Simulation models of gastrointestinal cancers: strategic approach to predicting and decision making
Introduction
The complexity of cancer and the vast amount of experimental data available have made computer-aided approaches necessary. Biomolecular modeling techniques and simulation are becoming increasingly easier to use, whereas hardware and software are becoming better and cheaper. Gastrointestinal (GI) cancers are the most common cancers (1-3). Information technology plays an important role in research and clinical issues into the cause of cancer, patterns and trends and planning and monitoring of cancer by reliable evidence (4). Health information technology plays an important role in research and clinical issues into the cause of cancer, patterns and trends and planning and monitoring of cancer by reliable evidence (4). Health managers need to accurate and up-to-date epidemiological information for analyzing of the burden of the different types of GI cancer for example colorectal, gastric, liver, pancreatic and esophageal cancers (5,6). Wireless connection permits easier simultaneous access to patient data, saving time in busy and distant areas (7-9). Wireless simulation is the imitation of the operation of a real-world process or system over time by wireless technology. In using discrete event simulation for planning services in the health sector, epidemiologists and clinicians were closely involved in model design, data collection, analysis, validation and experimentation (10-12).
Virtual reality simulation and learning of GI endoscopy
Training in GI endoscopy has been based upon a traditional or apprenticeship model. The growing awareness of the need for patient safety has brought the issue of simulation-based training. The use of simulation technology may have important educational and societal advantages; the effectiveness of virtual reality GI endoscopy simulators has yet to be clearly demonstrated. Virtual reality simulation can help training colonoscopy, diagnostic esophagogastroduodenoscopy and sigmoidoscopy for health care professions (13). Also virtual colorectal patients have been developed to train in enhanced recovery programs through pre- and post-operative cases. Such patients could be included in a whole pathway care training involving technical and non-technical skills (14).
The use of simulation for evaluation of chemotherapy qualification
Simulation models are effective educational tool. Modeling with wireless technology is used in schools of nursing and hospital-based education as a method of teaching clinical content, enhancing clinical skills, applying theory to practice, and validating competency. It provides a safe learning environment to enhance nurses’ clinical judgment and critical thinking skills in an increasingly complex care environment. Simulation can be used in the practice setting with experienced nurses to teach or reinforce complex information and allow the learner to practice without devastating consequences. Medical-surgical units in some institutions have dedicated beds for patients with cancer but may not be a full oncology unit. Evaluating chemotherapy and biotherapy competency is difficult when extensive time periods exist between chemotherapy administrations. One method for assessing annual chemotherapy competency is to use simulation (15).
Example of simulation models for colorectal cancer (CRC)
In cancer chemotherapy, it is important to design treatment strategies for drug protocols that ensure a desired rate of tumor cell kill without overdosing the host. Mathematical modeling was used for optimization this function (16).
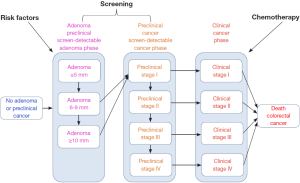
Two mathematical models are used to simulate the impact on CRC mortality of changing cancer prevention, early detection, and use of chemotherapy treatments among all populations. The two models explain risk factors, screening, and treatment influence the course of the disease and ultimately mortality. SimCRC and MISCAN are two computer models that simulate CRC disease progression. The models simulate colorectal disease progression in a large population of individuals from birth to death. The simulated population of individuals reflects observed distributions of characteristics in the real population.
Variables of models simulated as follow:
- Risk factor behavior: influences the risk of onset of adenomas and/or the progression of large adenomas to preclinical cancer. The probabilities of adenoma onset or adenoma progression are modified up or down depending on a simulated individual’s risk factor profile;
- Screening and surveillance: affect all preclinical disease stages, possibly leading to the removal of an adenoma (potentially preventing CRC) or to the early detection of a carcinoma with a more favorable prognosis;
- Chemotherapy: can postpone or even prevent death from CRC. Improvements in chemotherapy are modeled by a reduction in cancer-specific mortality rates based on published hazard ratios.
The models incorporate specific objectives for these interventions, termed upstream objectives. The simulations project the effects of changes to upstream factors on CRC mortality, the downstream objective. The SimCRC and MISCAN models share many characteristics. Both models simulate the US population from the 1970s to 2020 according to basic demographics, derived from census data and life table inputs (17). Figure 1 was illustrated simulation model of CRC.
Conclusions
GI cancers refer to malignant conditions that are leading cause of cancer mortality worldwide. But GI cancers are prevalent cancers. Simulation models are useful to both estimate up-to-date mortality trends, because of the normal reporting delays for mortality data, and to estimate future mortality trends of cancers. To understand cancer pathology and develop effective treatment strategies play significant roles in improving cancer survival rates. The simulation can determine the expected workload and the amount of vision loss prevented for any population group.
Computational prediction of cancer is now being used as a tool for detecting the probable oncogenes. Simulation models cancer screening improve prevention plans. Most patients treated curatively are placed on some type of surveillance program involving periodic follow-up testing to detect preclinical recurrence. For patients who will experience recurrence, prognosis, though generally poor, may be improved if detection occurs prior to symptom onset, particularly if surgical resection of metastatic disease is possible. Computer technologies, preliminarily estimate disease progression parameters by expert systems.
Some aspects of the natural such as the rate of progression from adenomatous polyp to cancers are unknown. The objective of simulation methodology is to estimate a set of parameters revealing some of these unknown characteristics of cancers. Cost-effectiveness analysis is typically performed by predetermined screening policies. A simulation-optimization model to determine the ages at which screening should be performed, resulting in dynamic, age-based screening policies. Complex modeling offer substantial economic savings in order to offer the same health benefits as equally spaced screening strategies. Incidental findings evaluate by simulation modeling. Alerting and reminding system improve by this approach. Oncologists and other decision makers make comprehensive decision by knowledge of quantitative and qualitative models. With further advancements in the computational simulation, it will become much easier to predict such mutations with higher accuracy level.
Acknowledgements
This research has been supported by Research Centre for Gastroenterology and Liver Disease of Shaheed Beheshti Medical University. The authors would like to thank Dr. Zali for very helpful comments on this work.
Disclosure: The authors declare no conflict of interest.
References
- Shaheen NJ, Hansen RA, Morgan DR, et al. The burden of gastrointestinal and liver diseases, 2006. Am J Gastroenterol 2006;101:2128-38. [PubMed]
- Keighley MR. Gastrointestinal cancers in Europe. Aliment Pharmacol Ther 2003;18 Suppl 3:7-30. [PubMed]
- Somi MH, Golzari M, Farhang S, et al. Gastrointestinal cancer incidence in East Azerbaijan, Iran: update on 5 year incidence and trends. Asian Pac J Cancer Prev 2014;15:3945-9. [PubMed]
- Elham M, Nasraran Z, Reza ZM. Health informatics and information system: an integrated evidence-base tool for colorectal cancer screening. Asian Pac J Cancer Prev 2008;9:537-40. [PubMed]
- Pourhoseingholi MA, Vahedi M, Moghimi-Dehkordi B, et al. Burden of hospitalization for gastrointestinal tract cancer patients - Results from a cross-sectional study in Tehran. Asian Pac J Cancer Prev 2009;10:107-10. [PubMed]
- Atrkar-Roushan Z, Kazemnejad A, Mansour-Ghanaei F, et al. Trend analysis of gastrointestinal cancer incidences in Guilan province: comparing rates over 15 years. Asian Pac J Cancer Prev 2013;14:7587-93. [PubMed]
- Mandal A, Asthana AK, Aggarwal LM. Experience of wireless local area network in a radiation oncology department. J Cancer Res Ther 2010;6:148-51. [PubMed]
- Han G, Xu H, Duong TQ, et al. Localization algorithms of wireless sensor networks: a survey. Telecommunication Systems 2013;52:2419-36.
- Chen X, Dai Z, Li W, et al. ProHet: A Probabilistic Routing Protocol with Assured Delivery Rate in Wireless Heterogeneous Sensor Networks. IEEE Transactions on Wireless Communications 2013;12:1524-31.
- Davies R, Brailsford S, Roderick P, et al. Using simulation modelling for evaluation screening services for diabetic retinopathy. J Oper Res Soc 2000;51:476-84.
- Kumar A, Purohit R. Computational investigation of pathogenic nsSNPs in CEP63 protein. Gene 2012;503:75-82. [PubMed]
- Kumar A, Purohit R. Use of long term molecular dynamics simulation in predicting cancer associated SNPs. PLoS Comput Biol 2014;10:e1003318. [PubMed]
- Walsh CM, Sherlock ME, Ling SC, et al. Virtual reality simulation training for health professions trainees in gastrointestinal endoscopy. Cochrane Database Syst Rev 2012;6:CD008237. [PubMed]
- Beyer-Berjot L, Patel V, Ziprin P, et al. Enhanced Recovery Simulation in Colorectal Surgery: Design of Virtual Online Patients. Society of American Gastrointestinal and Endoscopic Surgeons 2013;27:s347.
- Muehlbauer PM, Parr MB, Perkins AK. Using simulation to assess chemotherapy competency. Clin J Oncol Nurs 2013;17:392-6. [PubMed]
- Barbolosi D, Iliadis A. Optimizing drug regimens in cancer chemotherapy: a simulation study using a PK-PD model. Comput Biol Med 2001;31:157-72. [PubMed]
- Natinal cancer institute. Simulation Models for Colorectal Cancer (2014). Available online: http://cisnet.cancer.gov/profiles/