Clinical perspectives: leptin replacement therapy for the treatment of lipodystrophy-associated non-alcoholic fatty liver disease
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a chronic, severe, and progressive condition of the liver, characterized by the deposition of fat in the liver parenchyma, not associated with excessive alcohol intake (1). It is frequently found in conjunction with obesity and metabolic syndrome. Indeed, 90% of patients with NAFLD have at least one feature of metabolic syndrome, and one third of them meet the full criteria for the syndrome defined under the ATPIII criteria (2,3). Recent epidemiological data suggests that NAFLD affects approximately 15-30% of people from western countries, and as the prevalence of obesity and other obesity-related risk-factors is increasing worldwide, it is expected that NAFLD will increase in other countries as well (4).
At the cellular level, the accumulation of triglycerides (TG) in hepatocytes (steatosis) can progress to inflammation with hepatocyte injury (ballooning), with or without fibrosis-defined as non-alcoholic steatohepatitis (NASH). NAFLD is strongly linked to obesity and metabolic syndrome, and is an important risk factor for liver cancer (5). NAFLD is thought to increase the risk of hepatocellular carcinoma (HCC) by a number of molecular pathways previously reviewed by Michelotti and briefly summarised in the following (6). The nuclear factor κB (NF-κB) family of transcription factors is an important family of inflammatory mediators, and dysregulation of NF-κB has a profound effect on the course of NAFLD and NASH, including increased presence of natural killer T-cells in the liver amongst a pro-inflammatory milieu contributing to further liver damage. Inappropriate responses to insulin through altered signalling in PI3K, AKT and PTEN pathways can exacerbate lipid accumulation in the liver and may be a mechanism for lipotoxicity and subsequent liver damage. Also, microRNAs are emerging as important cellular regulators, with important actions in DNA stability. Some animal microRNA knockout models have shown impairment of lipid metabolism and uncontrolled cell proliferation. Overall these deficiencies represent a confluence of obesity and liver injury leading to HCC. Whereas obesity is the most common causal factor of NAFLD, lipid accumulation within the liver can also occur due to partial or complete leptin deficiency (Figure 1) (8,9).
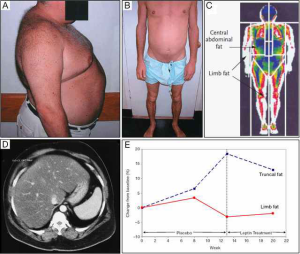
Leptin is one of the most expressed adipose-derived cytokines (adipokines). Leptin exerts key roles in regulating food intake, energy balance and body weight. Leptin also plays important roles in lipid and glucose metabolism, the gonadal, adrenal, somatotropic and thyroid axes, sympathetic tone, immunity, inflammation, and brain structure and function (10,11). Impaired leptin action, due to either leptin resistance or leptin deficiency, results in several metabolic abnormalities such as insulin resistance, hyperinsulinemia, diabetes, hypertriglyceridemia, and hepatic steatosis (12,13). Leptin is absent or in very low levels in cases of lipodystrophy, and also in patients with functional hypothalamic amenorrhea/anorexia nervosa, and genetic deficiency due to mutations in the leptin gene. Most of the phenotypic abnormalities seen in lipodystrophy are similar in those cases of leptin deficiency, including hepatic steatosis (14). Leptin replacement therapy (LRT) has been extensively evaluated in patients with genetic leptin deficiency due to mutations in the leptin gene, showing significant metabolic improvements (13). In one Austrian patient, it has also been shown that exogenous leptin administration reduces circulating levels of transaminases, total cholesterol, LDL, insulin resistance, and liver fat content (9). Likewise, it was shown that ob/ob mice that received daily leptin injections, or underwent white adipose tissue (WAT) grafting from wild-type donors showed improved glucose and insulin homeostasis, decreased serum TG, reduced lipogenesis, and increased lipolysis (15).
Lipodystrophy syndromes (LS) are a group of congenital and acquired disorders characterized by the generalized or partial absence of adipose tissue (Table 1). The subcutaneous adipose tissue is the most affected. In the absence of leptin, fat deposition is favoured in non-adipose tissues. The development of lipodystrophy is determined by molecular defects in genes that regulate adipocyte differentiation, lipid metabolism, and lipid droplet morphology. Acquired forms of lipodystrophy may also develop from underlying autoimmune conditions, HIV infection, or use of highly-active retroviral therapy (HAART) (16). The adipose tissue is an important endocrine organ that synthesizes hormones with cytokine-like actions (17). The absence of adipose tissue determines several metabolic defects, such as insulin resistance, diabetes, and hypertriglyceridemia. These abnormalities can lead to the development of atherosclerosis, acute pancreatitis, and may evolve into NAFLD. Similar to patients with NAFLD, 80% of patients with lipodystrophy meet the criteria for the diagnosis of metabolic syndrome (18). Thus, lipodystrophy represents an extreme version of the common, obesity-associated, “metabolic syndrome”. In contrast to the obesity-associated metabolic syndrome, patients with lipodystrophy have low levels of adipocyte-derived hormones, including leptin (14,19). Therefore, leptin deficiency represents a specific therapeutic target for metabolic abnormalities in patients with lipodystrophy, including NAFLD.

Full table
LRT is the FDA-approved treatment of choice for generalized forms of congenital and acquired lipodystrophy. LRT has been shown to be very effective in ameliorating hepatic and metabolic complications, as well as decreasing ectopic fat deposition in the liver and skeletal muscle (12,20). Specifically, leptin ameliorates the glucose and lipid abnormalities of these patients, and increases insulin sensitivity. Furthermore, LRT restores gonadotropin secretion (21). Coincident with the normalization of these hormonal systems, leptin treatment results in weight reduction by 1 to 2 kg (22). Overall, LRT can potentially reverse the severe pathology present in lipodystrophy, and limit against the risk of further development into NAFLD (21).
In this review, we report some of the molecular mechanisms by which leptin is thought to ameliorate lipodystrophy diseases, and outline the current literature on the use of exogenous leptin in improving liver and metabolic health in patients with lipodystrophy-associated NAFLD.
Molecular action of leptin in lipodystrophy-associated NAFLD
Leptin is a 16 kDa 167-amino acid protein synthesized predominantly by the adipose tissue, encoded by the leptin gene Ob (Lep), located on chromosome 7 in humans (23). The main role of leptin is to regulate feeding behavior by increasing satiety and reducing hunger. Leptin shares structural homology to some pro-inflammatory cytokines, and thus exhibits some important pro-inflammatory actions, such as increasing the synthesis and release of IL-6 (24) and TNF-α (25). In addition, leptin contributes to the regulation of endocrine systems such as the thyrotropic, gonadotropic and corticotropic axes, and affects hematopoiesis, angiogenesis, osteogenesis, and wound healing (10). Leptin’s actions are achieved by binding to its cognate receptor, Ob-R, which is expressed in several variants including one long isoform, four short isoforms and one soluble isoform. Only the long isoform is capable of producing signal transduction. These receptors are located in many tissues including the arcuate nucleus of the hypothalamus in the central nervous system (CNS). This region of the brain is responsible for regulating food intake, and in this way leptin exerts its metabolic effects via the CNS (23). The Ob-R receptor is also expressed in peripheral tissues, and in murine models of lipodystrophy, exogenous supplementation of leptin leads to increased fatty acid oxidation in skeletal muscle by activating AMP-activated protein kinase (AMPK), which in turn phosphorylates and inhibits the activity of acetyl-CoA carboxylase, decreasing the amount of malonyl-CoA and leading to the increase in fatty acid oxidation. Other studies suggest that leptin may activate fatty acid oxidation through AMPK activation within the heart and the liver; leptin is suggested to increase fatty acid oxidation by a mechanism dependent on peroxisome proliferator-activated receptor α (PPARα) (26,27). In a rodent model of lipodystrophy, leptin treatment leads to the increase of insulin-stimulated insulin receptor signaling and of insulin receptor substrate 2 (IRS-2) phosphorylation, IRS-2-associated PI3K activity, and Akt activity in the liver, suggesting that leptin can increase insulin sensitivity and may further contribute to correcting diabetes (28).
Numerous animal models of LS and leptin deficiency have shown that exogenous supplementation of leptin is effective in reversing the clinical manifestations of lipodystrophy (29). Our previous research has shown that in leptin-deficient mice, reversal of NAFLD can be achieved by transplantation of WAT from wild-type, leptin-sufficient donors (15). Compared to sham-operated ob/ob controls, leptin-deficient mice receiving WAT lost a significant amount of weight and displayed reduced plasma levels of fasting glucose and insulin. Additionally, they showed a significant reduction in liver injury scores when compared with sham-operated ob/ob mice (15). Overall, mice receiving leptin treatment (through WAT transplantation) were histologically and physiologically healthier. Furthermore, liver microarray data indicated that WAT transplantation induced the differential expression of several pathways thought to be critical in NAFLD pathogenesis. These included pathways involved in lipid and glucose metabolism, insulin signaling, inflammatory response, cytochrome P450 function, glutathione metabolism, and peroxisome proliferator-activated receptor signaling (15).
In humans with lipodystrophy, LRT is performed using the leptin analogue “recombinant methionyl human leptin” (r-metHuLeptin, metreleptin, AstraZeneca) and this has been evaluated by several clinical trials. This drug is under patent, marketed under the trade name “MYALEPT” in the United States. The first approval of metreleptin for lipodystrophy was in Japan, in-licensed by Shionogi & Co, Ltd. from Amylin Pharmaceuticals (30). Administered as a subcutaneous injection once or twice a day, metreleptin has been shown to reverse the metabolic abnormalities that are seen in lipodystrophy, leading to significant improvements in overall liver health (31-40).
FDA approves leptin to treat lipodystrophy
In late 2013, the US Food and Drug Administration (FDA) released a briefing document prepared by Bristol-Myers Squibb and AstraZeneca (22) for the “Metabolic Advisory Committee on Metreleptin”, into the integrated findings of one completed study (NIH Study 991265), and two ongoing studies (NIH Study 20010769 and FHA101). These studies were commissioned to assess the safety and efficacy of metreleptin for the treatment of patients with lipodystrophy, including the effects on metabolic parameters such as hypertriglyceridemia and/or diabetes mellitus inadequately controlled on a current therapy, and/or evidence of hepatic steatosis. The primary outcomes were the achievement of normal physiological levels of liver volume and transaminases, glycated hemoglobin (HbA1c), fasting plasma glucose (FPG) and TG. Data were captured from a combined cohort of 72 participants (Table 2). Leptin was administered at a starting dose of 0.02 mg/kg for patients weighing ≤40 kg and 1.25-2.5 mg/kg for patients weighing >40 kg. Following a 12-month trial period, all these outcome parameters were improved (Table 3).
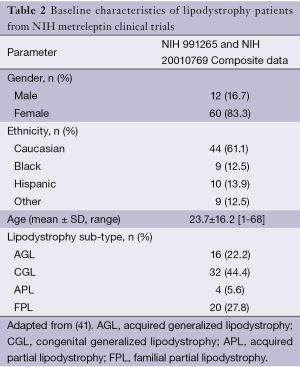
Full table
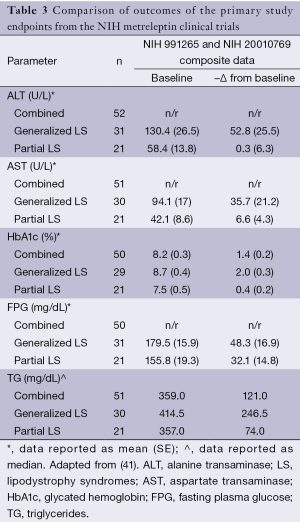
Full table
Therefore, existing data show that leptin replacement in patients with lipodystrophy leads to an improvement in glycemic control, a restoration toward a normal lipid profile, and improved liver function. In a sub-analysis, the cohort results were stratified according to the degree of elevation (from the normal range) of baseline metabolic parameters (Table 4). In this sub-analysis, it was noted that metreleptin determined better results, that is, more pronounced changes from baseline were seen in patients with a more severe metabolic phenotype. For example, after following the 12-month trial period, patients with generalized lipodystrophy that displayed a baseline HbA1c ≥6% achieved a mean reduction of 2.3%, whereas patients with a baseline HbA1c ≥8% displayed a significantly greater reduction of 2.7%. Similarly, in patients with partial lipodystrophy with a baseline HbA1c ≥6%, their mean reduction was reported as 0.8%; reductions reached a mean of 1.4% in patients with a baseline HbA1c ≥8%. A similar pattern of results was observed in all the parameters assessed, which lead the investigators to suggest that in recovering a healthy phenotype, the effect of metreleptin was more pronounced in those patients with more severe underlying metabolic abnormalities.
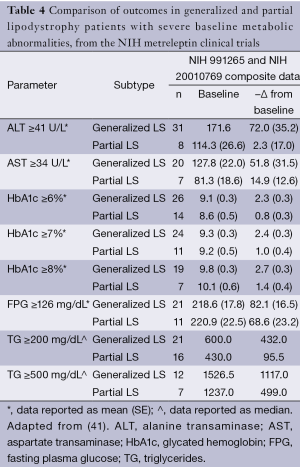
Full table
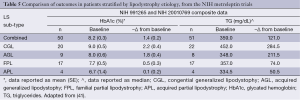
Furthermore, results suggested that the clinical efficacy of metreleptin was more related to the severity of the underlying metabolic abnormalities rather than to which form of lipodystrophy (generalized or partial) the patient had. To assess this claim, a further sub-analysis was performed whereby the data from the two main outcomes (HbA1c and TG) were stratified according to lipodystrophy sub-type (Table 5). This sub-analysis showed that more pronounced results were seen in patients with the generalized form, and similar results were seen in patients with congenital or acquired variants of each lipodystrophy sub-type. This suggests that metreleptin was more effective in ameliorating the condition of patients with the generalized lipodystrophy form, even though other results suggest the efficacy of metreleptin may be independent of lipodystrophy etiology. The report notes that patients with generalized lipodystrophy presented with more substantial mortality and morbidity due to complications arising from several severe metabolic abnormalities, and in this way, it is easier to determine and predict the benefit of metreleptin therapy in a patient with generalized lipodystrophy as opposed to a patient with partial lipodystrophy. Furthermore, patients with partial lipodystrophy presented with a more heterogeneous disease profile, as there was greater variability in the severity of the metabolic abnormalities. Taken together, these data comprehensively suggest that exogenous supplementation of leptin can return key liver parameters and biochemical markers to normal ranges in patients with lipodystrophy-associated NAFLD.
Full table
Clinical trials investigating the effects of leptin in treating patients with lipodystrophy
The NIH-funded studies represent the industry standard both in terms of procedural rigor and in data analysis and presentation. However, outside of these well-recognized studies, there are several studies in this area. The first reference of the use of metreleptin in treating patients with lipodystrophy associated NAFLD was reported in 2002 by Oral et al. (37), and the focus of this study was primarily on the safety of the drug and the influence of metreleptin on the endocrine system and metabolism.
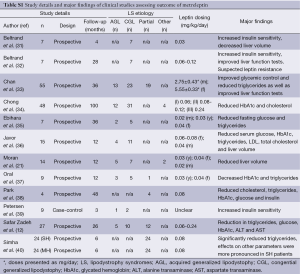
Several other studies assessed the effect of leptin (in the form of metreleptin) on liver health and other endpoints in patients with non-HAART lipodystrophy associated NAFLD (31-40). Study sample sizes range from n=4 to 55, and the mean number of participants was 18±16. Importantly, as lipodystrophy-associated NAFLD is an uncommon disease, most of these studies are very small in terms of patient population, and as such, not significantly powered. Therefore, it is necessary to consider results and perspectives from these studies together in order to overcome statistical error associated with small studies. Altogether, these studies make a composite population of 226 participants including 45 males and 181 females. Nine patients from two studies had diabetes as a metabolic co-morbidity (37,39). Follow-up times ranged from 3 to 100 months, where the mean time was 27±27 months. The majority of these studies were prospective cohort analyses; however there are a few case-control type studies (39). As per current recommendations, all studies administered the hormone via a subcutaneous injection, either daily or twice daily according to their study protocol. Starting doses of metreleptin ranged from 0.02 to 0.24 mg/kg/day for males and 0.04 to 0.24 mg/kg/day for females. Some studies did not stratify drug dosing according to gender, and in these instances the dosages ranged from 0.03 to 0.24 mg/kg/day (12,31,32,37,40). Overall, these studies assessed the effect of exogenous leptin on the same hepatic and metabolic endpoints as did the NIH studies. These data are summarized below, and a snapshot of the important information of these studies is provided in Table S1.
Full table
Effect of metreleptin on hepatic parameters
Significant reductions in mean liver volume following leptin therapy were seen in three studies and one study reported significant reductions in liver fat. Simha et al., stratified results according to the participants’ degree of hypoleptinemia, either severely or mildly hypoleptinemic, and both groups of patients exhibited significant decreases in mean liver fat percentage (40) (Table 5). In terms of biochemical parameters, serum albumin concentrations did not significantly alter from metreleptin treatment (21,37). Alanine transaminase (ALT) and aspartate transaminase (AST) were significantly decreased following LTR (12,31,40). In the study by Simha et al., neither cohort of participants showed significant reductions in ALT, but severely hypoleptinemic patients showed a significant reduction in AST levels (40).
Effect of metreleptin on metabolic parameters
Fasting glucose was significantly reduced in seven studies (33-35,37-40). The greatest reduction was seen in the study by Petersen et al. (39), where at baseline patients had a fasting glucose concentration of 234±14 mg/dL and following the three-month intervention, this declined to 122±21 mg/dL. Insulin responses varied, one study observed an increase but did not report whether this was significant (32). Four studies reported a significant reduction in HbA1c (12,34,36,37). Oral et al. reported the greatest reduction totalling 1.90% (41,42). Interestingly, HbA1c was reported to have increased by 1.77% but the study did not report whether this represented a significant increase from baseline (32). Eight studies reported significant reductions in serum TG (12,31,35-40). The largest reduction was in the Petersen et al. cohort where patients had an initial mean TG concentration of 5,851±5,079 mg/dL. This decreased to 1,134±523 mg/dL following three months of metreleptin therapy (39). Significant reductions in TG concentrations were observed in patients with mild and severe hypoleptinemic status (40). TC concentration was both reported to have declined (34,38), and increased (31) following metreleptin therapy. Seven studies reported changes in HDL-cholesterol following LRT (32-34,36,39,40), but none of them reported significant changes.
Perspectives on the studies assessing metreleptin to treat lipodystrophy
Taken together, these studies represent a significant pool of evidence supporting the view that LRT using metreleptin is effective at correcting the metabolic abnormalities seen in patients with lipodystrophy-associated NAFLD. LRT improved several hepatic parameters including transaminases, liver volume and liver fat content. Leptin therapy also improved lipid profile, as serum TG and cholesterol decreased. These effects translate into a reduction in the risk of the development of NASH. Glycemic measures such as glucose and HbA1c also improved as a result of LRT.
Overall, LRT leads to a significant increase in health in patients with lipodystrophy. Whereas this message is positive, the studies themselves were limited in several aspects and the overall quality of this body of research may be improved by considering some of the following points. Despite the fact that the dosage of metreleptin was largely uniform across the studies, the study that utilized the largest dose (33) also observed the greatest changes in biochemical outcomes. As the statistical significance of these changes was not reported, it limits a reviewer’s interpretation as to whether increases in leptin dosage may elicit greater changes. Significantly, there were large discrepancies in the mean or median follow-up times reported. This may be a reflection of the nature of the studies themselves as some were prospective in design, but were in reality case series of similar patients (39). Some studies (Table S1) involved follow-up times shorter than 12 months, which may be viewed as a limitation. However, longer trials such as the one conducted by Chong et al. observed similar results to shorter trials, suggesting that leptin has similar immediate and long-term effects (34). Importantly, mean baseline concentrations of leptin varied considerably amongst the studies (range 0.7-3.8 ng/mL). This may impact in comparing the effects of different metreleptin dosages in reaching target levels of hepatic and metabolic parameters.
A similar pattern of results is seen across all the studies, which would suggest metreleptin is safe and effective in patients with differing states of leptin-deficiency status. Therefore, it would be reasonable to suggest that leptin may be applicable for use in other non-lipodystrophy leptin-deficient conditions, especially considering the fact that lipodystrophy shares similar pathology to other diseases characterized by leptin insufficiency and inadequacy. Indeed, leptin has previously been investigated in functional hypothalamic amenorrhea associated with low leptin levels, with positive results (43).
In terms of liver morphology assessment, the method of determining liver volume and liver fat varied between using CT and MRI scans, and one study quantified these parameters through liver biopsy (11). Recent literature reports that MRI is the more sensitive method of assessment for these metrics, and thus standardized guidelines should be validated to limit discrepancies between studies (44,45). Finally, age and sex specific effects cannot be discounted as possible reasons for heterogeneity. Future clinical trials would do well to investigate these aspects, particularly given that some studies assessed here included only children and others only women (46).
The reporting of qualitative aspects such as the use of other medications by the patients was lacking in most of the studies. The use of some drugs, in particular glucose and lipid lowering drugs, may impact on metreleptin’s effects, and therefore this would further impact on interpreting the true effects of leptin in this setting. By far, the most significant limitation in the studies reviewed is the small population sizes, ranging from 4 to 55 participants. Therefore, in order to improve the research in this area and further develop metreleptin as a therapy for other conditions of leptin deficiency sharing similar pathology to lipodystrophy, large prospective trials are warranted.
Conclusions and future directions
Lipodystrophy is a leptin-deficient disease that presents with significant metabolic abnormalities, and can severely affect the liver and progress to NAFLD. NAFLD is an important risk factor for further liver complications including cancer. In animal models of the disease, as well in humans, LRT reverses the pathology of lipodystrophy, acting both peripherally (evidenced through fatty acid oxidation), and centrally (evidenced in differing brain activity following leptin stimulation). The effects of LRT have been demonstrated in several studies, suggesting that metreleptin improves several metabolic and hepatic outcomes, including glucose and lipid homeostasis, liver volume, liver fat content, and serum transaminases.
Metreleptin has been recently approved by the FDA for the treatment of patients with generalized forms of lipodystrophy, but not of patients with partial forms (due to the lack of strong evidence supporting its benefits in this population). Large prospective studies spanning several years are needed to determine the true benefit of LRT, and to further develop the drug for other leptin-deficient conditions. Indeed, the FDA requires seven post-marketing studies for Myalept, including a long-term prospective observational study (product exposure registry) of patients treated with Myalept, a study to assess for immunogenicity, and an assessment and analysis of spontaneous reports of potential serious risks related to the use of Myalept. Furthermore, an additional eight studies are being requested as post-marketing commitments.
Should larger sets of data from patients under LRT become available, metreleptin may become a therapeutic option for patients with other conditions associated with NAFLD, such as patients with common obesity or patients submitted to liver transplantation (47).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005-23. [PubMed]
- Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology 2001;121:91-100. [PubMed]
- Marchesini G, Bugianesi E, Forlani G, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology 2003;37:917-23. [PubMed]
- Schwenger KJ, Allard JP. Clinical approaches to non-alcoholic fatty liver disease. World J Gastroenterol 2014;20:1712-23. [PubMed]
- Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 2010;51:1820-32. [PubMed]
- Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol 2013;10:656-65. [PubMed]
- Fiorenza CG, Chou SH, Mantzoros CS. Lipodystrophy: pathophysiology and advances in treatment. Nat Rev Endocrinol 2011;7:137-50. [PubMed]
- Huang XD, Fan Y, Zhang H, et al. Serum leptin and soluble leptin receptor in non-alcoholic fatty liver disease. World J Gastroenterol 2008;14:2888-93. [PubMed]
- von Schnurbein J, Heni M, Moss A, et al. Rapid improvement of hepatic steatosis after initiation of leptin substitution in a leptin-deficient girl. Horm Res Paediatr 2013;79:310-7. [PubMed]
- Boguszewski CL, Paz-Filho G, Velloso LA. Neuroendocrine body weight regulation: integration between fat tissue, gastrointestinal tract, and the brain. Endokrynol Pol 2010;61:194-206. [PubMed]
- Dardeno TA, Chou SH, Moon HS, et al. Leptin in human physiology and therapeutics. Front Neuroendocrinol 2010;31:377-93. [PubMed]
- Safar Zadeh E, Lungu AO, Cochran EK, et al. The liver diseases of lipodystrophy: the long-term effect of leptin treatment. J Hepatol 2013;59:131-7. [PubMed]
- Paz-Filho G, Wong ML, Licinio J. Ten years of leptin replacement therapy. Obes Rev 2011;12:e315-23. [PubMed]
- Moon HS, Dalamaga M, Kim SY, et al. Leptin’s role in lipodystrophic and nonlipodystrophic insulin-resistant and diabetic individuals. Endocr Rev 2013;34:377-412. [PubMed]
- Paz-Filho G, Mastronardi CA, Parker BJ, et al. Molecular pathways involved in the improvement of non-alcoholic fatty liver disease. J Mol Endocrinol 2013;51:167-79. [PubMed]
- Vantyghem MC, Balavoine AS, Douillard C, et al. How to diagnose a lipodystrophy syndrome. Ann Endocrinol (Paris) 2012;73:170-89. [PubMed]
- Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol 2014;220:T47-59. [PubMed]
- Gorden P, Lupsa BC, Chong AY, et al. Is there a human model for the ‘metabolic syndrome’ with a defined aetiology? Diabetologia 2010;53:1534-6. [PubMed]
- Simha V. Metreleptin for metabolic disorders associated with generalized or partial lipodystrophy. Expert Rev Endocrinol Metab 2014;9:205-12.
- Simha V, Szczepaniak LS, Wagner AJ, et al. Effect of leptin replacement on intrahepatic and intramyocellular lipid content in patients with generalized lipodystrophy. Diabetes Care 2003;26:30-5. [PubMed]
- Moran SA, Patten N, Young JR, et al. Changes in body composition in patients with severe lipodystrophy after leptin replacement therapy. Metabolism 2004;53:513-9. [PubMed]
- Endocrinologic and Metabolic Drugs Advisory Committee Briefing Document 2013 [30/01/2014]. Available online: http://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm377929.pdf
- Paz-Filho G, Mastronardi C, Franco CB, et al. Leptin: molecular mechanisms, systemic pro-inflammatory effects, and clinical implications. Arq Bras Endocrinol Metabol 2012;56:597-607. [PubMed]
- Fenton JI, Hursting SD, Perkins SN, et al. Interleukin-6 production induced by leptin treatment promotes cell proliferation in an Apc (Min/+) colon epithelial cell line. Carcinogenesis 2006;27:1507-15. [PubMed]
- Mastronardi CA, Yu WH, McCann SM. Resting and circadian release of nitric oxide is controlled by leptin in male rats. Proc Natl Acad Sci U S A 2002;99:5721-6. [PubMed]
- Minokoshi Y, Kim YB, Peroni OD, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 2002;415:339-43. [PubMed]
- Suzuki A, Okamoto S, Lee S, et al. Leptin stimulates fatty acid oxidation and peroxisome proliferator-activated receptor alpha gene expression in mouse C2C12 myoblasts by changing the subcellular localization of the alpha2 form of AMP-activated protein kinase. Mol Cell Biol 2007;27:4317-27. [PubMed]
- Asilmaz E, Cohen P, Miyazaki M, et al. Site and mechanism of leptin action in a rodent form of congenital lipodystrophy. J Clin Invest 2004;113:414-24. [PubMed]
- Shimomura I, Hammer RE, Ikemoto S, et al. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 1999;401:73-6. [PubMed]
- Chou K, Perry CM. Metreleptin: first global approval. Drugs 2013;73:989-97. [PubMed]
- Beltrand J, Beregszaszi M, Chevenne D, et al. Metabolic correction induced by leptin replacement treatment in young children with Berardinelli-Seip congenital lipoatrophy. Pediatrics 2007;120:e291-6. [PubMed]
- Beltrand J, Lahlou N, Le Charpentier T, et al. Resistance to leptin-replacement therapy in Berardinelli-Seip congenital lipodystrophy: an immunological origin. Eur J Endocrinol 2010;162:1083-91. [PubMed]
- Chan JL, Lutz K, Cochran E, et al. Clinical effects of long-term metreleptin treatment in patients with lipodystrophy. Endocr Pract 2011;17:922-32. [PubMed]
- Chong AY, Lupsa BC, Cochran EK, et al. Efficacy of leptin therapy in the different forms of human lipodystrophy. Diabetologia 2010;53:27-35. [PubMed]
- Ebihara K, Kusakabe T, Hirata M, et al. Efficacy and safety of leptin-replacement therapy and possible mechanisms of leptin actions in patients with generalized lipodystrophy. J Clin Endocrinol Metab 2007;92:532-41. [PubMed]
- Javor ED, Ghany MG, Cochran EK, et al. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology 2005;41:753-60. [PubMed]
- Oral EA, Simha V, Ruiz E, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med 2002;346:570-8. [PubMed]
- Park JY, Javor ED, Cochran EK, et al. Long-term efficacy of leptin replacement in patients with Dunnigan-type familial partial lipodystrophy. Metabolism 2007;56:508-16. [PubMed]
- Petersen KF, Oral EA, Dufour S, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest 2002;109:1345-50. [PubMed]
- Simha V, Subramanyam L, Szczepaniak L, et al. Comparison of efficacy and safety of leptin replacement therapy in moderately and severely hypoleptinemic patients with familial partial lipodystrophy of the Dunnigan variety. J Clin Endocrinol Metab 2012;97:785-92. [PubMed]
- Javor ED, Cochran EK, Musso C, et al. Long-term efficacy of leptin replacement in patients with generalized lipodystrophy. Diabetes 2005;54:1994-2002. [PubMed]
- Oral EA, Javor ED, Ding L, et al. Leptin replacement therapy modulates circulating lymphocyte subsets and cytokine responsiveness in severe lipodystrophy. J Clin Endocrinol Metab 2006;91:621-8. [PubMed]
- Chou SH, Chamberland JP, Liu X, et al. Leptin is an effective treatment for hypothalamic amenorrhea. Proc Natl Acad Sci U S A 2011;108:6585-90. [PubMed]
- Machado MV, Cortez-Pinto H. Leptin in the treatment of lipodystrophy-associated nonalcoholic fatty liver disease: are we there already? Expert Rev Gastroenterol Hepatol 2013;7:513-5. [PubMed]
- Mutter D, Soler L, Marescaux J. Recent advances in liver imaging. Expert Rev Gastroenterol Hepatol 2010;4:613-21. [PubMed]
- Matarese G, La Rocca C, Moon HS, et al. Selective capacity of metreleptin administration to reconstitute CD4+ T-cell number in females with acquired hypoleptinemia. Proc Natl Acad Sci U S A 2013;110:E818-27. [PubMed]
- Casey SP, Lokan J, Testro A, et al. Post-liver transplant leptin results in resolution of severe recurrence of lipodystrophy-associated nonalcoholic steatohepatitis. Am J Transplant 2013;13:3031-4. [PubMed]


