Osteoporosis in gastrointestinal diseases
Introduction
Osteoporosis is a progressive bone disease. It affects the bone strength due to reduction of bone mass and microarchitectural disruption leading to fragile bones with increased risk of fractures (1). Patients with osteoporotic fractures have high morbi-mortality and are a great burden for the health system. In the United States, 10 million people have osteoporosis and 1.5 million of fragility fractures happen each year (2).
Primary osteoporosis is due to the physiological process of aging, and is exacerbated by normal menopause. Secondary osteoporosis denotes bone loss that is caused or aggravated by other diseases or medications. In patients with osteoporosis, 50-65% of men, 50% of premenopausal women and 30% of postmenopausal women have a secondary cause for bone loss (3,4).
Smoking, high alcohol intake, glucocorticoids (GC) use, malabsorption, calcium and vitamin D deficiencies and low body mass index (BMI) are well-known causes of secondary osteoporosis. Patients with gastrointestinal diseases (GID) may present multiple risk factors for bone disease. Systemic inflammation may exacerbate bone loss in diseases such as celiac disease (CD) and inflammatory bowel disease (IBD).The aim of this review is to discuss the epidemiology, pathogenesis, screening and treatment of osteoporosis in the most common GID.
Pathophysiology of osteoporosis
The diagnosis of osteoporosis is established by dual energy X-ray absorptiometry (DXA) of lumbar spine, proximal femur or forearm. For postmenopausal women and men aged 50 years or more, DXA results are compared to those of a young adult reference population (T score), and a standard deviation (SD) is generated. Osteopenia is defined as bone mass density (BMD) between –1 and –2.5 SD, and osteoporosis as BMD of –2.5 SD or below. For other populations, BMD results are compared to an age- and gender-matched reference population (Z-scores). Low bone mass is defined as a Z score of –2 SD or less (5). For each SD below normal, the fracture relative risk increases by 1.5- to 3-fold (6). However, not only a low BMD defines the fracture risk.
Bone strength is defined by a proper balance between bone tissue resorption and formation, a process of bone renovation called remodeling. Bone remodeling disruption, due to bone formation reduction or enhanced resorption, or both, may result in osteoporosis. In addition to bone remodeling, microdamage accumulation, microarchitecture, mineralization degree, mineral matrix composition and bone geometry also contribute to fracture risk (7).
Osteoclasts are responsible for bone resorption, osteoblasts, for bone formation (8), and osteocytes, for the detection of microdamages, microstructural and biomechanical alterations in the bone, signalizing the start of the remodeling process. The communication between osteoclasts and osteoblasts is intermediated by the receptor activator of NF-κB ligand (RANK-L), its soluble form (sRANK-L), and osteoprotegerin (OPG). Osteoblasts express RANK-L, which links to RANK present on the osteoclasts surface. RANK-L stimulates the differentiation and osteoclasts activity, hence initiating bone resorption. On the other hand, osteoblasts produce OPG, which binds to RANK, and antagonizes RANK-L action and bone resorption (9). Many inflammatory cells and cells of the immune system are able to express RANK-L, a member of the tumor necrosis factor (TNF) family and an important link between systemic inflammation and bone health (10). Besides, pro-inflammatory cytokines contribute to osteoporosis, by stimulating bone resorption (a mechanism that is osteoblast-independent). Interleukin (IL)-1β, IL-6 and TNFα act in synergy with RANK-L, stimulating bone resorption. Also, TNF-α inhibits bone formation, affecting the differentiation of osteoblasts (11). Chronic inflammation, T cells hyperactivation, increased levels of pro-inflammatory cytokines, and unbalanced RANK-L/RANK/OPG system are observed in patients with IBD and celiac disease, which could explain in part their susceptibility to osteoporosis.
Epidemiology and pathogenesis of osteoporosis in gastrointestinal diseases
Celiac disease
CD is an autoimmune disorder, which causes chronic inflammation of the small intestine due to sensitivity to gluten. The mucosa of small intestine has an abnormal architecture with lymphocytic infiltration in the epithelium. This inflammatory process results in atrophy of the intestinal mucosa and malabsorption (12). The prevalence of CD is increasing worldwide, mainly because the consumption of products with gluten has enhanced, reaching 0.2-1% in the U.S. and Europe (13-15).
CD has been associated with osteoporosis (16,17), even in patients without gastrointestinal symptoms or those following for years a gluten-free diet (GFD) (18). The prevalence of low BMD is high. Up to 70% of patients with CD have abnormal DXA (16,19-22). Results of studies assessing the fracture risk in patients with CD are divergent, depending on the length of the follow-up, the compliance to a GFD, the analysis of fracture history, and intestinal mucosal status. The fracture risk in patients with CD ranged from 1.3- to 10-fold higher than the general population (21,23-25). However, other authors did not find increased risk of fracture in patients with CD (26,27).
The pathogenesis of metabolic bone disease associated with CD is multifactorial. Chronic malabsorption leads to nutrient deficiency, secondary lactose intolerance and low BMI. Calcium absorption is particularly impaired because CD affects the region of the small intestine where it is absorbed. Vitamin D-dependent calcium-binding proteins, which are responsible for calcium uptake by enterocytes, were absent or diminished in patients with CD (28). In addition, unabsorbed fatty acid in the intestinal lumen can bind to calcium and prevent its absorption. Hyperparathyroidism secondary to deficiency of calcium and 25OH-vitamin D is common. Elevated PTH increases bone resorption and turnover, resulting in low BMD and impaired bone quality, especially in the cortical bone present in the appendicular skeleton (29).
Lactose intolerance occurs in 30-60% of newly diagnosed patients, who avoid ingestion of dairy products and consequently, calcium. Thus, CD may affect calcium metabolism in many ways. Furthermore, patients with adherence to GFD may have nutritional deficiencies even after the reestablishment of a normal intestinal absorption, since gluten-free products are poor in calcium, vitamin D, zinc and magnesium. Patients with CD who are newly diagnosed or have poor adherence to GFD may present weight loss and low BMI. Low BMI is strongly correlated with low BMD, and is a predictor of fractures in the general population (30,31). A longitudinal study with CD seropositive patients showed that BMI correlated with BMD at both diagnosis and follow-up of the disease, and after 2.4 years there was an increase in body fat, and an increase in BMD by 10.8% and 7.1% in the spine and hip, respectively (32). Lower systemic levels of insulin growth factor (IGF) 1 and leptin, which are associated with the stimulation of bone anabolism and reduction of mechanical load are some factors involved in the decrease of BMD caused by low BMI.
Disease activity evaluated by duodenal biopsy may also be an important predictor of bone disease in CD. In patients with follow-up biopsy, persistence of mucosal atrophy was correlated with fracture of the femur. Risk was increased in case of subtotal or total mucosal atrophy. In this study, 43% of patients who had subtotal or total mucosa atrophy at diagnosis had persistent mucosal atrophy over the following years (33). This suggests that patients with more severe disease at diagnosis should be monitored for bone disease.
In corroboration with the relation between CD activity/severity and bone loss, there are evidences that systemic inflammation, mainly T-cell-mediated, is involved in the pathogenesis of low bone mass. Serum levels of pro-inflammatory cytokines IL-1β (34), and IL-6 were higher in patients with CD without treatment, and IL-6 was inversely correlated with BMD (34,35). GFD was able to ameliorate the pro-inflammatory profile, with reduction of IL-1β and IL-6 and increase of IL-1 receptor antagonist, an anti-inflammatory cytokine (34).
Besides, circulating levels of RANK-L and OPG were increased in patients on GFD, as well as in newly diagnosed CD cases who were not on GFD yet. However, the RANK-L/OPG ratio was increased only in those with newly diagnosed CD. BMD Z-score was negatively correlated with serum IL-6 levels and RANK-L/OPG ratio, but not correlated with PTH levels. Osteoclasts multiplication and activity were stimulated in vitro when osteoclasts precursors from healthy donors were incubated with serum of newly diagnosed CD patients, with a more robust effect in osteoclastogenesis increase. Serum from CD patients, on GFD or not, stimulated osteoblast proliferation and activity in cell cultures. However, only serum from patients not on GFD decreased the levels of OPG (35). These pieces of evidence demonstrate that the pathogenesis of bone loss in CD may not be restricted to malabsorption and calcium-vitamin D-PTH disturbances. Inflammation may explain why even patients without gastrointestinal symptoms have higher risk of osteoporosis. In those patients, gluten withdrawal is fundamental for reducing the inflammatory status.
Furthermore, CD is related to other disorders that contribute to osteoporosis development. About 10% of patients with type 1 diabetes mellitus (T1DM) present CD (12). T1DM is a well-known cause of osteoporosis (36). Among other disorders with high frequency in patients with CD, delayed puberty, hypogonadism, autoimmune thyroiditis, primary hyperparathyroidism can negatively influence bone mass (12).
Inflammatory bowel disease
The term IBD includes Crohn’s disease and ulcerative colitis (UC), which are characterized by relapsing or persistent inflammation mediated by a hyperactivation of T-cells. In Crohn’s disease, the inflammation is transmural and may affect the entire gastrointestinal tract, whilst in UC, the inflammation is restricted to the mucosal layer of the rectum and/or colon (37). The prevalence of osteoporosis in IBD ranges from 4-40% (38-41), depending on the population, disease activity and exposure to GC. It has been shown that the incidence of osteoporotic fractures was 40% higher in patients with IBD than in the general population, and 74% higher when specifically analyzing spinal fractures (42).
Use of GC, chronic inflammation, deficiencies of vitamin D, K and calcium due to malabsorption or inadequate dietary intake, hypogonadism and immobility, are among causes of low BMD in IBD.
GC use is the most frequent cause of secondary osteoporosis. Bone loss in patients exposed to therapeutic doses of GC is more intense in the first 6 months, leading to an incidence of osteoporosis in GC users of 50% in this period (43,44). BMD can decrease in up to 12% in the first year of GC treatment, with losses of 2-3% per year afterwards (45). GC stimulate bone resorption through osteoclasts proliferation, differentiation and stimulation, and inhibit bone formation by promoting apoptosis of osteoblasts and blocking their multiplication and function. GC indirectly affect the skeleton, diminishing the intestinal calcium absorption and increasing calciuria. Also, GC decrease muscle mass and suppress growth hormone and sex steroids, important to maintain the bone mass (45). About 35% of patients with IBD receive GC during the first year diagnosis (46). Thus, GC use is a relevant cause of osteoporosis in patients with IBD, especially for those on doses equivalent to prednisone >10 mg/d for >3 months (47).
As is observed in CD, hyperactivated immune cells in IBD may alter the RANK/RANK-L/OPG system, causing metabolic bone disease (48). Serum levels of sRANK-L and OPG were increased in patients with Crohn’s disease. Also, sRANK-L and OPG release was significantly enhanced in cultures of colon from Crohn’s disease patients. OPG in supernatant of cultures positively correlated with histological inflammation and levels of TNF-α, IL-1β, IL-6, an anti-inflammatory cytokine (IL-10) and a marker of tissue destruction (MMP3). The expression of RANK-L was higher in dendritic cells and in activated macrophages from the colonic mucosa of patients Crohn’s disease than of controls (48). In an IL-2 deficient mice model of colitis, abnormally activated T cells secreted sRANK-L at early stages of the colonic disease. As they aged, these mice presented progressive bone loss, with decrease in the levels of sRANK-L. Conversely, OPG levels raised later in the course of the bowel disease without reaching a plateau (49). In spite of the lack of studies that demonstrate the longitudinal correlation between bone disease, the OPG/RANK/RANK-L system, and IBD in humans, Moschen et al. showed results that support this mice model. Serum levels of OPG and OPG/sRANK-L ratio were significantly increased in patients with IBD, with the highest levels observed in Crohn’s disease cases. OPG was inversely correlated with femur and spine BMD. Lower sRANK-L levels were found in IBD patients treated with GC alone or in combination with azathioprine, than in IBD patients without specific drug treatment. To confirm the source of OPG, cytokines secretion from colonic explant cultures were analyzed. Inflamed colonic regions from patients with Crohn’s disease and UC presented a 3.4-fold and a 3.8-fold increase of OPG in the media, respectively. There were no differences between the production of OPG by non-inflamed areas of IBD patients and healthy controls. Macrophages and dendritic cells were the primary sources of OPG, while RANK-L was mainly produced by T-cells. Although high levels of OPG seem controversial in the pathogenesis of osteoporosis, the production of OPG may represent an attempt to cease the progression of bone loss (50). Indeed, exogenous recombinant OPG was able to reverse bone loss and decrease T lymphocyte-induced colonic inflammation in IL-2 deficient mice colitis model (49). Moreover, other cytokines may contribute to bone disease in IBD. Circulating IL-6 levels were increased in children with IBD and were inversely correlated with BMD (51).
Deficiencies of vitamin D, K and calcium have been reported in patients with IBD. Particularly in Crohn’s disease, malabsorption of nutrients and fat due to ileal resection or ileal active disease may contribute to bone disease (52). Up to 70% of patients with for Crohn’s disease and up to 45% of UC patients present vitamin D deficiency (53-57). In a Japanese population, 25OH-vitamin D levels were lower in patients with long time of disease and in those in which the disease was active for long periods (58). 25OH-vitamin D is essential for bone mineralization and has many other physiologic roles. Regarding osteoporosis, 25OH-vitamin improves lower limbs function and body balance, decrease falls (59) and prevent fractures (60). As discussed, 25OH-vitamin D deficiency can induce secondary hyperparathyroidism and increase bone turnover and resorption. In addition to calcium malabsorption, a large number of patients with IBD do not have an adequate intake of calcium (61,62). Furthermore, diarrhea may lead to magnesium deficiency contributing to calcium malabsorption. Although less studied, vitamin K sufficiency is also important for bone health. The prevalence of vitamin K deficiency in IBD patients was 31% in one study (63). Vitamin K induces bone formation and mineralization as well as accumulation of collagen into osteoblasts. Besides bone formation, vitamin K is able to increase the production of OPG and alkaline phosphatase by osteoblasts, and induces the carboxylation and activation of osteocalcin, a protein produced by osteoblasts involved in bone mineralization. In addition to bone formation, vitamin K can inhibit bone resorption and osteoclastogenesis. However, there is no enough clinical evidence to support the use of vitamin K to improve the bone mass and prevent fractures neither in IBD or in the general population (64).
BMD seems to improve after colectomy in patients with UC, probably through inflammatory reduction and less need of GC therapy (65-67), whereas in Crohn’s disease, the role of intestinal resections on osteoporosis is less clear (27,68).
It is controversial whether IBD per se is able to decrease the bone mass and increase fracture incidence in clinical studies. In a study with patients with Crohn’s disease, low BMD only correlated with GC therapy and disease activity (defined as levels of C-reactive protein) (69). In patients with UC and Crohn’s disease with a prevalence of 25% of vertebral fractures, BMD, disease activity, GC and other clinical risk were not associated with increased fracture risk (70). In addition, a study with a follow-up of patients with Crohn’s disease for 20 years, did not find increased risk of osteoporotic fractures. The only clinical factor related with overall risk of fractures was age. Exposure to GC and intestinal resections were not associated with higher risk of fractures (71). In contrast, Crohn’s disease was associated with osteoporosis after the control of GC use and other factors known for interfering in the bone mass in a population-based database of the province of Manitoba, Canada. However, UC was not related to increased risk of low BMD in this population (72). Also, the fracture risk was increased by 2.5-fold in women with Crohn’s disease, but no enhanced risk was observed in males with Crohn’s disease or patients with UC. The length of exposure to GC also determined a higher fracture risk in Crohn’s disease, but not in UC (73).
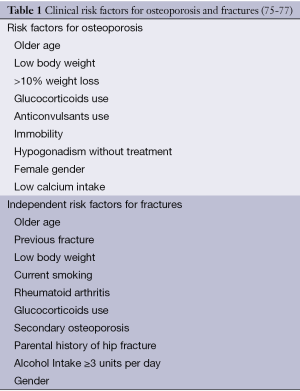
The 10-year fracture risk can be estimated in men and women older than 50 years from femoral neck BMD and clinical risk factors by the World Health Organization Fracture Risk Assessment tool (FRAX) (74). The inclusion of clinical risk factors independent associated with fracture risk (Table 1) can also predict the fracture risk without using BMD values (75). Analysing the Manitoba cohort, IBD was associated with 2-fold higher risk of femur fracture after controlling for FRAX fracture probability including the BMD or not. However, the risk of other osteoporotic fractures combined was related to low BMD and clinical factors, without differences determined by the presence or absence of IBD (78). Overall, these data suggest that IBD confers a small independent risk of fractures, probably more evident in patients with severe disease. The combination of other clinical factors may have a greater role in the pathogenesis of fractures in the IBD population.
Bariatric surgery with intestinal bypass
Surgeries that involve intestinal bypass were developed to produce malabsorption and weight loss. The most common procedure is the Roux-en-Y gastric bypass (RYGB). Among 60% to 70% of excess body weight is lost after RYGB. RYGB is a gastrojejunostomy and consists in the creation of a small gastric pouch attached to a transected jejunum that connects to a duodenal limb (79).
Data regarding the effects of biliopancreatic diversion with duodenal switch (BPDDS) on bone metabolism are scarce. In this surgical procedure, there is a partial vertical gastrectomy with preservation of the pylorus which is connected to an alimentary limb; and a long limb bypass with a portion of the duodenum attached to the pancreas and gallbladder is connected to a short common duct closer to the large intestine (80,81). The expected weight loss is 70-80% of the excess weight (82).
Many authors have shown BMD loss after bariatric surgery (83-85). In a RYGB prospective study with 1 year of follow-up, decreases of 9.2% of femoral neck BMD and of 8.0% at the total femur were observed. There was a strong correlation between BMD reduction and magnitude of weight loss. No changes occurred at spine and forearm BMD (86). In another study, the risk of fractures was studied in 258 patients from Olmsted County, Minnesota, who underwent bariatric surgery. The average follow-up was almost 9 years; patients were young, with a mean age of 44 years. The risk was 2-fold higher for osteoporotic fractures (forearm, vertebra and femur) and 2.3-fold higher for non-osteoporotic fractures at appendicular regions of the skeleton, in comparison with the general population. The estimated risk of any fractures in 10 years in individuals of this population was 35%. In addition, more than 50% of fractures occurred after 5 years of bariatric surgery (87).
Many causes play a role in the pathogenesis of metabolic bone disease after bariatric surgery. There are reports of increased bone remodelling after surgery. Studies have demonstrated elevated levels of markers for bone turnover in the post-operatory period, mainly of the bone resorption indicator N-telopeptide (86,88).The drastic and fast reduction of BMI leads to decreased mechanical load on the skeleton, an established cause for losing BMD and muscle mass (89,90). As explained, BMI has strong a correlation with BMD.
Calcium and 25OH-vitamin D absorption decrease early after the surgery (86) and seems to be worse in procedures with longer Roux limbs (91). Secondary hyperpathyroidism has been described in bariatric patients (92,93) especially if supplementation of calcium/25OH-vitamin D is inadequate. Indeed, those patients usually need higher doses of elemental calcium due to duodenal and proximal jejune bypass, and supra physiological doses of 25OH-vitamin D to avoid secondary hyperparathyroidism (94).
Irritable bowel syndrome
Few authors have investigated the association between irritable bowel syndrome (IBS) and risk of fractures. The risk of fractures was increased (OR =1.99, 95% CI, 1.24-3.19) in comparison with controls in a study that evaluated the medical records of patients with IBD from a health maintenance organization (95). Another study, which included more than 300,000 patients with IBS from the Nationwide Emergency Department Sample database showed a higher risk for osteoporosis (OR =4.28, 95% CI, 4.21-4.35) and fragility fractures (OR =4.28, 95% CI, 4.21-4.35) than patients without IBS (96). The authors discussed that a possible cause for osteoporosis in patients with IBS is the elevated levels of serotonin produced by the gastrointestinal tract. Serotonin may decrease bone formation mediated via LDL-receptor related protein 5 (LRP5) (97). In addition, elevated circulating levels of pro-inflammatory cytokines such as IL-6 and TNF-α have been demonstrated in patients with IBS (98,99). Besides, patients with IBS may avoid products with lactose because of gastrointestinal symptoms, limiting the intake of calcium (96).
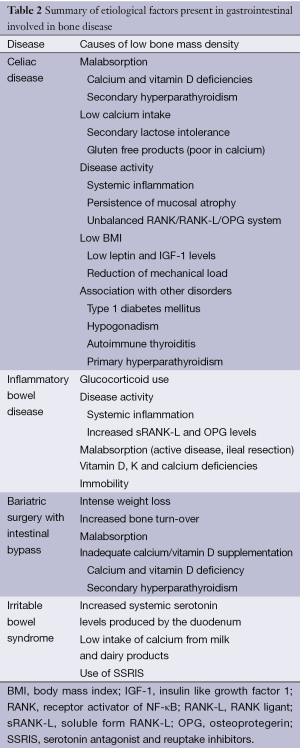
Table 2 summarizes the etiological factors for osteoporosis secondary to GID.
Full table
Screening
Celiac disease
There is no consensus among societies regarding measurement of DXA for osteoporosis screening in CD. The British Society of Gastroenterology recommends DXA for those at higher risk of osteoporosis, with 2 or more risk factors: poor adherence to GFD or persistence of symptoms on GFD for 1 year; weight loss >10%, BMI 70, or prior osteoporotic fracture (76).
According to the American Gastroenterological Association (AGA), adults with newly diagnosed CD should have the BMD evaluated with DXA after 1 year of GFD. In addition, the AGA recommends that levels of calcium, 25OH-vitamin D and PTH should be assessed in all newly diagnosed CD patients (41). If osteopenia or osteoporosis is identified, specific treatment for bone disease should be offered, as suggested by the guidelines (e.g., bisphosphonates) with regular follow-up. If BMD is abnormal without indication of treatment, its measurement should be repeated after three years. If BMD is normal, its measurement should be repeated at menopause in women, and after 55-year-old in males. Other authors recommend that all patients should have a DXA when CD is diagnosed (100). In men with osteoporosis, testosterone levels should be checked (41).
Inflammatory bowel disease
The AGA recommends that DXA scan should be performed for patients with one or more risk factors for osteoporosis (Table 1), which should be re-assessed in 2 to 3 years. If the patient is on high dose GC treatment, DXA scan should be repeated after 1 year. Serum levels of calcium should be evaluated in newly diagnosed patients. A broader investigation for secondary osteoporosis (blood count, total creatinine, 25OH-vitamin D level, protein electrophoresis, and testosterone level) should be done in those with osteoporosis or a previous osteoporotic fracture (41). The British Society suggests that DXA scan should be performed in patients at higher osteoporosis risk, with at least 2 risk factors (Table 1). Other authors discuss the use of the FRAX calculator to estimate the probability of fractures and to recommend the initiation of treatment (46).
Bariatric surgery
For both RYGB and BPDDS, DXA scan is recommended at 2 years after surgery. There is not enough evidence to indicate DXA scan at baseline for all patients, and the recommendation for osteoporosis screening should follow the recommendations of the National Osteoporosis Foundation (www.nof.org). The measurement of 25OH-vitamin D in the preoperatory period is advised, and a more extensive investigation, including calcium, albumin, phosphate and PTH in individual patients, is suggested for those at higher risk. During the routine follow-up, 25OH-vitamin D, iPTH and calcium should be regularly assessed. It is recommended to evaluate 24-hour urinary calcium excretion at 6 months after the surgery, and then annually (94).
Irritable bowel disease
There is not enough evidence to suggest routine evaluation of BMD and serum levels of 25OH-vitamin D in patients with IBS. The prevention of fractures should follow the standards for the general population.
Treatment and prevention of metabolic bone diseases
Celiac disease
GFD is the main treatment for bone disease associated with CD. It improves bone mass, but may not normalize BMD in all patients. Response depends on when the GFD is implemented. Patients with untreated CD in the two first decades of life may not reach their bone mass peak. In this situation, bone mass is unlikely to recover completely. In adults, particularly in postmenopausal women and men >55 years, BMD response will be related to the magnitude of bone damage at the diagnosis. BMD increases in an average of 5% in the first year of GFD, and is more evident in axial than appendicular skeleton. The increment of BMD will be lower in the subsequent years (77). It is important to emphasize that the reduction of the fracture risk in patients following the GFD is not only due to the BMD increase, but also because the overall nutritional status, body composition, BMI, and bone architecture are improved, and risk of falls is decreased (18).
Celiac disease and IBD
It is recommended a minimum intake of elemental calcium of 1,000 mg per day, and up to 1,200-1,500 mg per day for postmenopausal women and men >55 years. The intake of 25OH-vitamin should be 400-800 IU per day. Higher doses should be prescribed if necessary to maintain the 25OH-vitamin D levels >25 ng/mL (41,76), especially in those with fat malabsorption. General recommendations of weight bearing exercise, alcohol and tobacco avoidance should be enforced. In patients with osteoporosis at high risk of fractures (e.g., for patients who will be on GC treatment for ≥3 months) or with a previous fracture, treatment with bisphophonates (e.g., alendronate, risedronate), raloxifene or teriparatide should be prescribed. In men with osteoporosis and hypogonadism, testosterone should be offered (41). In patients with Crohn’s disease, it is advised to avoid oral GC and initiate alternative medications less aggressive for bone such as anti-TNFα and azathioprine if possible (41,76).
Bariatric surgery
All patients submitted to RYGB should receive calcium citrate, 1,200-1,500 mg/d early after the surgery. Citrate is the first choice because of hypocloridria caused by gastrectomy. All patients who had BPDDS and RYGB should be prescribed at least 3,000 UI/d of vitamin D. The dose should be titrated to maintain serum levels >30 ng/mL. The aim of calcium and 25OH-vitamin D supplementation is to avoid secondary hyperparathyroidism without inducing hypercalciuria (94).
In patients with osteoporosis or atraumatic fracture with calcium/vitamin sufficiency, bisphosphonates should be offered. The best options are intravenous bisphosphonates (zoledronate and ibandronate) since the absorption of oral bisphosphonates is impaired with hypocloridria. Also, oral bisphosphonates may cause anastomotic ulceration (94).
Conclusions
GID can lead to significant impact on bone health and are frequent causes of secondary osteoporosis. Normal intestinal function is essential for an adequate calcium metabolism. Malabsorption of calcium and vitamin D with consequent secondary hyperparathyroidism may occur in patients with CD, Crohn’s disease and bariatric surgery. Low calcium intake due to avoidance of milk and dairy products is also common in patients with GID, not only in those with malabsorption, but also in patients with disorders associated with chronic abdominal pain, altered bowel habits and abdominal bloating such as IBS and colitis ulcerative. Calcium and vitamin D status should be analysed and supplementation ensured when necessary. Use of GC, weight loss, disease activity, systemic inflammation and immobility present in patients with GID also play roles in the pathogenesis of bone disease.
There is not enough evidence to determine the independent risk of each GID for fractures. However, patients with GID frequently present multiple risk factors for bone disease. Since osteoporosis in general progresses slowly and bone loss is not always completely reverted with treatment, a high level of suspicion is required to prevent advanced bone loss and fractures. Thus, prompt evaluation and diagnosis are important for patients at risk of bone disease. Control of the primary GID, reinforcement of a healthy lifestyle and prescription of drugs which negatively affect bone metabolism with parsimony are good strategies to prevent bone disease. In the future, the validation of diagnostic tools, such as FRAX, in subgroups with specific GID will optimise the clinical judgement and indication of osteoporosis treatment, especially for patients who are not clearly at high risk of osteoporosis and fractures.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001;285:785-95. [PubMed]
- Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville: Department of Health and Human Services, 2004.
- Painter SE, Kleerekoper M, Camacho PM. Secondary osteoporosis: a review of the recent evidence. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists 2006;12:436-45.
- Seeman E. Pathogenesis of bone fragility in women and men. Lancet 2002;359:1841-50. [PubMed]
- WHO Scientific Group on the Assessment of Osteoporosis at Primary Health Care Level. Summary Meeting Report. Brussels, Belgium. 2004.
- Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996;312:1254-9. [PubMed]
- Seeman E, Delmas PD. Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med 2006;354:2250-61. [PubMed]
- Parfitt AM. The cellular basis of bone remodeling: the quantum concept reexamined in light of recent advances in the cell biology of bone. Calcif Tissue Int 1984;36:S37-S45. [PubMed]
- Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature 2003;423:337-42. [PubMed]
- Hardy R, Cooper M. Bone loss in inflammatory disorders. J Endocrinol 2009;201:309-20. [PubMed]
- Gilbert L, He X, Farmer P, et al. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology 2000;141:3956-64. [PubMed]
- Guandalini S, Assiri A. Celiac disease: a review. JAMA Pediatr 2014;168:272-8. [PubMed]
- Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology 2009;137:88-93. [PubMed]
- Catassi C, Kryszak D, Bhatti B, et al. Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Ann Med 2010;42:530-8. [PubMed]
- Mustalahti K, Catassi C, Reunanen A, et al. The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med 2010;42:587-95. [PubMed]
- Meyer D, Stavropolous S, Diamond B, et al. Osteoporosis in a North American adult population with celiac disease. Am J Gastroenterol 2001;96:112-9. [PubMed]
- Delcò F, El-Serag HB, Sonnenberg A. Celiac Sprue Among US Military Veterans (Associated Disorders and Clinical Manifestations). Dig Dis Sci 1999;44:966-72. [PubMed]
- Jafri MR, Nordstrom CW, Murray JA, et al. Long-term Fracture Risk in Patients with Celiac Disease: A Population-Based Study in Olmsted County, Minnesota. Dig Dis Sci 2008;53:964-71. [PubMed]
- Vilppula A, Kaukinen K, Luostarinen L, et al. Clinical benefit of gluten-free diet in screen-detected older celiac disease patients. BMC Gastroenterol 2011;11:136. [PubMed]
- Sategna-Guidetti C, Grosso SB, Grosso S, et al. The effects of 1-year gluten withdrawal on bone mass, bone metabolism and nutritional status in newly-diagnosed adult coeliac disease patients. Aliment Pharmacol Ther 2000;14:35-43. [PubMed]
- Moreno ML, Vazquez H, Mazure R, et al. Stratification of bone fracture risk in patients with celiac disease. Clin Gastroenterol Hepatol 2004;2:127-34. [PubMed]
- Ludvigsson JF, Michaelsson K, Ekbom A, et al. Coeliac disease and the risk of fractures-a general population-based cohort study. Aliment Pharmacol Ther 2007;25:273-85. [PubMed]
- Olmos M, Antelo M, Vazquez H, et al. Systematic review and meta-analysis of observational studies on the prevalence of fractures in coeliac disease. Dig Liver Dis 2008;40:46-53. [PubMed]
- Davie MW, Gaywood I, George E, et al. Excess non-spine fractures in women over 50 years with celiac disease: a cross-sectional, questionnaire-based study. Osteoporos Int 2005;16:1150-5. [PubMed]
- West J, Logan RF, Card TR, et al. Fracture risk in people with celiac disease: a population-based cohort study. Gastroenterology 2003;125:429-36. [PubMed]
- Thomason K, West J, Logan RF, et al. Fracture experience of patients with coeliac disease: a population based survey. Gut 2003;52:518-22. [PubMed]
- Vestergaard P, Mosekilde L. Fracture risk in patients with celiac disease, Crohn’s disease, and ulcerative colitis: a nationwide follow-up study of 16,416 patients in Denmark. Am J Epidemiol 2002;156:1-10. [PubMed]
- Staun M, Jarnum S. Measurement of the 10,000-molecular weight calcium-binding protein in small-intestinal biopsy specimens from patients with malabsorption syndromes. Scand J Gastroenterol 1988;23:827-32. [PubMed]
- Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266-81. [PubMed]
- Di Stefano M, Veneto G, Corrao G, et al. Role of lifestyle factors in the pathogenesis of osteopenia in adult coeliac disease: a multivariate analysis. Eur J Gastroenterol Hepatol 2000;12:1195-9. [PubMed]
- De Laet C, Kanis JA, Odén A, et al. Body mass index as a predictor of fracture risk: A meta-analysis. Osteoporos Int 2005;16:1330-8. [PubMed]
- Duerksen DR, Leslie WD. Longitudinal Evaluation of Bone Mineral Density and Body Composition in Patients With Positive Celiac Serology. J Clin Densitom 2011;14:478-83. [PubMed]
- Lebwohl B, Michaëlsson K, Green PH, et al. Persistent mucosal damage and risk of fracture in celiac disease. J Clin Endocrinol Metab 2014;99:609-16. [PubMed]
- Fornari MC, Pedreira S, Niveloni S, et al. Pre- and post-treatment serum levels of cytokines IL-1beta, IL-6, and IL-1 receptor antagonist in celiac disease. Are they related to the associated osteopenia? Am J Gastroenterol 1998;93:413-8. [PubMed]
- Taranta A, Fortunati D, Longo M, et al. Imbalance of osteoclastogenesis-regulating factors in patients with celiac disease. J Bone Miner Res 2004;19:1112-21. [PubMed]
- Vestergaard P. Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes—a meta-analysis. Osteoporos Int 2007;18:427-44. [PubMed]
- Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19 Suppl A:5A-36A.
- Leslie WD, Miller N, Rogala L, et al. Body mass and composition affect bone density in recently diagnosed inflammatory bowel disease: the Manitoba IBD Cohort Study. Inflamm Bowel Dis 2009;15:39-46. [PubMed]
- Bjarnason I, Macpherson A, Mackintosh C, et al. Reduced bone density in patients with inflammatory bowel disease. Gut 1997;40:228-33. [PubMed]
- Compston JE, Judd D, Crawley EO, et al. Osteoporosis in patients with inflammatory bowel disease. Gut 1987;28:410-5. [PubMed]
- Bernstein CN, Leslie WD, Leboff MS. AGA technical review on osteoporosis in gastrointestinal diseases. Gastroenterology 2003;124:795-841. [PubMed]
- Bernstein CN, Blanchard JF, Leslie W, et al. The incidence of fracture among patients with inflammatory bowel disease. A population-based cohort study. Ann Intern Med 2000;133:795-9. [PubMed]
- Epstein S, Inzerillo AM, Caminis J, et al. Disorders associated with acute rapid and severe bone loss. J Bone Miner Res 2003;18:2083-94. [PubMed]
- Mazziotti G, Canalis E, Giustina A. Drug-induced osteoporosis: mechanisms and clinical implications. Am J Med 2010;123:877-84. [PubMed]
- Henneicke H, Gasparini SJ, Brennan-Speranza TC, et al. Glucocorticoids and bone: local effects and systemic implications. Trends Endocrinol Metab 2014;25:197-211. [PubMed]
- Targownik LE, Bernstein CN, Leslie WD. Inflammatory bowel disease and the risk of osteoporosis and fracture. Maturitas 2013;76:315-9. [PubMed]
- Azzopardi N, Ellul P. Risk factors for osteoporosis in crohn’s disease: infliximab, corticosteroids, body mass index, and age of onset. Inflamm Bowel Dis 2013;19:1173-8. [PubMed]
- Franchimont N, Reenaers C, Lambert C, et al. Increased expression of receptor activator of NF-C ligand (RANKL), its receptor RANK and its decoy receptor osteoprotegerin in the colon of Crohn’s disease patients. Clin Exp Immunol 2004;138:491-8. [PubMed]
- Ashcroft AJ, Cruickshank SM, Croucher PI, et al. Colonic dendritic cells, intestinal inflammation, and T cell-mediated bone destruction are modulated by recombinant osteoprotegerin. Immunity 2003;19:849-61. [PubMed]
- Moschen AR, Kaser A, Enrich B, et al. The RANKL/OPG system is activated in inflammatory bowel disease and relates to the state of bone loss. Gut 2005;54:479-87. [PubMed]
- Paganelli M, Albanese C, Borrelli O, et al. Inflammation is the main determinant of low bone mineral density in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2007;13:416-23. [PubMed]
- Stein E, Shane E. Secondary osteoporosis. Endocrinol Metab Clin North Am 2003;32:115-34. [PubMed]
- Haderslev KV, Jeppesen P, Sorensen H, et al. Vitamin D status and measurements of markers of bone metabolism in patients with small intestinal resection. Gut 2003;52:653-8. [PubMed]
- Gilman J, Shanahan F, Cashman K. Determinants of vitamin D status in adult Crohn’s disease patients, with particular emphasis on supplemental vitamin D use. Eur J Clin Nutr 2006;60:889-96. [PubMed]
- Leslie WD, Miller N, Rogala L, Bernstein CN. Vitamin D status and bone density in recently diagnosed inflammatory bowel disease: the Manitoba IBD Cohort Study. Am J Gastroenterol 2008;103:1451-9. [PubMed]
- Siffledeen JS, Siminoski K, Steinhart H, et al. The frequency of vitamin D deficiency in adults with Crohn’s disease. Can J Gastroenterol 2003;17:473-8. [PubMed]
- Sentongo TA, Semaeo EJ, Stettler N, et al. Vitamin D status in children, adolescents, and young adults with Crohn disease. Am J Clin Nutr 2002;76:1077-81. [PubMed]
- Tajika M, Matsuura A, Nakamura T, et al. Risk factors for vitamin D deficiency in patients with Crohn’s disease. J Gastroenterol 2004;39:527-33. [PubMed]
- Boyé NDA, Oudshoorn C, Van Der Velde N, et al. Vitamin D and physical performance in older men and women visiting the emergency department because of a fall: Data from the improving medication prescribing to reduce risk of FALLs (IMPROveFALL) study. J Am Geriatr Soc 2013;61:1948-52. [PubMed]
- Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. JAMA 2005;293:2257-64. [PubMed]
- Vagianos K, Bector S, McConnell J, et al. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr 2007;31:311-9. [PubMed]
- Filippi J, Al-Jaouni R, Wiroth JB, et al. Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm Bowel Dis 2006;12:185-91. [PubMed]
- Krasinski SD, Russell R, Furie B, et al. The prevalence of vitamin K deficiency in chronic gastrointestinal disorders. Am J Clin Nutr 1985;41:639-43. [PubMed]
- Kuwabara A, Tanaka K, Tsugawa N, et al. High prevalence of vitamin K and D deficiency and decreased BMD in inflammatory bowel disease. Osteoporos Int 2009;20:935-42. [PubMed]
- Abitbol V, Roux C, Guillemant S, et al. Bone assessment in patients with ileal pouch–anal anastomosis for inflammatory bowel disease. Br J Surg 1997;84:1551-4. [PubMed]
- Kuisma J, Luukkonen P, Järvinen H, et al. Risk of osteopenia after proctocolectomy and ileal pouch-anal anastomosis for ulcerative colitis. Scand J Gastroenterol 2002;37:171-6. [PubMed]
- van Staa TP, Cooper C, Brusse LS, et al. Inflammatory bowel disease and the risk of fracture. Gastroenterology 2003;125:1591-7. [PubMed]
- Frei P, Fried M, Hungerbuhler V, et al. Analysis of risk factors for low bone mineral density in inflammatory bowel disease. Digestion 2006;73:40-6. [PubMed]
- Jahnsen J, Falch JA, Mowinckel P, et al. Bone Mineral Density in Patients with Inflammatory Bowel Disease: A Population-Based Prospective Two-Year Follow-Up Study. Scand J Gastroenterol 2004;39:145-53. [PubMed]
- Heijckmann AC, Huijberts MSP, Schoon EJ, et al. High prevalence of morphometric vertebral deformities in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol 2008;20:740-7. [PubMed]
- Loftus EV Jr, Crowson CS, Sandborn WJ, et al. Long-term fracture risk in patients with Crohn’s disease: a population-based study in Olmsted County, Minnesota. Gastroenterology 2002;123:468-75. [PubMed]
- Targownik LE, Bernstein CN, Nugent Z, et al. Inflammatory bowel disease has a small effect on bone mineral density and risk for osteoporosis. Clin Gastroenterol Hepatol 2013;11:278-85. [PubMed]
- Vestergaard P, Krogh K, Rejnmark L, et al. Fracture risk is increased in Crohn’s disease, but not in ulcerative colitis. Gut 2000;46:176-81. [PubMed]
- Kanis JA, Oden A, Johnell O, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int 2007;18:1033-46. [PubMed]
- Kanis JA, McCloskey E, Johansson H, et al. FRAX® with and without Bone Mineral Density. Calcif Tissue Int 2012;90:1-13. [PubMed]
- Lewis N, Scott B. Guidelines for Osteoporosis in Inflammatory Bowel Disease and Coeliac Disease 2007; Available online: http://www.bsg.org.uk/clinical-guidelines/ibd/guidelines-for-osteoporosis-in-inflammatory-bowel-disease-and-coeliac-disease.html.
- American Gastroenterological Association medical position statement: Guidelines on osteoporosis in gastrointestinal diseases. Gastroenterology 2003;124:791-4. [PubMed]
- Targownik LE, Bernstein CN, Nugent Z, et al. Inflammatory bowel disease and the risk of fracture after controlling for FRAX. J Bone Miner Res 2013;28:1007-13. [PubMed]
- Schauer PR, Ikramuddin S, Gourash W, et al. Outcomes after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Ann Surg 2000;232:515. [PubMed]
- Marceau P, Hould FS, Simard S, et al. Biliopancreatic diversion with duodenal switch. World J Surg 1998;22:947-54. [PubMed]
- Hess DS, Hess DW. Biliopancreatic diversion with a duodenal switch. Obes Surg 1998;8:267-82. [PubMed]
- Nelson DW, Blair KS, Martin MJ. Analysis of obesity-related outcomes and bariatric failure rates with the duodenal switch vs gastric bypass for morbid obesity. Arch Surg 2012;147:847-54. [PubMed]
- Valderas JP, Velasco S, Solari S, et al. Increase of bone resorption and the parathyroid hormone in postmenopausal women in the long-term after Roux-en-Y gastric bypass. Obes Surg 2009;19:1132-8. [PubMed]
- Vilarrasa N, De Gordejuela AGR, Gómez-Vaquero C, et al. Effect of bariatric surgery on bone mineral density: Comparison of gastric bypass and sleeve gastrectomy. Obes Surg 2013;23:2086-91. [PubMed]
- Carrasco F, Ruz M, Rojas P, et al. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg 2009;19:41-6. [PubMed]
- Fleischer J, Stein E, Bessler M, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab 2008;93:3735-40. [PubMed]
- Nakamura KM, Haglind EGC, Clowes JA, et al. Fracture risk following bariatric surgery: A population-based study. Osteoporos Int 2014;25:151-8. [PubMed]
- Coates PS, Fernstrom JD, Fernstrom MH, et al. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab 2004;89:1061-5. [PubMed]
- Suva LJ, Gaddy D, Perrien DS, et al. Regulation of bone mass by mechanical loading: microarchitecture and genetics. Curr Osteoporos Rep 2005;3:46-51. [PubMed]
- Huiskes R, Ruimerman R, Van Lenthe GH, et al. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature 2000;405:704-6. [PubMed]
- Ott MT, Fanti P, Malluche HH, et al. Biochemical Evidence of Metabolic Bone Disease in Women Following Roux-Y Gastric Bypass for Morbid Obesity. Obes Surg 1992;2:341-8. [PubMed]
- Hamoui N, Kim K, Anthone G. The significance of elevated levels of parathyroid hormone in patients with morbid obesity before and after bariatric surgery. Arch Surg 2003;138:891-7. [PubMed]
- Hamoui N, Anthone G, Crookes PF. Calcium metabolism in the morbidly obese. Obes Surg 2004;14:9-12. [PubMed]
- Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient-2013 update: Cosponsored by American association of clinical endocrinologists, the obesity society, and American society for metabolic & bariatric surgery. Obesity 2013;21:S1-S27. [PubMed]
- Whitehead WE, Palsson OS, Levy RR, et al. Comorbidity in irritable bowel syndrome. Am J Gastroenterol 2007;102:2767-76. [PubMed]
- Stobaugh DJ, Deepak P, Ehrenpreis ED. Increased risk of osteoporosis-related fractures in patients with irritable bowel syndrome. Osteoporos Int 2013;24:1169-75. [PubMed]
- Yadav VK, Ryu JH, Suda N, et al. Lrp5 Controls Bone Formation by Inhibiting Serotonin Synthesis in the Duodenum. Cell 2008;135:825-37. [PubMed]
- Dinan TG, Quigley EM, Ahmed SM, et al. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: plasma cytokines as a potential biomarker? Gastroenterology 2006;130:304-11. [PubMed]
- Dinan TG, Clarke G, Quigley EM, et al. Enhanced cholinergic-mediated increase in the pro-inflammatory cytokine IL-6 in irritable bowel syndrome: role of muscarinic receptors. Am J Gastroenterol 2008;103:2570-6. [PubMed]
- Napier C, Pearce SHS. How should I approach standard endocrine evaluation in patients with coeliac disease? Clin Endocrinol (Oxf) 2013;79:464-7. [PubMed]