,10). However, the prevalence varies considerably according to the prevalence of obesity, T2D, ethnicity and other co-factors.
Obesity and T2D
Most individuals with NAFLD are overweight or obese, and the prevalence of NAFLD and NASH approaches 75% and 20%, respectively (4). In morbidly obese patients, the corresponding figures are 90% and 30%, respectively (11). Lean individuals are not exempt. Some lean patients may have a genetic predisposition to developing fatty liver, while in others this is likely a definitional issue (12). The average body mass index (BMI) of 24 kg/m2 would classify an Asian patient with NAFLD as being “lean”, when he/she should really be considered “overweight”. The distribution of obesity is also important, with central obesity (reflecting visceral obesity which has strong ties with insulin resistance) often present in otherwise “lean” Asian subjects with NAFLD (12).
The relationship between NAFLD and T2D is important. Both NAFLD (40% to 70%) and NASH (60% to 80%) are highly prevalent among patients with T2D (13). Up to 40% of patients with fatty liver will have diabetes or a family history of diabetes (5). Of those without overt diabetes, up to one-third show abnormalities of glucose tolerance (pre-diabetes or T2D) (5). Patients with T2D are more likely to progress to cirrhosis and are over-represented among cases of fatty liver-related hepatocellular carcinoma (13).
Metabolic syndrome
Several groups including ours have suggested that NAFLD is the hepatic manifestation of the metabolic syndrome (5,14). The majority (up to 87%) of patients with NAFLD fulfil criteria for the metabolic syndrome, and those who do are likely to have NASH or advanced hepatic fibrosis than individuals who do not (14). Further, the metabolic syndrome can pre-date the onset of NAFLD by several years and confers a 4-11-fold future risk of developing fatty liver (15). Likewise, fatty liver is associated with an increased future risk of prediabetes, diabetes, hypertension and death from ischaemic heart disease (16-19).
Clinical presentation
Most patients with fatty liver are asymptomatic. The presence of the disorder is often first noted as an incidental finding on an abdominal ultrasound examination or by increased serum aminotransferases (AT). Occasionally, patients may complain of right upper quadrant abdominal discomfort. Findings such as an enlarged firm liver, splenomegaly, ascites and other features of decompensated cirrhosis are present only in advanced cases.
Natural history of NAFLD
Simple steatosis (SS)
Patients with SS do not usually develop progressive liver disease (4). Most of these individuals can be reassured. This assertion has been challenged by a French study comparing paired liver biopsies over a period of 3.2 years. Some patients showed progression to NASH. However, these findings needed to be cautiously interpreted because this was a retrospective study involving tertiary centres (20). Further, sampling errors may have missed more significant changes that may have been present on the first biopsy. Nevertheless, one of the key messages is that, as compared to those without progression, those developing NASH tended to have a higher BMI (30.1 vs. 26.2 kg/m2) and frequency of diabetes (38% vs. 18%) at baseline and were more likely to have gained weight or developed diabetes. On the other hand, improvement or resolution of NASH was observed with weight loss (median weight loss, 2 kg).
Nonalcoholic steatohepatitis (NASH)
The course of patients with NASH is less predictable, with resolution, progression or no change described in longitudinal studies. Over an average follow up period of 7 years, up to a third of patients will show progression in fibrotic severity, while 16% to 29% may show regression of fibrosis (21,22). Overall, about 11% will develop cirrhosis over 15 years (23). Predictors for progressive fibrosis include older age, increasing BMI and T2D (14,24), polycystic ovarian syndrome (25), hypothalamo-pituitary disease (26) and obstructive sleep apnoea (27).
Earlier studies showed an association between NASH and liver-related death (~10% over 10 years). A reanalysis of these studies showed that liver-related deaths were most closely linked to the degree of hepatic fibrosis than to the extent of the inflammatory activity alone (28).
Cirrhosis
In time, patients with NAFLD-associated cirrhosis will develop hepatic decompensation. The rate of decompensation is lower than for comparable patients with chronic hepatitis C-cirrhosis, but the overall mortality is similar (29).
A growing concern is the development of hepatocellular carcinoma (HCC). By themselves, obesity and T2D are associated with an increased risk of HCC. Compared to those with a normal BMI, the risk of dying from liver cancer is increased for men and women with a BMI >35 kg/m2 (relative risk, 4.52 and 1.68 respectively) (30). While the absolute rates of HCC (2.6%/year) (31) are lower than for cases with chronic hepatitis C, the proportion of HCC attributable to NAFLD in the community is still substantial (40%). Most tend to occur at an older age (median age, 72 years) (32), are diagnosed late and consequently are less likely to receive a liver transplant. While most arise in patients with cirrhosis, cases of HCC in non-cirrhotic cases have also emerged (33). In one pathologic study of liver cancer arising in non-cirrhotic livers, the authors compared the background rates of steatosis and steatohepatitis between cases with HCC and controls with intrahepatic cholangiocarcinoma (34). They found significantly higher rates of steatosis and steatohepatitis (54% vs. 27%, and 15% vs. 1%, respectively) among cases with HCC as compared to controls. This suggests that NASH itself may be enough to facilitate carcinogenesis.
Extrahepatic complications
Ischaemic heart disease, ahead of cancer and liver-related mortality, is the leading cause of death in patients with fatty liver. The risk of heart disease remains even after adjustment for classical risk factors. There is some evidence that NASH is more closely associated with cardiovascular disease than SS alone (35). The idea that fatty liver is correlated with a pro-atherogenic milieu is supported by independent links with surrogate markers of atherosclerosis such as carotid intima-media thickness, endothelial function or coronary calcium score (36), and its association with many pro-inflammatory and pro-thrombotic characteristics including increased levels of tumour necrosis factor-alfa (TNF-α) and interleukin-6 (IL-6), hyperinsulinemia, low adiponectin and pro-thrombotic factors such as fibrinogen and plasminogen activator inhibitor-1 (17,37).
Other associations of NAFLD include chronic kidney disease (1.5-2-fold), colonic adenomas and obstructive sleep apnoea (16).
Diagnosis
Liver ultrasound and/or elevated serum liver enzymes usually call attention to the diagnosis of NAFLD. However, sole reliance on these modalities can be misleading.
Imaging
Fatty liver on liver ultrasound is characterised by increased hepatic echogenicity relative to the kidney (bright liver), progressive vascular blurring and poor visualisation of the diaphragm. Liver ultrasound has high specificity (>90%) but is insensitive to lesser degrees of hepatic steatosis (38). Currently, a research tool, magnetic resonance spectroscopy is the most accurate non-invasive method of determining liver fat, with NAFLD defined by an intrahepatic triglyceride fat content of >5.6% (39). All these techniques cannot differentiate SS from NASH, nor accurately assess fibrotic severity.
Of the newer imaging modalities, TE using a dedicated instrument (Fibroscan,™ EchoSens, Paris, France) or with conventional ultrasound equipment (acoustic radiation force imaging, ARFI) are now used in staging liver fibrosis (40). These involve shear wave generation using a cutaneous probe placed over the liver area and measuring the velocity of its passage across a defined block of the liver. This provides an estimate of liver stiffness (liver stiffness measurement, LSM), which correlates with the fibrosis stage. The negative predictive value for cirrhosis is high (>90%) and liver biopsies can be avoided in some cases. However, there is a ‘gray’ zone of LSM where there is uncertainty about the liver fibrosis stage and biopsies may be still necessary. Failure to obtain validated readings is also an issue in obese individuals, but a new XL probe has been helpful in overcoming this limitation. Another promising development is using the same equipment to detect mild steatosis (10%) and assess the steatosis grade. This is scored by the controlled attenuation parameter (CAP), a measure of the attenuation of the ultrasound beam by hepatic steatosis (41). However, the cut-off thresholds have yet to be conclusively established.
Liver function tests
Aminotransferase changes are not reliable in predicting liver histology. The entire histologic spectrum has been described in patients with normal liver tests (42). Serum alanine aminotransferase (ALT) values typically exceed those of aspartate aminotransferase (AST) (unlike for alcoholic liver disease) and are usually less than 10-fold over the limit. Cases with ALT exceeding 1,000 IU/L should prompt consideration of viral, autoimmune or drug-induced hepatitis. There is an increased likelihood of advanced fibrosis if the AST/ALT ratio exceeds 1. An isolated rise in alkaline phosphatase or gamma glutamyl transpeptidase may also occasionally occur.
Laboratory tests
This should include a full blood count, liver function tests, blood glucose, serum lipid profile, glucose tolerance test in non-diabetic patients, prothrombin time and tests to exclude other liver disorders. Antinuclear and/or smooth muscle antibodies may be present in 25%. In some cases, a liver biopsy may be necessary to exclude autoimmune hepatitis. An elevated serum ferritin (with normal transferrin saturation) is often present in patients with NASH but this is usually not related to iron overload (43). Haemochromatosis gene mutation testing should be considered in appropriate ethnic groups. Some have found a correlation between serum ferritin and histologic severity and have suggested that such individuals should undergo liver biopsy (44). However, others have found noninvasive fibrosis scores to be superior to serum ferritin as a marker of advanced fibrosis (45).
Making the diagnosis
It is important to seek supportive evidence to make a positive diagnosis of NAFLD (metabolic syndrome features) (Table 1) and to exclude those that negate this possibility (significant alcohol use, presence of other liver diseases or drugs known to be associated with steatosis including chronic hepatitis C, amiodarone, tamoxifen, methotrexate, lipodystrophies, Wilson’s disease, jejuno-ileal bypass, abetalipoproteinemia).

Full table
Alcohol
Excluding significant alcohol use is critical for defining NAFLD. Different thresholds have been suggested but most guidelines limit alcohol use to ≤20 g/d for men and ≤10 g/d for women) (46). As compared to non-drinkers, moderate alcohol drinkers have a reduced prevalence and severity of fatty liver (OR for NASH, 0.56) (47). Therefore, most doctors would not proscribe alcohol but concerns remain in patients with significant liver fibrosis/cirrhosis for fear of worsening liver failure and the risk of HCC. In one study, persons reporting regular alcohol use were more likely (hazard ratio 3.6; 95% CI, 1.5-8.3) to develop HCC as compared to non-drinkers (31).
Serum biomarkers
Separating SS and NASH
Current methods fall well short of achieving this and biopsy still remains the only reliable method. Cytokeratin 18 which reflects hepatocyte apoptosis, an important mode of cell death in NASH, showed initial promise but larger studies have been disappointing (48).
Staging hepatic fibrosis
Several different scoring systems have been proposed (NAFLD fibrosis score, FIB-4, APRI and others). These involve a combination of clinical and laboratory variables and their performance vary considerably. The NAFLD score is the most widely validated scoring system and involves a combination of age, BMI, AST, ALT, platelet count, albumin and impaired fasting glucose/diabetes (49). Derived from a cohort of 733 patients with NAFLD, a low cut-off score (–1.455) had a high negative predictive value in excluding advanced hepatic fibrosis (NPV, 88-93%) and a score of 0.676 can rule in advanced hepatic fibrosis with high accuracy (positive predictive value of 82-90%). The authors concluded that the majority of their patients (75%) could have avoided undergoing liver biopsy (accuracy, 90%).
Liver biopsy: is it still necessary?
From an initial defining role, the role of liver biopsy has receded. In addition to the known risks, the validity of a liver biopsy as a gold standard has been questioned by studies showing inter- and intra-observer variation in assessing inflammation and fibrosis (50). Indications for liver biopsy are listed in Table 2.
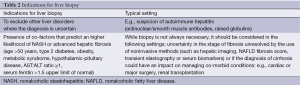
Full table
Pathogenesis
A brief overview of the pathogenesis will be presented (Table 3). This has been reviewed in detail elsewhere (51-53). NAFLD was initially conceptualized as a series of “hits”, with the first hit being the development of hepatic steatosis. In turn, this set the stage for a second or sequential hits culminating in NASH. The “hits” involved a wide array of mechanistic processes including oxidative stress, adipocytokine alterations, mitochondrial toxicity and small intestinal bacterial overgrowth. As steatosis has a benign outcome, this above sequence seems less tenable. In fact, accumulation of intrahepatic triglyceride (which underlies hepatic steatosis) itself is now considered to be a protective mechanism (52). Preventing hepatic triglyceride formation actually worsens steatohepatitis. Thus, molecules other than triglycerides are important and multiple parallel “hits” are likely to be involved (53).
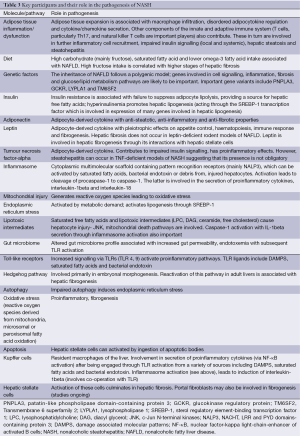
Full table
Current favours lipotoxicity as a key factor in development of progressive liver injury. Lipotoxicity refers to cellular dysfunction and injury resulting from fat deposition in non-adipose tissues. With triglyceride production now considered to be a protective adaption to FFA influx, attention has focussed on other lipid intermediates [diacetylglycerol (DAG), ceramide and free cholesterol] as being the main activators of pro-inflammatory pathways. Once triggered, there are a number of cellular processes that amplify and perpetuate the hepatic necro-inflammatory activity. Key participants include oxidative stress (reactive oxygen species derived from mitochondrial, microsomal or peroxisomal oxidation of FFA), mitochondrial injury, endoplasmic reticulum stress, impaired autophagy, hedgehog pathway signalling, specific microRNAs and Kupffer cell cytokine release through activation of certain toll-like receptors (TLR 4). Cell death eventually (mainly through apoptosis) results. These apoptotic bodies and other processes can activate hepatic stellate cells leading to progressive hepatic fibrosis and cirrhosis.
How does all this begin? A combination of a genetic predisposition and environmental factors including high caloric, saturated fat or high fructose-laden diets and decreased physical activity set the stage for obesity. The origins of lipotoxicity are linked to obesity-induced adipose tissue dysfunction. Although de novo hepatic synthesis of FFA does occur, the bulk of serum FFA are of adipose tissue or dietary origin. Adipose tissue, a very bioactive organ, secretes several adipocytokines (adipokines) and chemokines that have profound effects on glucose and lipid metabolism, insulin sensitivity and inflammation. In obese individuals, the adipose tissue expansion is associated with adipocyte dysfunction and dysregulated adipokine secretion. Chemokine-driven macrophage infiltration with a shift (polarisation) of the macrophage population from the anti-inflammatory M2 to the pro-inflammatory M1 phenotype of adipose tissue is associated with cytokine secretion (TNF-α and IL-6). Levels of the anti-inflammatory cytokine adiponectin decrease. Other cells include CD8+ T cells, Th17 cells, and NKT cells are also involved. These macrophage and other cell-derived products enter the systemic circulation and liver. This leads to insulin resistance (with failure to suppress adipocyte lipolysis and FFA release, unimpeded hepatic gluconeogenesis) and hyperinsulinemia. The latter along with hyperglycemia promotes hepatic lipogenesis through actions on specific transcription factors (sterol regulatory binding-element protein-1c, carbohydrate-response element-binding protein). The lipid-laden liver contributes further to systemic insulin resistance creating a vicious cycle.
Genetic factors
Familial predisposition and ethnic variations in the prevalence of NAFLD are well described. In the Dallas Heart Study (39), the prevalence figures for Mexicans, African-Americans and non-Hispanic Whites were 46%, 24% and 33%, respectively. Although these point to clear genetic influences (likely PNPLA 3 in this case), the genetic contribution to NAFLD has not been easy to quantify. Candidate gene studies have identified polymorphisms associated with various steps relating to appetite regulation, hepatic lipid metabolism/export, oxidative stress, fibrogenesis, inflammatory response, cytokine receptors and others (54). Their contribution has not always been well validated across multiple ethnic groups. Stronger associations have been derived from genome wide association studies (GWAS). The best studied of these is a single nucleotide polymorphism I148M (rs738409 C/G) in relation to the patatin-like phospholipase domain-containing protein 3 (PNPLA 3) gene (55). This missense variant is associated with hepatic steatosis and ALT levels but surprisingly not with insulin resistance. Further, when compared with patients carrying the CC genotype, patients carrying the GG genotype had a 3-fold greater risk of developing steatohepatitis and fibrosis when compared with those with CC. Some of the other genes identified by GWAS include glucokinase regulatory protein (GCKR), lysophospholipase-like protein 1 (LYPLAL 1) and NCAN (encoding neurocan) (56). These are involved in aspects of cell adhesion, triglyceride metabolism and anthropometric traits. The results have not always been consistent across different populations and some of the genes may be in linkage disequilibrium with other as yet unidentified variants.
Management
Optimising lifestyle (diet and exercise) is the mainstay of management. The lifestyle program should take into account co-morbid conditions and general fitness. Involvement of a personal trainer and participation in a group improves adherence and effectiveness of the program. Managing other components of the metabolic syndrome is also important from the perspective of reducing cardiovascular complications. These are managed along conventional lines but a few aspects of statin use will be discussed later.
Diet/exercise
Several studies have shown an inverse correlation between physical activity and the risk of developing NAFLD. In one recent Korean study of over 3,700 individuals, the risk of developing NAFLD was significantly lower the highest two quartiles of physical activity (OR 0.68 and 0.74, respectively) as compared with participants in the lowest quartile (57).
A number of different dietary schedules have been recommended, including low fat, low carbohydrate, high protein and “Mediterranean” diet plans (58). Some have combined diet and exercise schedules while others have been single-arm studies of diet or exercise alone. Although there may be some initial differences in efficacy between these diets, these differences are not sustained over a 2-year period. There is general agreement that refined sugar intake, particularly dietary fructose should be reduced (58), along with restrictions in total and saturated fat. Crash diets can exacerbate NASH and should be avoided.
Like diet, the ideal type and duration of exercise is debated, with studies showing comparable results with either aerobic or resistance training (59). Weight loss of 5% to 10% is correlated with loss of liver fat and improvement in liver inflammatory activity, respectively (57). The lack of impact on hepatic fibrosis is not surprising as most of the studies have been of short duration. Anaerobic exercise (resistive exercise, or weight training), even in the absence of weight loss, can reduce hepatic steatosis (60,61). In any case, improved overall cardiorespiratory fitness rather than weight loss alone should be the goal. Recommendations aimed at improving cardiovascular fitness, i.e., increasing exercise to 150 minutes of moderate activity/week, seems appropriate. In the light of data showing the benefits of resistance training alone, this is a valid alternative or an adjunct to aerobic exercise.
Vitamin E
The rationale of using vitamin E is to abrogate oxidative stress, whether this is its only mode of action is unclear. Two key randomized controlled trials in NAFLD have been published [PIVENS (in adults), TONIC (in children)] (62,63). The TONIC trial involved 173 children and compared vitamin E (800 IU/d) with metformin (1,000 mg/d) or placebo for a period of 48 weeks. The primary outcome was a decrease of ALT to vs. 58%, P=0.006) but not with metformin. The PIVENS trial involved 247 non-diabetic adult patients with NASH randomized to pioglitazone (30 mg/d), vitamin E (800 mg/d) or placebo for 48 weeks. The primary endpoint was a composite of: reduction in hepatocyte ballooning by 1 grade, nonalcoholic steatosis score (NAS) (a composite index of the grades of steatosis, lobular inflammation and ballooning) by ≥2 and hepatic fibrosis stage by ≥1. This was not achieved either with pioglitazone or vitamin E. However, significant resolution of NASH (a secondary endpoint) was observed in the vitamin E group (43% vs. 19% for placebo) but not with pioglitazone (34% vs. 19%, P=0.04, prespecified outcome was P
Several questions remain. The studies did not include patients with diabetes or cirrhosis. Long-term safety data are lacking. Further, the enthusiasm for vitamin E is tempered by controversies such as its association with increasing all-cause mortality (doses of >400 mg/d). While this assertion has been contested (64), there are other ongoing issues such as a possible link with increasing prostate cancer (by 17% in the SELECT trial) (65) and haemorrhagic stroke (by 22%) (66).
Insulin sensitising agents
Metformin improved liver tests and some aspects of histology (mainly reduced liver fat), but most of these studies were small and non-randomized (67). Metformin is no longer recommended primarily for NAFLD but has a possible role in liver cancer prevention, particularly among patients with T2D. A meta-analysis of eight studies showed a 50% reduction in HCC among metformin users (OR 0.50, 95% CI: 0.34-0.73) (68). Interestingly, the risks of HCC were increased in those receiving insulin (OR 2.61, 95% CI, 1.46-4.65) or sulfonylureas (OR 1.62, 95% CI, 1.16-2.24).
Thiazolidinediones (TZD)
TZD are peroxisome-proliferator activated receptor-γ agonists with pleiotrophic effects. Their effects on improving insulin sensitivity protect non-adipose tissues including the liver from lipotoxicity. Unlike the first generation TZD (troglitazone), the second generation TZD do not have a predilection for significant liver injury. Rosiglitazone has been withdrawn due to cardiac toxicity but pioglitazone remains in use. The PIVENS trial has been mentioned earlier (see section on Vitamin E). A meta-analysis of four randomized controlled trials showed that TZD improved hepatic steatosis (OR 3.39), inflammation (OR 2.58), and ballooning (OR 2.11) (69). As expected, given the short duration of the trials (12-24 months), all but one showed no improvement in hepatic fibrosis. Most of the improvement occurred within the first 2 years and reached a plateau thereafter (70). On the other hand, TZD discontinuation leads to recrudescence of hepatic steatosis. Long-term issues also include weight gain (average, 4.4 kg), congestive heart failure, osteoporosis and bladder cancer. The risk of bladder cancer is mainly attributed to pioglitazone, and is correlated with dose (>28 g) (OR 1.64) and duration of use of >12 months (OR 1.41-1.51) (71).
Incretins
Glucagon-like peptide-1 (GLP-1) is secreted by ileal L-cells. It is involved in postprandial pancreatic insulin release, leading to lowering of plasma glucose and improved insulin sensitivity. Other effects include decreasing glucagon secretion, inducing satiety, slowing gastric emptying and suppression of hepatic lipogenesis (72). GLP-1 receptors have been identified on human hepatocytes and there is evidence showing that patients with NAFLD have a diminished response to glucose-induced GLP-1 (73). GLP-1 has a short half-life and cannot be used in practice. Instead, analogues that mimic endogenous GLP-1 action (exenatide, liraglutide) through acting on the GLP-1 receptor or drugs that inhibit inactivation of GLP-1 by dipeptidyl peptidase-4 (“gliptins”, e.g., sitagliptin) are used.
While rodent studies with GLP-1 agonists have confirmed their antisteatotic potential, human data are limited to small studies, with liraglutide and sitagliptin, showing resolution or improvement of steatohepatitis and fibrosis (74,75). Controversies have arisen in relation to possible links between incretin therapies, acute pancreatitis and pancreatic cancer (76,77). But recent cohort studies, meta-analyses and independent reviews by the FDA and EMEA, have not confirmed this (78-81).
Obeticholic acid
Farnesoid X receptor (FXR) is a nuclear receptor involved in glucose and lipid metabolism (82). Bile acids are endogenous ligands for FXR. In animal studies, FXR agonists have reduced liver fat content and improved insulin sensitivity. A proof-of-concept phase II study has tested the efficacy of obeticholic acid, a FXR agonist and semi-synthetic derivative of chenodeoxycholic acid, an endogenous bile acid (83). Patients were assigned to placebo (n=23) or to two groups treated with obeticholic acid at doses of 25 mg (n=20) and 50 mg, respectively (n=21). Improved insulin sensitivity was observed in nearly a quarter of the obeticholic acid-treated patients whereas this worsened in 5% of those receiving placebo. Improvements in transaminases and markers of liver fibrosis were also seen in the bile acid treated group. However, a rise in LDL-cholesterol was also observed and has raised concerns about its suitability in a cohort at risk of ischaemic heart disease. A larger 72-week multi-centre study (FLINT) comparing obeticholic acid and placebo over 72 weeks was halted by the data safety monitoring board as the primary endpoint (reduction in the NAS by two points without worsening of fibrosis) was achieved. The study findings have not been fully published. Detailed lipid data are awaited with interest.
Fish oil (n-3 polyunsaturated fatty acids)
N-3 polyunsaturated fatty acids (PUFA) have anti-inflammatory, antioxidant and antilipogenic properties. Some dietary surveys have shown that patients with NAFLD tend to consume disproportionately more n-6 PUFA than n-3 PUFA. Further, in small uncontrolled studies, n-3 PUFA supplementation was associated with improved liver tests and reduced hepatic steatosis (84). However, a recent double-blind placebo controlled randomized trial did not show any positive effects of n-3 PUFA on histology or insulin sensitivity as compared with placebo (85).
Pentoxifylline
TNF-α contributes to insulin resistance and hepatic inflammatory activity in NASH. In a mouse model of methionine-choline induced NASH, pentoxifylline (PTX) decreased hepatic steatosis and reduced TNF-α expression (86). Initial human studies noted improvement in liver steatosis and liver histology (87) but in one study, 40% of patients discontinued the drug due to gastrointestinal side-effects (88). Even so, PTX continues to be evaluated in NASH. A recent “positive” study showed reduction in liver fat and lobular inflammation but not ballooning or fibrosis (89). Interestingly, serum TNF-α levels were not significantly reduced but the authors showed that PTX could function as an antioxidant reducing free radical-mediated lipid oxidation (90). A meta-analysis of five studies (n=147) showed no improvement in steatosis, hepatocyte ballooning and liver fibrosis (91). Overall, the data supporting PTX use are not robust, involvement of small population samples and impact on key histologic features has not been demonstrated.
Probiotics
Small intestinal bacterial overgrowth, increased intestinal permeability, portal endotoxemia and TLR 4 signalling and activation of hepatic pro-inflammatory pathways have been demonstrated in NASH (92). In animal studies, probiotics have been shown to modulate the gut flora and reverse some of these changes, along with improvement in AT and liver histology. Human pilot studies have also demonstrated a reduction in liver steatosis and a reduction in AT (93). A small randomized controlled trial in children comparing a probiotic (VSL#3) and placebo showed that VSL#3 could induce a significant reduction in liver fat and BMI after just four months. However, there were no differences between the groups with respect to ALT, triglycerides or insulin sensitivity and histologic data were lacking (94).
Statins and hyperlipidemia
The 3-hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) are widely used in managing hypercholesterolemia. Small case series noted improvement in liver tests and hepatic steatosis with statins. However, definitive studies demonstrating improvement in liver inflammation or fibrosis are lacking. Therefore, they have not been endorsed for treating NAFLD. However, like metformin, they remain useful allies in managing dyslipidemia in these patients. Concerns of hepatic safety have seen underprescribing of statins in patients with fatty liver (95). These concerns stem from interpreting increased AT (seen in 1% to 3%) as indicative of clinically significant liver injury. The latter is rare (1 per million) and the US FDA no longer mandates routine liver function tests in patients on statins.
The safety of statin use in patients with chronic liver diseases, including NAFLD with baseline elevations of AT, has been reiterated (96,97). As cardiovascular disease is the leading cause of death, control of dyslipidaemia is critical. A post-hoc analysis of the GREACE study endorses this view. A subgroup of patients with NAFLD (with raised AT) were significantly less likely (10% vs. 30%) to experience a cardiovascular event than similar untreated patients with NAFLD (98). Finally, like metformin, statin use has been associated with a reduced risk of HCC in patients with T2D (adjusted OR 0.74) (99).
Other drugs
A number of other drugs (betaine, ursodeoxycholic acid, angiotensin receptor blockers) have either not been subjected to nor have survived the scrutiny of randomized controlled trials, or have failed to show demonstrate histologic improvement. These drugs are no longer being actively evaluated in treating fatty liver.
Bariatric surgery
This is a valid option for morbidly obese individuals (BMI >40 kg/m2) or those with a BMI >35 kg/m2 with co-morbid metabolic disorders. Surgical approaches vary from laparoscopic gastric banding, sleeve gastrectomy to roux-en-Y gastric bypass procedures. Weight loss is achieved with all techniques, with the greatest amount of weight reduction with bypass procedures. Overall, along with the improvement in the metabolic profile, there is also a reduction or resolution in the degree of liver fat (~90%) and inflammatory activity (~80%) (100). There may be a slight worsening of fibrosis stage in some cases but this is usually mild (F1 stage), and improvement even in fibrosis stage (~65%) may occur and this proportion could increase with a longer duration of follow up. Bariatric surgery can be performed in well-compensated cirrhotic patients but decompensated liver disease is a contra-indication. In some centres, bariatric procedures are performed at the time of liver transplantation but most surgeons would prefer to undertake these after liver transplantation.
Liver transplantation
NAFLD is the primary indication in 10% to 13% of patients undergoing liver transplantation in the USA (101). The short-term outcome is excellent (78% to 84%, 1- and 3-year survival rates) and is comparable to that for patients undergoing transplantation for other indications. However, nearly half the recipients develop hepatic steatosis within 5 years, 7% to 30% develop NASH and cirrhosis rates approach 10% at 10 years (102). Therefore, aggressive management of the metabolic syndrome is warranted.
Follow up
Annual review should include screening for the metabolic syndrome, T2D, cardiovascular disease, common cancers (breast and more recently, colonic adenomas and colorectal carcinoma) and signs of cirrhosis. Specialists usually manage patients with established cirrhosis but primary care physicians can assist in ensuring patient adherence with agreed management protocols (blood tests, periodic hepatic imaging, arranging dietician reviews and screening for osteoporosis).
Future directions
As the progressive form of fatty liver, the pathogenesis of NASH remains the main focus of current research. The definition of principal pro-inflammatory signalling pathways and cross-talk between these pathways and traditional (e.g., leptin, adiponectin, TNF-α) and recently identified adipokines (e.g., lipocalin-2, chemerin) and myokines (irisin) awaits clarification. The genetic and epigenetic (e.g., prenatal influences) modifiers of NAFLD are also being actively investigated. Many important gene variants (apolipoprotein C3) have shown not to be relevant in populations other than those in which they were first studied. Defining the function(s) of identified genes is also an ongoing area of research. For example, the PNPLA 3 variant has been variously considered to be a triglyceride lipase or as a gain-of-function mutation promoting lipogenesis. Is it possible that it has multiple functions depending on the source of FFA? How does this gene interact with environmental factors such as diet or physical activity?
Clinical areas of research are engaged in deriving, defining and refining serum and genetic biomarkers to identify NASH or stage fibrosis and to predict long-term outcomes. For example, should one target those carrying the PNPLA 3 variant because this is associated with a higher risk of hepatocellular carcinoma? Treatment strategies being explored include those targeting the inflammatory (caspase inhibitors) and/or fibrotic aspects of NASH (lysyl oxidase homolog 2 inhibitors, e.g., simtuzumab), the gut microbiome (probiotics, synbiotics) or the lipotoxic intermediates (statins, ezetimibe). Like many common cancers, NAFLD/NASH is a heterogeneous disorder and treatment strategies may need to be individualized based on the underlying pathogenic process.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Withrow D, Alter DA. The economic burden of obesity worldwide: a systematic review of the direct costs of obesity. Obes Rev 2011;12:131-41. [PubMed]
- Colagiuri S, Lee CM, Colagiuri R, et al. The cost of overweight and obesity in Australia. Med J Aust 2010;192:260-4. [PubMed]
- Goossens N, Negro F. The impact of obesity and metabolic syndrome on chronic hepatitis C. Clin Liver Dis 2014;18:147-56. [PubMed]
- Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 2006;43:S99-S112. [PubMed]
- Chitturi S, Abeygunasekera S, Farrell GC, et al. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology 2002;35:373-9. [PubMed]
- Brunt EM. Histopathology of non-alcoholic fatty liver disease. Clin Liver Dis 2009;13:533-44. [PubMed]
- Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology 2005;42:641-9. [PubMed]
- Poonawala A, Nair SP, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case-control study. Hepatology 2000;32:689-92. [PubMed]
- van der Poorten D, Samer CF, et al. Hepatic fat loss in advanced nonalcoholic steatohepatitis: are alterations in serum adiponectin the cause? Hepatology 2013;57:2180-8. [PubMed]
- Farrell GC, Wong VW, Chitturi S. NAFLD in Asia-as common and important as in the West. Nat Rev Gastroenterol Hepatol 2013;10:307-18. [PubMed]
- Rabl C, Campos GM. The impact of bariatric surgery on nonalcoholic steatohepatitis. Semin Liver Dis 2012;32:80-91. [PubMed]
- Chitturi S, Wong VWS, Farrell G. Nonalcoholic fatty liver in Asia: Firmly entrenched and rapidly gaining ground. J Gastroenterol Hepatol 2011;26 Suppl 1:163-72. [PubMed]
- Doycheva I, Patel N, Peterson M, et al. Prognostic implication of liver histology in patients with nonalcoholic fatty liver disease in diabetes. J Diabetes Complications 2013;27:293-300. [PubMed]
- Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003;37:917-23. [PubMed]
- Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 2005;143:722-8. [PubMed]
- Armstrong MJ, Adams LA, Canbay A, et al. Extrahepatic complications of nonalcoholic fatty liver disease. Hepatology 2014;59:1174-97. [PubMed]
- Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010;363:1341-50. [PubMed]
- Fan JG, Li F, Cai XB, et al. Effects of non-alcoholic fatty liver disease on the development of metabolic disorders. J Gastroenterol Hepatol 2007;22:1086-91. [PubMed]
- Zelber-Sagi S, Lotan R, Shibolet O, et al. Non-alcoholic fatty liver disease independently predicts prediabetes during a 7-year prospective follow-up. Liver Int 2013;33:1406-12. [PubMed]
- Ratziu V, Bugianesi E, Dixon J, et al. Histological progression of non-alcoholic fatty liver disease: a critical reassessment based on liver sampling variability. Aliment Pharmacol Ther 2007;26:821-30. [PubMed]
- Adams LA, Sanderson S, Lindor KD, et al. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol 2005;42:132-8. [PubMed]
- Ekstedt M, Franzén LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865-73. [PubMed]
- Angulo P. The natural history of NAFLD. In: Farrell GC, McCullough AJ, Day CP. eds. Non-alcoholic fatty liver disease. Chichester: Wiley-Blackwell, 2013:37-45.
- Angulo P, Keach JC, Batts KP, et al. Independent predictors of liver fibrosis in patients with non-alcoholic steatohepatitis. Hepatology 1999;30:1356-62. [PubMed]
- Hossain N, Stepanova M, Afendy A, et al. Non-alcoholic steatohepatitis (NASH) in patients with polycystic ovarian syndrome (PCOS). Scand J Gastroenterol 2011;46:479-84. [PubMed]
- Adams LA, Feldstein A, Lindor KD, et al. Nonalcoholic fatty liver disease among patients with hypothalamic and pituitary dysfunction. Hepatology 2004;39:909-14. [PubMed]
- Mishra P, Nugent C, Afendy A, et al. Apnoeic-hypopnoeic episodes during obstructive sleep apnoea are associated with histological nonalcoholic steatohepatitis. Liver Int 2008;28:1080-6. [PubMed]
- Angulo P. Diagnosing steatohepatitis and predicting liver-related mortality in patients with NAFLD: Two distinct concepts. Hepatology 2011;53:1792-4. [PubMed]
- Bhala N, Angulo P, van der Poorten D, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology 2011;54:1208-16. [PubMed]
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med 2003;348:1625-38. [PubMed]
- Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. Hepatology 2010;51:1972-8. [PubMed]
- Graham J, Reeves H. Non-alcoholic fatty liver disease, hepatocellular cancer, and other cancers. In: Farrell GC, McCullough AJ, Day CP. eds. Non-alcoholic fatty liver disease. Chichester: Wiley-Blackwell, 2013:192-205.
- Torres DM, Harrison SA. Nonalcoholic steatohepatitis and noncirrhotic hepatocellular carcinoma: fertile soil. Semin Liver Dis 2012;32:30-8. [PubMed]
- Alexander J, Torbenson M, Wu TT, et al. Non-alcoholic fatty liver disease contributes to hepatocarcinogenesis in non-cirrhotic liver: a clinical and pathological study. J Gastroenterol Hepatol 2013;28:848-54. [PubMed]
- Söderberg C, Stål P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology 2010;51:595-602. [PubMed]
- Chitturi S, Farrell GC. Clues from the carotids: an appraisal of cardiovascular disease risk in non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2009;24:1315-7. [PubMed]
- Verrijken A, Francque S, Mertens I, et al. Prothrombotic factors in histologically proven nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2014;59:121-9. [PubMed]
- Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed) 1986;292:13-5. [PubMed]
- Browning JD, Szcepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology 2004;40:1387-95. [PubMed]
- Wong VWS, Vergniol J, Wong GLH, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010;51:454-62. [PubMed]
- Shen F, Zheng RD, Mi YQ, et al. Controlled attenuation parameter for non-invasive assessment of hepatic steatosis in Chinese patients. World J Gastroenterol 2014;20:4702-11. [PubMed]
- Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 2003;37:1286-92. [PubMed]
- Chitturi S, Weltman M, Farrell GC, et al. HFE mutations, hepatic iron, and fibrosis: ethnic-specific association of NASH with C282Y but not with fibrotic severity. Hepatology 2002;36:142-9. [PubMed]
- Kowdley KV, Belt P, Wilson LA, et al. Serum ferritin is an independent predictor of histologic severity and advanced fibrosis in patients with nonalcoholic fatty liver disease. Hepatology 2012;55:77-85. [PubMed]
- Angulo P, George J, Day CP, et al. Serum ferritin levels lack diagnostic accuracy for liver fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2014;12:1163-9. [PubMed]
- Farrell GC, Chitturi S, Lau GK, et al. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region. Executive Summary. J Gastroenterol Hepatol 2007;22:775-7. [PubMed]
- Sookoian S, Castaño GO, Pirola CJ. Modest alcohol consumption decreases the risk of non-alcoholic fatty liver disease: a meta-analysis of 43 175 individuals. Gut 2014;63:530-2. [PubMed]
- Cusi K, Chang Z, Harrison S, et al. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol 2014;60:167-74. [PubMed]
- Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846-54. [PubMed]
- Ratziu V, Bugianesi E, Dixon J, et al. Histological progression of non-alcoholic fatty liver disease: a critical reassessment based on liver sampling variability. Aliment Pharmacol Ther 2007;26:821-30. [PubMed]
- Farrell GC, van Rooyen D, Gan L, et al. NASH is an Inflammatory Disorder: Pathogenic, Prognostic and Therapeutic Implications. Gut Liver 2012;6:149-71. [PubMed]
- Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis (NASH): clinical implications. Gastroenterology 2012;142:711-25. [PubMed]
- Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 2010;52:1836-46. [PubMed]
- Anstee QM, Day CP. The genetics of NAFLD. Nat Rev Gastroenterol Hepatol 2013;10:645-55. [PubMed]
- Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology 2011;53:1883-94. [PubMed]
- Speliotes EK, Yerges-Armstrong LM, Wu J, et al. Genome-wide association analysis identifies variants associated with nonalcoholic fatty liver disease that have distinct effects on metabolic traits. PLoS Genet 2011;7:e1001324. [PubMed]
- Kwak MS, Kim D, Chung GE, et al. Role of physical activity in nonalcoholic fatty liver disease in terms of visceral obesity and insulin resistance. Liver Int 2014. [Epub ahead of print]. [PubMed]
- Thoma C, Day C, Trenell MI. Lifestyle interventions for the treatment of non-alcoholic fatty liver disease in adults: a systematic review. J Hepatol 2012;56:255-66. [PubMed]
- Vos MB, Lavine JE. Dietary fructose in nonalcoholic fatty liver disease. Hepatology 2013;57:2525-31. [PubMed]
- Bacchi E, Negri C, Targher G, et al. Both resistance training and aerobic training reduce hepatic fat content in type 2 diabetic subjects with nonalcoholic fatty liver disease (the RAED2 Randomized Trial). Hepatology 2013;58:1287-95. [PubMed]
- Hallsworth K, Fattakhova G, Hollingsworth KG, et al. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut 2011;60:1278-83. [PubMed]
- Keating SE, Hackett DA, George J, et al. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol 2012;57:157-66. [PubMed]
- Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 2011;305:1659-68. [PubMed]
- Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675-85. [PubMed]
- Abner EL, Schmitt FA, Mendiondo MS, et al. Vitamin E and all-cause mortality: a meta-analysis. Curr Aging Sci 2011;4:158-70. [PubMed]
- Klein EA, Thompson IM Jr, Tangen CM, et al. Vitamin E and the risk of prostate cancer: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011;306:1549-56. [PubMed]
- Schürks M, Glynn RJ, Rist PM, et al. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ 2010;341:c5702. [PubMed]
- Shyangdan D, Clar C, Ghouri N, et al. Insulin sensitisers in the treatment of non-alcoholic fatty liver disease: a systematic review. Health Technol Assess 2011;15:1-110. [PubMed]
- Singh S, Singh PP, Singh AG, et al. Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol 2013;108:881-91. [PubMed]
- Boettcher E, Csako G, Pucino F, et al. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2012;35:66-75. [PubMed]
- Ratziu V, Charlotte F, Bernhardt C, et al. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology 2010;51:445-53. [PubMed]
- Turner RM, Kwok CS, Chen-Turner C, et al. Thiazolidinediones and associated risk of Bladder Cancer: a Systematic Review and Meta-analysis. Br J Clin Pharmacol 2014;78:258-73. [PubMed]
- Samson SL, Bajaj M. Potential of incretin-based therapies for non-alcoholic fatty liver disease. J Diabetes Complications 2013;27:401-6. [PubMed]
- Bernsmeier C, Meyer-Gerspach AC, Blaser LS, et al. Glucose-induced glucagon-like Peptide 1 secretion is deficient in patients with non-alcoholic fatty liver disease. PLoS One 2014;9:e87488. [PubMed]
- Eguchi Y, Kitajima Y, Hyogo H, et al. Pilot study of liraglutide effects in non-alcoholic steatohepatitis and non-alcoholic fatty liver disease with glucose intolerance in Japanese patients (LEAN-J). Hepatol Res 2014. [Epub ahead of print]. [PubMed]
- Yilmaz Y, Yonal O, Deyneli O, et al. Effects of sitagliptin in diabetic patients with nonalcoholic steatohepatitis. Acta Gastroenterol Belg 2012;75:240-4. [PubMed]
- Singh S, Chang HY, Richards TM, et al. Glucagonlike peptide 1-based therapies and risk of hospitalization for acute pancreatitis in type 2 diabetes mellitus: a population-based matched case-control study. JAMA Intern Med 2013;173:534-9. [PubMed]
- Elashoff M, Matveyenko AV, Gier B, et al. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology 2011;141:150-6. [PubMed]
- Li L, Shen J, Bala MM, et al. Incretin treatment and risk of pancreatitis in patients with type 2 diabetes mellitus: systematic review and meta-analysis of randomised and non-randomised studies. BMJ 2014;348:g2366. [PubMed]
- Faillie JL, Azoulay L, Patenaude V, et al. Incretin based drugs and risk of acute pancreatitis in patients with type 2 diabetes: cohort study. BMJ 2014;348:g2780. [PubMed]
- Montori VM. The safety of incretin based drugs. BMJ 2014;348:g2779. [PubMed]
- Egan AG, Blind E, Dunder K, et al. Pancreatic safety of incretin-based drugs--FDA and EMA assessment. N Engl J Med 2014;370:794-7. [PubMed]
- Fuchs M. Non-alcoholic Fatty liver disease: the bile Acid-activated farnesoid x receptor as an emerging treatment target. J Lipids 2012;2012:934396.
- Mudaliar S, Henry RR, Sanyal AJ, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013;145:574-82. [PubMed]
- Bargut TC, Frantz ED, Mandarim-de-Lacerda CA, et al. Effects of a diet rich in n-3 polyunsaturated fatty acids on hepatic lipogenesis and beta-oxidation in mice. Lipids 2014;49:431-44. [PubMed]
- Dasarathy S, Dasarathy J, Khiyami A, et al. Double-blind Randomized Placebo-controlled Clinical Trial of Omega 3 Fatty Acids for the Treatment of Diabetic Patients With Nonalcoholic Steatohepatitis. J Clin Gastroenterol 2014. [Epub ahead of print]. [PubMed]
- Koppe SW, Sahai A, Malladi P, et al. Pentoxifylline attenuates steatohepatitis induced by the methionine choline deficient diet. J Hepatol 2004;41:592-8. [PubMed]
- Adams LA, Zein CO, Angulo P, et al. A pilot trial of pentoxifylline in nonalcoholic steatohepatitis. Am J Gastroenterol 2004;99:2365-8. [PubMed]
- Satapathy SK, Sakhuja P, Malhotra V, et al. Beneficial effects of pentoxifylline on hepatic steatosis, fibrosis and necroinflammation in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol 2007;22:634-8. [PubMed]
- Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology 2011;54:1610-9. [PubMed]
- Zein CO, Lopez R, Fu X, et al. Pentoxifylline decreases oxidized lipid products in nonalcoholic steatohepatitis: new evidence on the potential therapeutic mechanism. Hepatology 2012;56:1291-9. [PubMed]
- Du J, Ma YY, Yu CH, et al. Effects of pentoxifylline on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol 2014;20:569-77. [PubMed]
- Iacono A, Raso GM, Canani RB, et al. Probiotics as an emerging therapeutic strategy to treat NAFLD: focus on molecular and biochemical mechanisms. J Nutr Biochem 2011;22:699-711. [PubMed]
- Alisi A, Bedogni G, Baviera G, et al. Randomised clinical trial: the beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2014;39:1276-85. [PubMed]
- Younoszai Z, Li Z, Stepanova M, et al. Statin use is not associated with liver related mortality. Ann Hepatol 2013;13:84-90. [PubMed]
- Lewis JH, Mortensen ME, Zweig S, et al. Efficacy and safety of high-dose pravastatin in hypercholesterolemic patients with well-compensated chronic liver disease: Results of a prospective, randomized, double-blind, placebo-controlled, multicenter trial. Hepatology 2007;46:1453-63. [PubMed]
- Chalasani N, Aljadhey H, Kesterson J, et al. Patients with elevated liver enzymes are not at higher risk for statin hepatotoxicity. Gastroenterology 2004;126:1287-92. [PubMed]
- Athyros VG, Tziomalos K, Gossios TD, et al. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet 2010;376:1916-22. [PubMed]
- El-Serag HB, Johnson ML, Hachem C, et al. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology 2009;136:1601-8. [PubMed]
- Mummadi RR, Kasturi KS, Chennareddygari S, et al. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2008;6:1396-402. [PubMed]
- Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology 2011;141:1249-53. [PubMed]
- Watt KD. Liver transplantation and nonalcoholic fatty liver disease. Clin Liv Dis 2012;1:130-2.


