Acromegaly and colorectal cancer
Introduction
Acromegalic patients are exposed to chronic growth hormone (GH) hypersecretion mostly associated to pituitary adenomas (1,2). The disease has a subclinical course, and the delay on diagnosis is associated with high morbidity and with premature mortality related to increased cardiovascular risk, sleep apnea, metabolic comorbidities, and cancer (3-5).
Overall and cancer mortality in acromegaly have been shown to correlate with the degree of GH control. Several studies have suggested increased risk of colon cancer and polyps in acromegalic patients (6-9). Prospective studies using colonoscopy showed a three times higher prevalence of intestinal polyps and up to four times increased presence of colorectal cancer (CRC) in acromegaly than in normal controls, independently of sex, age, duration of disease and clinical status of the patients (8). Data from registry-based cohorts in Europe showed increased risks for digestive system cancers [standardized incidence ratio (SIR) =2.1, 95% CI, 1.6-2.7), notably of the small intestine (SIR =6.0, 95% CI, 1.2-17.4), colon (SIR =2.6, 95% CI, 1.6-3.8), and rectum (SIR =2.5, 95% CI, 1.3-4.2) (10).
CRC is one of the most prevalent malignancy worldwide (11) and among patients with acromegaly (7-10), in whom it implies a mortality rate higher than that expected for the general population (6). The main objective of the current article is to review the most relevant aspects concerning prevalence, pathogenesis and screening of CRC in acromegalic patients.
Pathogenesis of colorectal cancer (CRC) in acromegaly
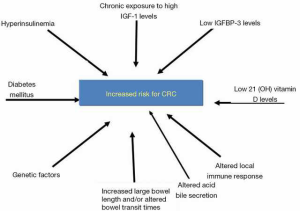
The mechanisms involved in cancer initiation in acromegalic patients remain unclear. Several hypothesis have been investigated and they may be related to sustained increase of GH and insulin-like growth factor 1 (IGF-1) levels, metabolic disorders, and genetic factors (7,8,12) (Figure 1).
GH-IGF-1 axis
Overall and cancer mortality in acromegaly have been shown to correlate with the degree of GH control (2,6,7). Some authors have described increased risk for benign and malignant tumors in digestive tract in acromegalics, and they found that the odds ratio for the presence of hyperplastic polyps was 8.3, for adenomas 4.2 and for colon carcinomas 9.8, showing an association with higher serum GH levels (12).
A recent study performed in a cohort of Japanese acromegalic patients has shown that increased mean area under the curve (AUC) for GH was associated with an increased risk for colon adenocarcinomas (13).
An attractive explanation for the increased risk of CRC in acromegaly has been the link to IGF-1. Plasma GH triggers the production of IGF-1 from the liver, which in turn stimulates the growth of organs and tissues, through its known mitogenic and antiapoptotic properties. Any imbalance in the tight control between epithelial cell turn-over and cell death could result in epithelial hyperproliferation, promoting the formation of hyperplastic polyps and colorectal adenomas (14). IGF-1 receptors, as well as IGF-1 mRNA, have been identified in human CRC cell lines (15).
IGF-1 can stimulate growth of CRC cells in vitro, whereas the blockade of its effect by the alpha IR3, a neutralizing monoclonal antibody against the human IGF-1 receptor, inhibits cell growth in the same model (15,16). Cats et al. (17) reported that patients with acromegaly had an increased proliferation index of colonic epithelium proportional to their circulating IGF-1 levels. A recent study found that elevated levels of serum IGF-1 are associated with increased proliferation in the superficial crypt cells and stronger immunostaining to Ki67 in colonic epithelial cells (18). These results suggest that colonic neoplasia in acromegaly would result from increased proliferation rather than deceased apoptosis (18).
The role of chronic exposure to elevated IGF-1 levels and cancer development involves several hypotheses. Cohen et al. (19) discussed three important mechanisms. First, an effect of IGF-1 causing symptomatic benign tissue hyperplasia may result in an ascertainment bias leading to an initiation of procedures resulting in the diagnosis of asymptomatic cancers. Second, elevated serum IGF-1 in cancer patients may originate within the tumor (as suggested by some animal studies). Thirdly, serum IGF-1 may actually be a surrogate marker of tissue IGF-1 levels or of nutritional factors, which are not under GH control and may be involved in cancer initiation (19).
Studies on the IGF-1 signal transduction pathways have suggested that the IGF-1 receptor and the activation of tyrosine kinase may be a potential substrate for steroid receptor coactivator (SRC) oncogenes and may be associated to the mechanisms of dedifferentiation (15,18,19).
IGF binding protein-3 (IGFBP-3) regulates the bioavailability of IGF-1 an IGF-2 and has both anti-proliferative and pro-apoptotic properties (7,20). Elevated plasma IGFBP-3 has therefore been associated with reduced risk of CRC. By contrast, excess GH causes an elevated IGF-I to IGFBP-3 ratio, which is expected to increase cancer risk (15,20). Increased circulating levels of IGF-2 and IGFBP-2 are also believed to play a role in the pathogenesis of colonic neoplasms in acromegaly (15,16).
Metabolic disorders
Factors such as hyperinsulinaemia, diabetes mellitus, altered acid bile secretion, altered local immune response, increased large bowel length and/or altered bowel transit times could also contribute to an adenoma occurrence/recurrence in patients with acromegaly (7,15).
In vivo experimental studies (21,22) demonstrated growth-promoting effects of exogenous insulin, dietary-induced hyperinsulinemia, and hypertriglyceridemia on colon cancer and aberrant crypt foci, a putative precursor of colon cancer. Moreover, insulin has been shown to increase the growth of colon epithelial and carcinoma cells in vitro (23).
It has also been suggested that insulin may promote colorectal carcinogenesis directly by activating its own receptor, the receptors for IGF-1, or hybrid insulin/IGF-1 receptors (24), all of which are expressed by colorectal epithelial and carcinoma cells (25). In addition, chronic hyperinsulinemia may indirectly promote colorectal carcinogenesis by inducing pathophysiologic changes in concentrations of circulating IGF-1 and IGF binding proteins (IGFBPs) (20,26).
Recent prospective observational studies (27,28) have shown that colorectal adenomas and cancer are positively, albeit moderately, associated with type 2 diabetes. Accordingly, there have been some reports that hyperglycemia is associated with an increased risk of CRC (29,30). These results have led to the suggestion that hyperinsulinemia might underlie the link between type 2 diabetes and CRC (7,15). Indeed, both cross-sectional and prospective population studies have found that CRC is more common in people with hyperinsulinemia and its metabolic correlates, including type 2 diabetes and hypertriglyceridemia (27,28). The study by Colao et al. (31) suggested that increase in fasting insulin levels is associated with an 8.6- to 14.8-fold increased risk of presenting with colonic adenomas in acromegaly. Diabetes or impaired glucose tolerance was also a risk factor for the development of colonic lesions (31). However, in another Italian study, fasting insulin, 25(OH)-D3, folate, and homocysteine levels did not differ in acromegaly patients with or without colonic adenomas (32).
Epidemiological studies have revealed that low serum 25(OH) D levels, i.e., vitamin D deficiency/insufficiency, are associated with higher incidence in colon cancer, which is associated with poor prognosis (33,34). The protective role of vitamin D3 against cancer has been attributed to its influence of on cell proliferation, differentiation, apoptosis, DNA repair mechanisms, inflammation and immune function (33,34). However, clinical studies so far have not demonstrated any effects of vitamin D supplementation on cancer incidence or prognosis (11,33).
Genetic factors
The investigation of cancer-related proteins may identify protein biomarkers or therapeutic targets. A recent study described the proteogenomic characterization of human colon and rectal cancers, and highlighted potential candidates at chromosome 20, including HNF4A (hepatocyte nuclear factor 4), TOMM34 (translocase of outer mitochondrial membrane 34) and SRC proto-oncogene (35).
Other authors have suggested the association of CRC and chromosomal instability and using SNP microarrays (36).
In acromegalic patients, few studies have been performed to associate polymorphisms or gene mutations and colorectal tumors (CRT). A recent study evaluated the polymorphism of C677T in methylenetetrahydrofolate reductase (MTHFR) gene, which is a well-documented risk factor for CRT in the general population. It was found that patients with TT genotype showed a 2.4 higher odd ration for CRT (95% CI, 0.484-11.891; P = NS) than C-allele carriers among patients with low plasma folate levels (37).
The association of the Ser326Cys polymorphism in the 8-oxoguanine glycosylase (OGG1) gene with a colon carcinoma and diabetes mellitus has been examined and results suggest that the Cys allele may influence the colon polyp risk in acromegalic patients (38).
Germline mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene, known to be a tumor suppressor gene, are related to pituitary adenoma predisposition, and may be involved in the pathogenesis of prolactin (PRL) or GH over secreting pituitary adenomas (39). However, somatic AIP mutations are not common in colorectal, breast, and prostate cancers (40). Indeed, among the 52 CRCs samples initially screened, a heterozygous missense change, R16H (47G > A) in exon 1 was detected in two samples (40).
Potential genetic disorders involved in the occurrence of CRC and polyps in patients with acromegaly are summarized in Table 1.

Full table
Epidemiologic findings
Acromegalic patients may be at an increased risk for malignancies in several systems including the thyroid, digestive tract, brain, kidney, breast and prostate (8,26-30). CRC incidence (2,6-10,41-44) and mortality rates (6,7,43) have been reported to be higher in acromegalics than expected. However, reported relative risks of CRC vary significantly depending on the study population and the study design. Moreover, the reported higher indices of colorectal neoplasia in acromegalics have not been a universal finding (45-47).
Several studies have demonstrated that in acromegalic patients there is a considerable incidence of colonic neoplasms, included the CRC (48-56). Among the first studies published on these topic, two may be highlighted. The first one found an incidence of colonic neoplasms of 41%, among which 29% of adenomatous polyps and 12% of CRC (48). Ituarte et al. (49) demonstrated that in a total of 33 acromegalics, 12 were submitted to a colonoscopy. Among the colonoscopy findings, three patients presented adenomatous polyps and three had colon cancer (49).
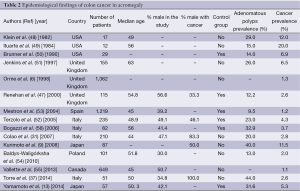
These alarming results raised various posterior studies about the incidence of the colonic neoplasia in acromegaly (6,13,31,37,47,50-56). These findings are summarized in Table 2. Overall, the prevalence of CRC ranged from 1.07% to 20%. In the series by Renehan et al. (47), of the 115 patients with complete examinations, adenocarcinomas were discovered in 3 (2.6%), and at least 1 adenoma was found in 11, giving an overall prevalence of neoplasia of 12% (14 of 115). Prevalence rates for age bands 30-40, 40-49, 50-59, 60-69, and 70+ yr were 0%, 8%, 12%, 20%, and 21%, respectively. Compared with the two control models, the prevalence of occult CRC was not significantly increased (acromegalics vs. models 1 and 2, 2.6% vs. 2.3% and 0.9%), nor was there an increase in the prevalence of adenomas in any age band. Pathological characteristics showed some differences, in that adenomas in acromegalics tended to be right sided (68% vs. 57% and 56%), larger (for ≥10 mm, 27% vs. 13% and 9%), and of advanced histology (for tubulovillous, 27% vs. 4% and 22%) (47).
Full table
A retrospective chart analysis was performed on 140 patients with active acromegaly who had attended a single Japanese institute and confirmed that patients with acromegaly have an increased risk of colon cancer and polyps (9). Indeed, colon cancer was found in 10 patients, thyroid cancer in 5, breast cancer in 4 and gastric cancer in 2. When compared with the local population, the SIRs for thyroid cancer in patients with acromegaly were 61.74 [95% confidence interval (CI), 0.51-114.63] for females and 272.4 (95% CI, 29.12-876.71) for males. The SIRs for colon cancer in the acromegalic patients were 17.4 (95% CI, 4.74-44.55) for females and 19.0 (95% CI, 5.18-48.64) for male patients in comparison with the local population. Of the benign tumors, multinodular goiter and colonic, gastric and gallbladder polyps were observed in 57% (47/83), 40% (35/87), 23% (10/43), and 14% (11/77) of the patients, respectively (9).
In the meta-analysis done by Rokkas et al. (57), data from 701 patients with acromegaly and 1,573 controls were gathered. The pooled results of this study clearly showed that acromegalic patients are at a significantly increased risk of developing colorectal adenomatous and hyperplastic polyps as well as CRC compared with controls. They also highlighted the true increased prevalence of colon cancer compared to adenoma in acromegaly (57). These findings might support the hypothesis of an increased risk of malignant transformation in acromegaly.
Analysis of prospective colonoscopic screening studies involving almost 700 subjects with acromegaly has shown a 2.4-fold increased risk of colonic adenomas and a 7.4-fold greater risk of cancer with an overall prevalence of CRC of 3.7% (58,59).
Among consecutive 57 acromegalic patients who had undergone full-length colonoscopy at the time of diagnosis, 22 (38.6%), 18 (31.6%) and 3 (5.3%) patients were diagnosed with hyperplastic polyps, adenomas, and adenocarcinomas, respectively and the prevalence was significantly higher than in a historical control group, Chinese patients with irritable bowel syndrome (the odds ratio was 4.0, 8.7, and 17.5, respectively) (13). The prevalence of adenocarcinomas was also significantly higher in these patients than in the general Japanese population (odds ratio 14.5). Patients with acromegaly who had colorectal neoplasms had longer disease duration than those without colorectal neoplasms (13).
In the study by Wassenaar et al. (60), colonic diverticula were present in 37% of patients, dolichocolon in 34%, and adenomatous polyps in 34%, which was increased compared with controls (odds ratio 3.6, 95% CI, 1.4-5.7; 12.4, 95% CI, 6.8-18.0; 4.1, 95% CI, 1.9-6.4, respectively).
By contrast, two studies have failed to demonstrate an increased prevalence of neoplasia in acromegaly (45,47). In both studies patients were predominantly younger and colonoscopy was incomplete with caecal intubation rate of 70% (45,47).
Acrochordons (skin tags) are markers for the colonic lesions and have been found in most patients harboring these lesions (2,8).
Colorectal cancer (CRC) screening in acromegaly
Why to screen?
CRC is the third most frequent cancer in men, after lung and prostate cancer, and is the second most frequent cancer in women after breast cancer (11,61). It is also the third cause of death in men and women separately, and is the second most frequent cause of death by cancer if both genders are considered together (61,62). In 2014, an estimated 71,830 men and 65,000 women will be diagnosed with CRC and 26,270 men and 24,040 women will die of the disease in USA (62). CRC accounts for approximately 10% of deaths by cancer (11).
Several studies have shown an increased prevalence of pre-cancerous and cancerous colonic lesions in patients with acromegaly compared to the general population (irrespective of diet, age of onset, disease duration and ethnicity, increased propensity for malignant transformation, and worse case prognosis of CRC) (7,15,41-44,48-53).
In non-acromegalic individuals, the majority of colon cancers develop as a result of multi-step malignant transformation of benign adenomatous colonic polyps, which takes approximately 10-15 years (15). The onset of GH hypersecretion is difficult to ascertain, but usually precedes the diagnosis of acromegaly by at least 7 to 10 years (1,2,7). Therefore, one may speculated that if acromegaly is associated with increased incidence of colon polyps, there is ample time for premalignant lesions to transform into a cancer (15).
A large retrospective cohort study (n=1,362) has shown that overall cancer mortality was not increased but patients with acromegaly through concurrent colon cancer had nearly a 2.5-fold higher colon cancer specific mortality rate compared to the general population [standardized mortality rate (SMR) =2.47] (6).
Data from St. Bartholomew’s Hospital, in London, demonstrated that patients with an initial adenoma at the initial screening had a 4.4 and 8.8 fold increased risk of developing a new adenoma at the second and the third colonoscopy respectively, while patients with a normal initial colonoscopy and elevated IGF-1 level had 7.5-fold risk of a subsequent adenoma compared to those with a normal colonoscopy at the initial screening and inactive disease (15,58). Notably, despite a normal baseline colonoscopy 50-100% of patients went on to have adenoma detected at interval colonoscopies. Of all patients who had an adenoma at the second, third and fourth colonoscopy, 50%, 75% and 100% respectively had an adenoma at the initial colonoscopy implying that 50% and 25% of patients with new polyps at the second and third colonoscopy respectively, had a normal initial colonoscopy (15,58,59). These findings strongly support an evidence base for a regular surveillance programme in all patients with acromegaly, irrespectively of the findings from the initial colonoscopy (15).
By contrast, Bogazzi et al. (56) have shown that if colonic adenomas were not present initially, it was unlikely that they develop thereafter, regardless the metabolic control of acromegaly. Conversely, new lesions were frequent (and often multiple) in patients who already had colonic adenomas at baseline, particularly if acromegalic disease was poorly controlled by treatment (56).
How to screen?
Fecal occult blood testing (FOBT) is the most common mass screening test for CRC (63,64). It is a simple, cheap and safe laboratory test that relies on the assumption that asymptomatic CRC and large adenomas may bleed (63,64). False negative results may be due to incorrect storage of sample or drug assumption, whereas hemorrhoids, diet and medications are causes of false positive results (63-65).
Other screening tests, such as optical colonoscopy (OC) and computed tomography colonography (CTC) are highly accurate for examining the entire colon for adenomas and CRC (63,64). OC is widely accepted as the gold standard procedure for detection of colorectal neoplasia, and there are indirect data showing that this strategy may contribute to a 76% to 90% decrease of the incidence for CRC (15,63,64). Moreover, screening with OC in selected cohorts of subjects by detection and removal of most advanced adenomas could allow long screening intervals (15,63,64).
Colonoscopy was shown to be superior to FOBT in detecting colonic lesions at the first diagnosis of acromegaly. In the study by Bogazzi et al. (66) FOBT, which was positive in 16 (18.8%) out of 85 patients, identified 2 patients with colonic adenocarcinoma and 2 with adenoma; the remaining 12 patients had no detectable colonic lesions. Colonoscopy revealed colonic lesions in 29 patients: 3 (3.5%) cancers, 11 (12.9%) adenomas, and 15 (17.6%) hyperplastic polyps. The remaining 56 acromegalic patients had no detectable lesions. A patient with cancer and 9 patients with adenoma were missed if screened only by FOBT (66).
Unlike the general population, 25% of adenomas and 50% of carcinomas seem to occur in the ascending and transverse colon in patients with acromegaly, therefore a total colonoscopy is required rather than sigmoidoscopy or limited colonoscopy (15,47,57).
The major disadvantages of OC as a screening test are its complications, including bleeding and perforation, and the discomfort due to both full bowel preparation and the procedure itself (63,67,68). Moreover, there are some technical challenges for colonoscopy in acromegalic patients. Indeed, colonic transit time in these subjects is more than twice that of normal subjects, so that standard bowel preparation is often inadequate leading to suboptimal assessment (52,69,70). Furthermore, the increased bowel length and the intestinal loop complexity seen in acromegalic patients may lead to higher levels of technical difficulties and increase the risks of complications at conventional colonoscopy (15,70,71). Finally, the estimate death rate associated with the colonoscopic procedure in acromegalic patients can be as high as 1 in 2,898 exams (1 in 10,000 for the general population) (72,73).
An alternative procedure to OC is the CTC, also named virtual colonoscopy, whose main disadvantages are the fact that it does not allow polyp resection or biopsy, and delivers a significant amount of radiation therefore unsuitable for a screening programme (15,63). However, it is a safe and very accurate procedure (74,75). A review and meta-analysis assessing the sensitivity of both CTC colonography and OC for CRC detection found that primary CT colonography may be more suitable than OC for initial investigation of suspected CRC (76). Nevertheless, according to most experts, CTC should be reserved for patients with incomplete or unfeasible colonoscopy (15,63,77).
In the study by Ramos et al. (74), which evaluated 21 acromegalic patients, CTC showed 88% sensitivity, 75% specificity and 81% accuracy in detection of colonic polyps. This procedure was performed without complications and a complete and safe colorectal evaluation was possible in all acromegalic patients (74). Similar results were reported by Resmini et al. (78).
Other new technology such as colon capsule endoscopy may aid endoscopists in the challenge of completing the evaluation of the colon in those patients with an incomplete colonoscopy (63). Finally, there have been large studies which examine the performance characteristics of the so-called non-invasive CRC screening tests such as fecal immunochemical test (FIT) and fecal DNA (11,63). The performance of these new technologies in acromegalic patients has not yet been demonstrated.
When to screen?
Repeated colonoscopic screening of patients with acromegaly has demonstrated that they are at high risk of developing a new colonic neoplasia, especially in those with an adenoma at the initial screening and/or who have uncontrolled disease with persistently abnormal GH and IGF-1 levels (15,52,58). Furthermore, acromegalic patients are at an increased risk of malignant transformation of benign adenomatous colon polyps to CRC, which then reaches a higher mortality rate compared to the general population (15,57). For all these reasons, the guidelines from different institutions and societies recommend early colonoscopic screening starting at the time of diagnosis (or at the age of 40 years considering this is the mean age at diagnosis) (15).
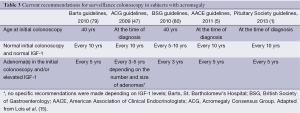
The most commonly referenced guidelines for colonoscopic screening and surveillance in patients with acromegaly are those published by the Acromegaly Consensus Group (ACG) in 2009 (47), a group of experts from the St. Bartholomew’s Hospital (Barts) in 2010 (79), the British Society of Gastroenterology (BSG) in 2010 (80), the American Association of Clinical Endocrinologists (AACE) in 2011 (5) and the Pituitary Society in 2013 (1). According to Barts and the BSG guidelines colonoscopic surveillance should be commenced at the age of 40 (47,80). The ACG, the Pituitary Society and AACE state, however, that the baseline colonoscopy should be performed at the time of acromegaly diagnosis (1,5,47). If a patient has normal findings on initial colonoscopy and normal IGF-1 levels, all guidelines but those from ACG recommend that further colonoscopies should be performed every 10 years (every 5-10 years for ACG). However, if baseline or subsequent surveillance colonoscopy reveals the presence of an adenoma, Barts, the Pituitary Society and the AACE recommend 5-yearly surveillance, BSG recommend 3-yearly colonoscopy while the ACG guidelines recommend further colonoscopies every 3-5 years depending of the number and size of adenoma (1,5,47,79,80). Recently, these guidelines were elegantly reviewed by Lois et al. (15). The current recommendations for surveillance colonoscopy in acromegaly are summarized in Table 3.
Full table
Management and prevention
The options for the management of CRC include surgery, chemotherapy and radiotherapy, and they do not differ in patients with or without acromegaly. CRC largely can be prevented by the early detection and removal of adenomatous polyps (11). Indeed, several cohort studies demonstrate that polyps removal lowers the incidence of CRC by 76-90% (11).
As the incidence of polyps and CRC is higher in patients with active acromegaly, normalization of IGF-1 levels, regardless the kind of treatment (surgery or medical therapy), is always beneficial (7,8,51,57,58).
The role of aspirin in the prevention of the development of colonic neoplasms in acromegaly has not yet been fully evaluated. However, the results of a Cochrane review, which included three randomized control trials (RCTs), showed that aspirin (acetylsalicylic acid) significantly lowers the recurrence of adenomas after a three-year follow-up in the general population (RR =0.77; 95% CI, 0.61-0.96) (81). Moreover, the joint analysis of the British doctors aspirin trial and the UK-TIA aspirin trial indicates that taking aspirin in doses of ≥300 mg/day for at least five years is an effective primary prevention method against CRC with a 10-year latency period (82). Although the pharmacological mode of aspirin action is unclear, inhibition of COX-1 and/or COX-2 is most likely involved (11,82).
Conclusions
Patients with acromegaly are at high risk for benign and malignant colonic neoplasms (6,7,58,59). Furthermore, these patients are at an increased risk of malignant transformation of benign adenomatous colon polyps to CRC, whose mortality rate in higher than that seen in the general population (6,57). Therefore, guidelines from different institutions and societies recommend early colonoscopic screening starting at the time of diagnosis (or at the age of 40 years, considering this is the mean age at diagnosis). Interval colonic surveillance depends on the findings from the baseline colonoscopy and on IGF-1 levels. Firm evidence and outcome based confirmation of the best approach is still lacking (15).
The mechanisms involved in cancer development and progression in acromegalic patients are still unclear. Chronic exposure to elevated IGF-1 levels seems to be the most important (15,47,56,58). In addition, hyperinsulinemia, diabetes mellitus, altered acid bile secretion, altered local immune response, increased large bowel length and/or altered bowel transit times, and genetic factors could also play a role (7,15,31).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Melmed S, Casanueva FF, Klibanski A, et al. A consensus on the diagnosis and treatment of acromegaly complications. Pituitary 2013;16:294-302. [PubMed]
- Lugo G, Pena L, Cordido F. Clinical manifestations and diagnosis of acromegaly. Int J Endocrinol 2012;2012:540398.
- Vilar L, Naves LA, Costa SS, et al. Increase of classic and nonclassic cardiovascular risk factors in patients with acromegaly. Endocr Pract 2007;13:363-72. [PubMed]
- Rodrigues MP, Naves LA, Casulari LA, et al. Using clinical data to predict sleep hypoxemia in patients with acromegaly. Arq Neuropsiquiatr 2007;65:234-9. [PubMed]
- Katznelson L, Atkinson JL, Cook DM, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of acromegaly--2011 update. Endocr Pract 2011;17 Suppl 4:1-44. [PubMed]
- Orme SM, McNally RJ, Cartwright RA, et al. Mortality and cancer incidence in acromegaly: a retrospective cohort study. United Kingdom Acromegaly Study Group. J Clin Endocrinol Metab 1998;83:2730-4. [PubMed]
- Webb SM, Casanueva F, Wass JA. Oncological complications of excess GH in acromegaly. Pituitary 2002;5:21-5. [PubMed]
- Scialpi C, Mosca S, Malaguti A, et al. Acromegaly and intestinal neoplasms. Minerva Endocrinol 1999;24:123-7. [PubMed]
- Kurimoto M, Fukuda I, Hizuka N, et al. The prevalence of benign and malignant tumors in patients with acromegaly at a single institute. Endocr J 2008;55:67-71. [PubMed]
- Baris D, Gridley G, Ron E, et al. Acromegaly and cancer risk: a cohort study in Sweden and Denmark. Cancer Causes Control 2002;13:395-400. [PubMed]
- Tárraga López PJ, Albero JS, Rodríguez-Montes JA. Primary and secondary prevention of colorectal cancer. Clin Med Insights Gastroenterol 2014;7:33-46. [PubMed]
- Matano Y, Okada T, Suzuki A, et al. Risk of colorectal neoplasm in patients with acromegaly and its relationship with serum growth hormone levels. Am J Gastroenterol 2005;100:1154-60. [PubMed]
- Yamamoto M, Fukuoka H, Iguchi G, et al. The prevalence and associated factors of colorectal neoplasms in acromegaly: a single center based study. Pituitary 2014. [Epub ahead of print]. [PubMed]
- Jenkins PJ, Mukherjeet A, Shalet SM. Does growth hormone cause cancer? Clin Endocrinol (Oxf) 2006;64:115-21. [PubMed]
- Lois K, Bukowczan J, Perros P, et al. The role of colonoscopic screening in acromegaly revisited: review of current literature and practice guidelines. Pituitary 2014. [Epub ahead of print]. [PubMed]
- Lahm H, Amstad P, Wyniger J, et al. Blockade of the insulin-like growth-factor-I receptor inhibits growth of human colorectal cancer cells: evidence of a functional IGF-II-mediated autocrine loop. Int J Cancer 1994;58:452-9. [PubMed]
- Cats A, Dullaart RP, Kleibeuker JH, et al. Increased epithelial cell proliferation in the colon of patients with acromegaly. Cancer Res 1996;56:523-6. [PubMed]
- Dutta P, Bhansali A, Vaiphei K, et al. Colonic neoplasia in acromegaly: increased proliferation or deceased apoptosis? Pituitary 2012;15:166-73. [PubMed]
- Cohen P, Clemmons DR, Rosenfeld RG. Does the GH-IGF axis play a role in cancer pathogenesis? Growth Horm IGF Res 2000;10:297-305. [PubMed]
- Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst 2002;94:972-80. [PubMed]
- Koohestani N, Tran TT, Lee W, et al. Insulin resistance and promotion of aberrant crypt foci in the colon of rats fed on a high-fat diet. Nutr Cancer 1997;29:69-76. [PubMed]
- Tran TT, Medline A, Bruce WR. Insulin promotion of colon tumors in rats. Cancer Epidemiol Biomarkers Prev 1996;5:1013-15. [PubMed]
- Koenuma M, Yamori T, Tsuruo T. Insulin and insulin-like growth factor 1 stimulate proliferation of metastatic variants of colon carcinoma 26. Jpn J Cancer Res 1989;80:51-8. [PubMed]
- Giovannucci E. Insulin and colon cancer. Cancer Causes Control 1995;6:164-79. [PubMed]
- Khandwala HM, McCutcheon IE, Flyvbjerg A, et al. The effects of insulin-like growth factors on tumorigenesis and neoplastic growth. Endocr Rev 2000;21:215-44. [PubMed]
- Kaaks R, Toniolo P, Akhmedkhanov A, et al. Serum C-peptide, IGF-I, IGFBPs, and colorectal cancer risk in women. J Natl Cancer Inst 2000;92:1592-600. [PubMed]
- Hu FB, Manson JE, Liu S, et al. Prospective study of adult onset diabetes mellitus and risk of colorectal cancer in women. J Natl Cancer Inst 1999;91:542-7. [PubMed]
- Will JC, Galuska DA, Vinicor F, et al. Colorectal cancer: another complication of diabetes mellitus? Am J Epidemiol 1998;147:816-25. [PubMed]
- Schoen RE, Tangen CM, Kuller LH, et al. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst 1999;91:1147-54. [PubMed]
- Yamada K, Araki S, Tamura M, et al. Relation of serum total cholesterol, serum triglycerides and fasting plasma glucose to colorectal carcinoma in situ. Int J Epidemiol 1998;27:794-8. [PubMed]
- Colao A, Pivonello R, Auriemma RS, et al. The association of fasting insulin concentrations and colonic neoplasms in acromegaly: a colonoscopy-based study in 210 patients. J Clin Endocrinol Metab 2007;92:3854-60. [PubMed]
- Lombardi M, Scattina I, Sardella C, et al. Serum factors associated with precancerous colonic lesions in acromegaly. J Endocrinol Invest 2013;36:545-9. [PubMed]
- Feldman D, Krishnan AV, Swami S, et al. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342-57. [PubMed]
- Di Rosa M, Malaguarnera M, Zanghì A, et al. Vitamin D3 insufficiency and colorectal cancer. Crit Rev Oncol Hematol 2013;88:594-612. [PubMed]
- Zhang B, Wang J, Wang X, et al. Proteogenomic characterization of human colon and rectal cancer. Nature 2014;513:382-7. [PubMed]
- Jasmine F, Rahaman R, Dodsworth C, et al. A genome-wide study of cytogenetic changes in colorectal cancer using SNP microarrays: opportunities for future personalized treatment. PLoS One 2012;7:e31968. [PubMed]
- Torre ML, Russo GT, Ragonese M, et al. MTHFR C677T polymorphism, folate status and colon cancer risk in acromegalic patients. Pituitary 2014;17:257-66. [PubMed]
- Zengi A, Karadeniz M, Cetintas VB, et al. Is there any association between the Ser326Cys polymorphism of the 8-oxoguanine glycosylase 1 (OGG1) gene and risk of colon polyp and abnormal glucose tolerance in acromegaly patients? Genet Test Mol Biomarkers 2013;17:267-73. [PubMed]
- Daly AF, Vanbellinghen JF, Khoo SK, et al. Aryl hydrocarbon receptor-interacting protein gene mutations in familial isolated pituitary adenomas: analysis in 73 families. J Clin Endocrinol Metab 2007;92:1891-6. [PubMed]
- Georgitsi M, Karhu A, Winqvist R, et al. Mutation analysis of aryl hydrocarbon receptor interacting protein (AIP) gene in colorectal, breast, and prostate cancers. Br J Cancer 2007;96:352-6. [PubMed]
- Loeper S, Ezzat S. Acromegaly: re-thinking the cancer risk. Rev Endocr Metab Disord 2008;9:41-58. [PubMed]
- Pines A, Rozen P, Ron E, et al. Gastrointestinal tumors in acromegalic patients. Am J Gastroenterol 1985;80:266-9. [PubMed]
- Ron E, Gridley G, Hrubec Z, et al. Acromegaly and gastrointestinal cancer. Cancer 1991;68:1673-7. [PubMed]
- Barzilay J, Heatley GJ, Cushing GW. Benign and malignant tumors in patients with acromegaly. Arch Intern Med 1991;151:1629-32. [PubMed]
- Ladas SD, Thalassinos NC, Ioannides G, et al. Does acromegaly really predispose to an increased prevalence of gastrointestinal tumours? Clin Endocrinol (Oxf) 1994;41:597-601. [PubMed]
- Ortego J, Vega B, Sampedro J, et al. Neoplastic colonic polyps in acromegaly. Horm Metab Res 1994;26:609-10. [PubMed]
- Renehan AG, Bhaskar P, Painter JE, et al. The prevalence and characteristics of colorectal neoplasia in acromegaly. J Clin Endocrinol Metab 2000;85:3417-24. [PubMed]
- Klein I, Parveen G, Gavaler JS, et al. Colonic polyps in patients with acromegaly. Ann Intern Med 1982;97:27-30. [PubMed]
- Ituarte EA, Petrini J, Hershman JM. Acromegaly and colon cancer. Ann Intern Med 1984;101:627-8. [PubMed]
- Brunner JE, Johnson CC, Zafar S, et al. Colon cancer and polyps in acromegaly: increased risk associated with family history of colon cancer. Clin Endocrinol (Oxf) 1990;32:65-71. [PubMed]
- Jenkins PJ, Fairclough PD, Richards T, et al. Acromegaly, colonic polyps and carcinoma. Clin Endocrinol (Oxf) 1997;47:17-22. [PubMed]
- Terzolo M, Reimondo G, Gasperi M, et al. Colonoscopic screening and follow-up in patients with acromegaly: A multicenter study in Italy. J Clin Endocrinol Metab 2005;90:84-90. [PubMed]
- Mestron A, Webb SM, Astorga R, et al. Epidemiology, clinical characteristics, outcome, morbidity and mortality in acromegaly based on the Spanish Acromegaly Registry (Registro Español de Acromegalia, REA). Eur J Endocrinol 2004;151:439-46. [PubMed]
- Bałdys-Waligórska A, Krzentowska A, Gołkowski F, et al. The prevalence of benign and malignant neoplasms in acromegalic patients. Endokrynol Pol 2010;61:29-34. [PubMed]
- Vallette S, Ezzat S, Chik C, et al. Emerging trends in the diagnosis and treatment of acromegaly in Canada. Clin Endocrinol (Oxf) 2013;79:79-85. [PubMed]
- Bogazzi F, Cosci C, Sardella C, et al. Identification of acromegalic patients at risk of developing colonic adenomas. J Clin Endocrinol Metab 2006;91:1351-6. [PubMed]
- Rokkas T, Pistiolas D, Sechopoulos P, et al. Risk of colorectal neoplasm in patients with acromegaly: A meta-analysis. Risk of colorectal neoplasm in patients with acromegaly: A meta-analysis. World J Gastroenterol 2008;14:3484-9. [PubMed]
- Jenkins PJ, Frajese V, Jones AM, et al. Insulin-like growth factor I and the development of colorectal neoplasia in acromegaly. J Clin Endocrinol Metab 2000;85:3218-21. [PubMed]
- Jenkins PJ, Besser M. Clinical perspective: acromegaly and cancer: a problem. J Clin Endocrinol Metab 2001;86:2935-41. [PubMed]
- Wassenaar MJ, Cazemier M, Biermasz NR, et al. Acromegaly is associated with an increased prevalence of colonic diverticula: a case-control study. J Clin Endocrinol Metab 2010;95:2073-9. [PubMed]
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of the cancer incidence and mortality in Europe in 2008. Eur J Cancer 2010;46:765-81. [PubMed]
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64:104-17. [PubMed]
- Anderson JC, Shaw RD. Update on colon cancer screening: recent advances and observations in colorectal cancer screening. Curr Gastroenterol Rep 2014;16:403. [PubMed]
- Sali L, Grazzini G, Carozzi F, et al. Screening for colorectal cancer with FOBT, virtual colonoscopy and optical colonoscopy: study protocol for a randomized controlled trial in the Florence district (SAVE study). Trials 2013;14:74. [PubMed]
- Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996;348:1467-71. [PubMed]
- Bogazzi F, Lombardi M, Scattina I, et al. Comparison of colonoscopy and fecal occult blood testing as a first-line screening of colonic lesions in patients with newly diagnosed acromegaly. J Endocrinol Invest 2010;33:530-3. [PubMed]
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The national polyp study workgroup. N Engl J Med 1993;329:1977-81. [PubMed]
- Brenner H, Chang-Claude J, Seiler CM, Hoffmeister M. Long-term risk of colorectal cancer after negative colonoscopy. J Clin Oncol 2011;29:3761-7. [PubMed]
- Nelson DB, McQuaid KR, Bond JH, et al. Procedural success and complications of large-scale screening colonoscopy. Gastrointest Endosc 2002;55:307-14. [PubMed]
- Renehan AG, Painter JE, Bell GD, et al. Determination of large bowel length and loop complexity in patients with acromegaly undergoing screening colonoscopy. Clin Endocrinol (Oxf) 2005;62:323-30. [PubMed]
- Renehan AG, O’Connell J, O’Halloran D, et al. Acromegaly and colorectal cancer: a comprehensive review of epidemiology, biological mechanisms, and clinical implications. Horm Metab Res 2003;35:712-25. [PubMed]
- Bowles CJ, Leicester R, Romaya C, et al. A prospective study of colonoscopy practice in the UK today: are we adequately prepared for national colorectal cancer screening tomorrow? Gut 2004;53:277-83. [PubMed]
- Renehan AG, Odwyer ST, Shalet SM. Screening colonoscopy for acromegaly in perspective. Clin Endocrinol (Oxf) 2001;55:731-3. [PubMed]
- Ramos O Jr, Boguszewski CL, Teixeira S, et al. Perfomance of computed tomographic colonography for the screening of colorectal polyp in acromegalic patients:a prospectiva study. Arq Gastroenterol 2009;46:90-6. [PubMed]
- Plumb AA, Halligan S, Pendsé DA, et al. Sensitivity and specificity of CT colonography for the detection of colonic neoplasia after positive faecal occult blood testing: systematic review and meta-analysis. Eur Radiol 2014;24:1049-58. [PubMed]
- Pickhardt PJ, Hassan C, Halligan S, et al. Colorectal cancer: CT colonography and colonoscopy for detection--systematic review and meta-analysis. Radiology 2011;259:393-405. [PubMed]
- Kriza C, Emmert M, Wahlster P, et al. An international review of the main cost-effectiveness drivers of virtual colonography versus conventional colonoscopy for colorectal cancer screening: is the tide changing due to adherence? Eur J Radiol 2013;82:e629-36. [PubMed]
- Resmini E, Tagliafico A, Bacigalupo L, et al. Computed tomography colonography in acromegaly. J Clin Endocrinol Metab 2009;94:218-22. [PubMed]
- Dworakowska D, Gueorguiev M, Kelly P, et al. Repeated colonoscopic screening of patients with acromegaly: 15-year experience identifies those at risk of new colonic neoplasia and allows for effective screening guidelines. Eur J Endocrinol 2010;163:21-8. [PubMed]
- Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010;59:666-89. [PubMed]
- Asano TK, McLeod RS. Non steroidal anti-inflammatory drugs (NSAID) and aspirin for preventing colorectal adenomas and carcinomas. Cochrane Database Syst Rev 2004;CD004079. [PubMed]
- Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet 2007;369:1603-13. [PubMed]


