Oral tyrosine kinase inhibitors targeting VEGF-receptors in patients with metastatic colorectal cancer
Our understanding of cancer genomics and proteomics associated with normal and malignant cell growth and angiogenesis has increased exponentially in recent years and has resulted in the identification of several critical molecular events that are fundamentally involved in carcinogenesis and tumor progression. Targeting these key ligands, receptors and molecular pathways offers survival benefit in several cancers such as breast cancer, colon cancer and lung cancer.
It is a decade ago since the first targeted drugs proved their efficacy in the treatment of patients with metastatic colorectal cancer (mCRC) (1) and since then Food and Drug Administration (FDA) and European Medicines Agency (EMA) have approved a limited number of targeted drugs (cetuximab, panitumumab, bevacizumab, aflibercept, regorafenib) for clinical use in patients with mCRC and a much larger number are in various phases of clinical development.
The anti-epidermal growth factor receptor (EGFR) antibodies—cetuximab and panitumumab—were first successfully implemented in the later line of therapies, and then moved forward into first line therapy. In contrast, the anti-angiogenic antibody bevazicumab was directly introduced in the first line setting and subsequently showed its efficacy in later lines.
In the pivotal BOND study (2), the combination of cetuximab with irinotecan (CetIri) significantly increased response rate (RR) and prolonged progression free survival (PFS) and based on these data CetIri was approved for patients with irinotecan-resistant disease in US and Europe in 2004. Soon after, the benefit of cetuximab and panitumumab as monotherapy was confirmed in patients with chemo-resistant mCRC (1) and as second line in combination with chemotherapy (3,4). Ligand-induced activation of EGFR achieves most of its effect via the RAS-RAF-MAPK pathways, which promote proliferation, invasion, migration and neovascularisation. KRAS mutation in exon 2, found in approximately 40% of mCRC patients, is now an established predictive marker of resistance to anti–EGFR therapy (5,6), but in addition patients with KRAS mutations may even experience inferior outcome if combined with oxaliplatin-containing regimens (7,8). Based on data from a number of phase III studies, cetuximab and panitumumab was subsequently approved in the first line treatment of mCRC patients with KRAS wild-type tumors, in combination with chemotherapy (9,10).
The advantage of anti-EGFR and anti-angiogenic therapy led to hope for additional progress, and it was obvious to test if multi-blockade with a combination of anti-angiogenic and anti-EGFR therapy could further improve survival.
This “add-on principle” was supported by promising data from preclinical models suggesting that increased angiogenic potential may be involved in the resistance to anti-EGFR antibodies (7). Clinical data supported the hypothesis of an increased efficacy of combined therapy as a randomized phase II study (8) comparing the combination of irinotecan, cetuximab and bevazicumab to cetuximab and bevazicumab in patients with pre-treated mCRC showed a higher RR and longer PFS compared to historical data on cetuximab and irinotecan in the BOND study (2,8).
However, despite the above-mentioned promising results on double-blockade in preclinical models and from early clinical data, two large phase III studies—the CAIRO2 and the PACCE studies—failed to confirm this and both trials actually showed that addition of bevazicumab to an anti-EGFR antibody and chemotherapy in chemo-naïve patients was associated with an inferior outcome compared to an anti-EGFR antibody and chemotherapy (11,12).
Another way of achieving multi-blockade is by the use of oral multi-targeted receptor tyrosine kinase (TKI) inhibitors including sunitinib, sorafenib, regorafenib, valatinib, axatinib, cediranib, and brivanib (13-22).
Sunitinib is an inhibitor of several TKI receptors including platelet-derived growth factor receptors (PDGF-R), the vascular endothelial growth factors receptors (VEGFRs), c-KIT, RET and FLT3. Saltz et al. published a phase II trial with sunitinib as mono-therapy in 82 patients with chemo-resistent mCRC (23). One patient achieved a partial response. Median PFS in the prior bevacizumab and bevacizumab-naıve cohorts was 2.2 and 2.5 months, respectively, whereas median overall survival (OS) was 7.1 and 10.2 months, respectively. The authors concluded that sunitinib did not demonstrate a meaningful single-agent activity, but the mechanisms of action, the relative mild safety profile and easy administration warranted further study in combination with standard chemotherapy regimens for mCRC.
In a phase I study, the maximum tolerated dose (MTD) of sunitinib combined with FOLFIRI for untreated mCRC was 37.5 mg/day when administered 4 weeks on and 2 weeks off (24). The predominant dose limiting toxicity (DLT) was neutropenia. The authors concluded that the combination had acceptable tolerability and showed preliminary antitumor activity and based on these promising data a large phase III study comparing FOLFIRI plus placebo or FOLFIRI and sunitinib was initiated—without a phase II study—to confirm the activity of sunitinib in mCRC. The primary aim was to prolong PFS from 8.0 months to 10.8 months (35% improvement), which would require 568 events (16).
Two interim analyses were planned at 25% and 60% of the 568 PFS events, and the stopping boundary for futility at the second interim analysis was a hazard ratio (HR) of ≥0.88. A final analysis was planned after inclusion of 720 patients.
Enrolment began in July 2007. At the second interim analysis in June 2009, after enrolment was complete and 367 PFS events had occurred, the HR for PFS was 1.095 in favour of the placebo arm. There were also increased toxic events (including neutropenia and diarrhoea and numerically a larger number of toxic deaths) in patients receiving sunitinib plus FOLFIRI.
As mentioned, two interim analyses were planned. The authors do not disclose the result of the first interim analysis, and they do not explain why 48 supplementary patients were included. Shortening of the time to approval of new drugs is crucial, however it is important that interim analyses can terminate a trial before inclusion of the planned number of patients—especially if a phase III study is built directly upon a phase I study.
As shown in Table 1, sunitinib is not the only oral multi-TKI inhibitor that has failed to improve OS in mCRC patients. So far the only randomized phase III study in which an oral multi-TKI inhibitor has prolonged PFS and OS is the CORRECT trial (21), in which regorafenib monotherapy prolonged PFS from 1.7 to 1.9 months (HR, 0.49) and OS from 5.0 to 6.4 months (HR, 0.77).
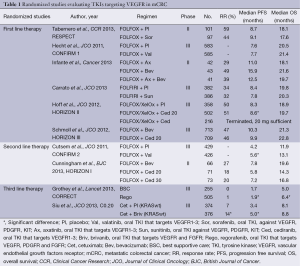
Full table
One of the most important advances in recent years in the treatment of patients with mCRC is the translational studies discovering the impact of the KRAS mutational status on efficacy of anti-EGFR therapy as described above. Recently, retrospective analyses of prospective randomized studies have demonstrated that additional mutations in KRAS and NRAS predict a lack of efficacy to anti-EGFR therapy. Therefore the European label for panitumumab (10) and cetuximab (25) was recently modified to require testing for KRAS and NRAS mutations and in addition a meta-analysis suggests that mutation in BRAF and PIK3CA and a non-functional PTEN also predict resistance to anti-EGFR therapies (26). Some of the multi-TKI inhibitors have improved PFS; however without translation into improvements in OS (17,19,22) and thus may have efficacy in subgroups of patients.
It is therefore very important that clinical studies—also in late lines of therapy—are combined with translational studies in order to improve our knowledge of the biology of mCRC and the identification of new predictive markers. However, it is important that these marker studies do not solely focus on the targeted agents but as well aim to identify predictive markers for the “classic cytostatics” in order to further improve outcome for patients with mCRC.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Pfeiffer P, Qvortrup C, Eriksen JG. Current role of antibody therapy in patients with metastatic colorectal cancer. Oncogene 2007;26:3661-78. [PubMed]
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337-45. [PubMed]
- Sobrero AF, Maurel J, Fehrenbacher L, et al. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:2311-9. [PubMed]
- Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:4706-13. [PubMed]
- Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med 2009;360:1408-17. [PubMed]
- Douillard JY, Siena S, Cassidy J, et al. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 2010;28:4697-705. [PubMed]
- Viloria-Petit A, Crombet T, Jothy S, et al. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: a role for altered tumor angiogenesis. Cancer Res 2001;61:5090-101. [PubMed]
- Saltz LB, Lenz HJ, Kindler HL, et al. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 study. J Clin Oncol 2007;25:4557-61. [PubMed]
- Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023-34. [PubMed]
- Bokemeyer C, Van Cutsem E, Rougier P, et al. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer 2012;48:1466-75. [PubMed]
- Tol J, Koopman M, Cats A, et al. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med 2009;360:563-72. [PubMed]
- Hecht JR, Mitchell E, Chidiac T, et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol 2009;27:672-80. [PubMed]
- Tabernero J, Garcia-Carbonero R, Cassidy J, et al. Sorafenib in combination with oxaliplatin, leucovorin, and fluorouracil (modified FOLFOX6) as first-line treatment of metastatic colorectal cancer: the RESPECT trial. Clin Cancer Res 2013;19:2541-50. [PubMed]
- Hecht JR, Trarbach T, Hainsworth JD, et al. Randomized, placebo-controlled, phase III study of first-line oxaliplatin-based chemotherapy plus PTK787/ZK 222584, an oral vascular endothelial growth factor receptor inhibitor, in patients with metastatic colorectal adenocarcinoma. J Clin Oncol 2011;29:1997-2003. [PubMed]
- Infante JR, Reid TR, Cohn AL, et al. Axitinib and/or bevacizumab with modified FOLFOX-6 as first-line therapy for metastatic colorectal cancer: a randomized phase 2 study. Cancer 2013;119:2555-63. [PubMed]
- Carrato A, Swieboda-Sadlej A, Staszewska-Skurczynska M, et al. Fluorouracil, leucovorin, and irinotecan plus either sunitinib or placebo in metastatic colorectal cancer: a randomized, phase III trial. J Clin Oncol 2013;31:1341-7. [PubMed]
- Hoff PM, Hochhaus A, Pestalozzi BC, et al. Cediranib plus FOLFOX/CAPOX versus placebo plus FOLFOX/CAPOX in patients with previously untreated metastatic colorectal cancer: a randomized, double-blind, phase III study (HORIZON II). J Clin Oncol 2012;30:3596-603. [PubMed]
- Schmoll HJ, Cunningham D, Sobrero A, et al. Cediranib with mFOLFOX6 versus bevacizumab with mFOLFOX6 as first-line treatment for patients with advanced colorectal cancer: a double-blind, randomized phase III study (HORIZON III). J Clin Oncol 2012;30:3588-95. [PubMed]
- Van Cutsem E, Bajetta E, Valle J, et al. Randomized, placebo-controlled, phase III study of oxaliplatin, fluorouracil, and leucovorin with or without PTK787/ZK 222584 in patients with previously treated metastatic colorectal adenocarcinoma. J Clin Oncol 2011;29:2004-10. [PubMed]
- Cunningham D, Wong RP, D'Haens G, et al. Cediranib with mFOLFOX6 vs bevacizumab with mFOLFOX6 in previously treated metastatic colorectal cancer. Br J Cancer 2013;108:493-502. [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [PubMed]
- Siu LL, Shapiro JD, Jonker DJ, et al. Phase III randomized, placebo-controlled study of cetuximab plus brivanib alaninate versus cetuximab plus placebo in patients with metastatic, chemotherapy-refractory, wild-type K-RAS colorectal carcinoma: the NCIC Clinical Trials Group and AGITG CO.20 Trial. J Clin Oncol 2013;31:2477-84. [PubMed]
- Saltz LB, Rosen LS, Marshall JL, et al. Phase II trial of sunitinib in patients with metastatic colorectal cancer after failure of standard therapy. J Clin Oncol 2007;25:4793-9. [PubMed]
- Starling N, Vázquez-Mazón F, Cunningham D, et al. A phase I study of sunitinib in combination with FOLFIRI in patients with untreated metastatic colorectal cancer. Ann Oncol 2012;23:119-27. [PubMed]
- Tejpar S, Lenz HJ, Köhne CH, et al. Effect of KRAS and NRAS mutations on treatment outcomes in patients with metastatic colorectal cancer (mCRC) treated first-line with cetuximab plus FOLFOX4: New results from the OPUS study. J Clin Oncol 2014;32:abstr LBA444.
- Therkildsen C, Bergmann TK, Henrichsen-Schnack T, et al. The predictive value of KRAS, NRAS, BRAF, PIK3CA and PTEN for anti-EGFR treatment in metastatic colorectal cancer: A systematic review and meta-analysis. Acta Oncol 2014;53:852-64. [PubMed]


