Emerging issues in multimodality treatment of gastric cancer
Introduction
Gastric cancer is the fifth most common malignancy worldwide with large geographic differences in incidence (1-3). The highest incidences are encountered in Eastern Asia (3), 35.4 and 5.4 per 100,000 per year for males and females respectively (2). In descending order are the incidences per 100,000 persons per year for males and females respectively 20.3 and 0.8 in Central-Eastern Europe (2), 14.2 and 2.0 in South America (2), 5.5 and 1.1 in Northern America, and 3.3 and 0.4 in Western Africa (2,3). Overall, gastric cancer is twice as common in men compared to women (2,3). These differences in gastric cancer incidence reflect etiologic heterogeneity (3,4).
Gastric cancer is worldwide the third most common cause of cancer death and responsible for 9% of cancer-related death yearly (2). Despite large geographic differences in survival (1-3), overall, mortality rates almost resemble the incidence rates (3). Whereas, the 5-year overall survival (OS) is around 25% in Europe and the United States, this is up to 60% in Asia (2,3,5). The higher survival rates in Asia are ascribed to mass screening programs in Japan, high accuracy of staging that is accompanied by stage migration, and high quality of surgery (3,6-9).
For gastric cancer, surgery remains indispensable for curative treatment. Patients with non-metastasized gastric cancer at diagnosis are eligible for potentially curative surgery if the tumor can be resected with free margins, i.e., resectable gastric cancer. However, even after potentially curative surgery gastric cancer patients have a high risk of locoregional recurrence, peritoneal carcinomatosis and distant metastases, in both Asian and Western countries (10-14). This risk increases with advanced tumor stage and can be as high as 88% locoregional recurrence, 44% peritoneal carcinomatosis, and 49% distant metastases in autopsy series (10). Recurrence patterns are also histological type dependent (15). For example, patients with a diffuse type gastric cancer (16) have a higher risk of peritoneal carcinomatosis than patients with an intestinal type, especially when the tumor has infiltrated the serosa (15).
Different multimodality treatments added to surgery have been investigated for locally advanced resectable gastric cancer. Whereas multiple multimodality strategies have been proven beneficial, which gastric cancer patients benefit most from which treatment modality remains a matter of debate. This review covers a comprehensive analysis of outcome and toxicity of clinical trials investigating multimodality treatment for locally advanced resectable gastric cancer to provide insight in patient groups that may benefit from certain treatments.
Surgery
The obvious goal of surgery is to achieve a microscopically complete resection of the primary tumor, known as an R0 resection, and full clearance of possibly affected regional lymph nodes (17). A microscopically tumor positive luminal resection margin, known as an R1 resection, has been reported in 2-22% of patients (18-21). Irrespective of its association with advanced tumor stage and aggressive tumor biology, an R1 resection has frequently been identified as an independent poor prognostic factor (18,21-24), justifying the use of peroperative frozen sections (25). Clear guidelines regarding patient management in case of an R1 resection are lacking. When an R1 resection is assessed by frozen section examination during surgery and a tumor negative resection margin can still be obtained, extended surgery is a clear option (26). Extended surgery is, however, disputable if that entails a distal esophagectomy or pancreaticoduodenectomy both carrying substantially increased morbidity (27). When an R1 resection is assessed postoperatively, options vary from watchful waiting (28), to re-resection in patients with limited nodal disease (23) or re-resection whenever feasible (20,24). The possible benefit of performing a re-resection is mainly based on the rationale that obtaining tumor negative margins can negate the adverse prognostic impact of tumor positive margins (29).
The development of gastric cancer surgery entailed the selection of patients who could benefit from a partial, instead of a total gastrectomy. Currently it is standard of care to perform a partial gastrectomy when tumor free margins can be obtained in distally located tumors as this is proven safely with regard to tumor control, and is accompanied by beneficial effects on nutritional status, quality of life (30,31) and reduced surgical morbidity and mortality (32,33). However, the risk of an R1 resection in diffuse type gastric cancer according to the Lauren classification (16) is high and may be reason to extend the surgical resection or even to consider a total gastrectomy irrespective of the tumor location, especially in young patients (21).
The extent of the lymph node dissection (LND) has been subject of extensive research. Traditionally, in the East more extended LND, i.e., D2 (lymph node stations 1-11 according to the Japanese classification of gastric cancer) or D3 (lymph node stations 1-14) (34), are routinely performed and their benefit regarding OS is confirmed by randomized controlled trials (13,14). An even more extended lymphadenectomy including para-aortic lymph nodes, i.e., D4 (lymph node stations 1-16), does not seem to add to the survival benefit (13). In Western countries, a D1 LND (lymph node stations 1-6) used to be common practice and a shift towards standard performance of a D2 LND has in recent years (12,35). The benefit of a D2 LND was not adopted until the 15-year follow-up results of the randomized Dutch Gastric Cancer Trial showed that a D2 LND was associated with significantly less gastric cancer-related death and less local recurrences compared to a D1 LND (12). Short term results had not shown an OS benefit for patients who had undergone a D2 LND compared to a D1 LND (36,37). A similar observation was made in the MRC randomized trial (33). In both trials the lack of benefit on OS was explained by the higher postoperative mortality in the D2-group that was caused by the higher percentages of pancreatico-splenectomies to enable dissection of lymph node stations 10 and 11 (33,37); i.e., the higher short-term mortality offset the long-term benefit on OS. This hypothesis was confirmed by a subgroup analysis of patients who had undergone a D1 or D2 LND without pancreatico-splenectomy that showed a significantly higher 15-year OS in those who had a D2 LND (22% vs. 35%; HR, 1.34; 95% CI: 1.09-1.65; P=0.006) (12). Patients with advanced disease and lymph node metastases may benefit more from a D2 LND than those with limited disease (37,38), except for patients with lymph node metastases in the splenic hilus (lymph node station 10). Nodal metastases at this site indicate a very poor prognosis which will not improve after removal of the affected lymph nodes that necessitates a splenectomy (25,37). At current times, surgeons are advised to perform a D2 LND involving lymph node stations 1-9 and 11 with the removal of at least 15 nodes without routine spleen and pancreatic tail resection, sometimes also nominated as a D1+ LND (12,17). With this approach, surgical mortality and morbidity rates can be reduced, as confirmed by an Italian randomized D1-D2 trial (38). Taken together, in recent years this has led to the adoption of the standard performance of a D2 LND in Western countries.
In general, a D2 LND reduces the risk of locoregional recurrence down to 7-28% (11,13,14), but does not influence the risk of peritoneal carcinomatosis or distant metastases (11-14). Also, although gastric cancer surgery has been optimized and the 5-year OS has been improved, the prognosis still remains dismal. Hence, disappointing long-term results after optimal surgery emphasize the need to develop multimodality treatments that are more effective.
Chemotherapy
Postoperative chemotherapy
The rationale for adding postoperative chemotherapy to the treatment of locally advanced resectable gastric cancer is to improve OS by eradicating remaining micrometastases that upon outgrowth are responsible for relapse. Multiple, predominantly fluoropyrimidine-based, postoperative chemotherapy regimens have been investigated resulting in conflicting evidence of efficacy, with mainly positive results for trials conducted in Asia and negative results for trials conducted in Western countries (Table 1).
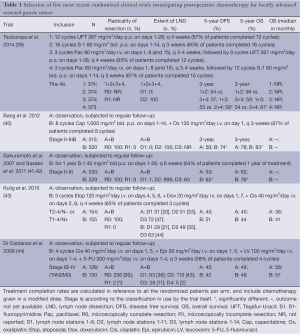
Full table
One of the first clinical trials that clearly showed survival benefit by adding postoperative chemotherapy was conducted in Japan (41). Patients (n=1,059) were randomized after potentially curative surgery including at least a D2 LND, for observation-only vs. postoperative treatment with S-1 monotherapy for 1 year. The results of the first interim analysis were disclosed because the 3-year OS in the S-1 group was significantly higher: 80.1% vs. 70.1% (HR, 0.68; 95% CI: 0.52-0.87; P=0.003) (41). This was later confirmed by a significantly higher 5-year OS: 71.7% vs. 61.1% (HR, 0.67; 95% CI: 0.54-0.83) (42). These results have led to standard postoperative treatment with S-1 after surgery for stage II and III gastric cancer patients in Japan and other East-Asian countries (45).
Another postoperative chemotherapy regimen for stage II and III gastric cancer consists of capecitabine in combination with oxaliplatin (CAPOX) that has been investigated in Korea (40,45). Data of this so-called CLASSIC trial (n=1,035) have not been finalized yet, but the results of the first interim analysis were also disclosed because the 3-year disease free survival (DFS) was significantly higher in patients randomized for 6 months capecitabine and oxaliplatin than those randomized for observation-only after surgery in combination with a D2 LND: 74% vs. 59% (HR, 0.56; 95% CI: 0.44-0.72; P<0.0001). A trend towards improved OS in the CAPOX-arm was also observed after 3 years (HR, 0.72; 95% CI: 0.52-1.00; P=0.0493). The data are however immature and patient follow-up is ongoing (40). The addition of postoperative CAPOX seemed to reduce locoregional recurrences and distant metastases, but not peritoneal carcinomatosis (40). The addition of postoperative S-1, on the other hand, significantly reduced locoregional recurrences and peritoneal carcinomatosis, but not distant metastases (41). Together, these large-scale Asian trials provide sufficient evidence for the efficacy of postoperative fluoropyrimidine-based chemotherapy after potentially curative surgery in combination with a D2 LND.
The most recently published Asian trial investigating postoperative chemotherapy for gastric cancer, Stomach cancer Adjuvant Multi-Institutional group Trial (SAMIT, n=1,495), compared four treatment groups in a two-by-two factorial design (39). The four treatments consisted of UFT-monotherapy, S-1 monotherapy, paclitaxel followed by UFT, and paclitaxel followed by S-1. Sequential chemotherapy treatment did not improve DFS nor OS compared to monotherapy. S-1 seemed superior to UFT (3-year DFS UFT: 53.0%; 95% CI: 49.2-56.6; S-1: 58.2%; 95% CI: 54.4-61.8; HR, 0.81; 95% CI: 0.70-0.93; P=0.0048; P non-inferiority =0.151).
Multiple randomized controlled trials investigating postoperative chemotherapy for resected gastric cancer have been conducted in the West (43,44,46-49). And yet, none of these has provided similar positive results as the Asian trials. The lack of effectiveness was initially ascribed to the use of old regimens (46,47) but newer regimens did not prove to be effective either (44,48,49). Multiple factors have been suggested to play a role in the different outcomes after chemotherapy for Asian and Western populations, among others patient- and tumor characteristics including ethnic variability in genes regarding the drug metabolizing enzymes (40,42,50,51), the poor compliance of patients to the full chemotherapy regimen (44), the use of different surgical techniques (45) or the small sample sizes. Several meta-analyses have been performed to investigate a possible positive effect of postoperative chemotherapy, but also showed conflicting results (52-56). The (subgroup) meta-analysis that included only Western trials showed a non-significant small benefit of postoperative chemotherapy for resectable gastric cancer (53,55,57). Hence, postoperative chemotherapy is not routinely advised for gastric cancer patients in the West (35).
Preoperative and perioperative chemotherapy
The main rationale for administration of preoperative chemotherapy is to improve OS by eradicating micrometastases as early as possible and to improve surgical results by downsizing and/or downstaging of the tumor (Table 2). The first randomized controlled trials that observed a beneficial effect of adding perioperative chemotherapy for resectable gastric cancer was the British Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC, n=503) trial (60). Patients were randomly assigned to surgery-only or preoperative chemotherapy followed by surgery and postoperative chemotherapy, consisting of epirubicine, cisplatin and fluorouracil. The results showed significantly improved R0 resection rates, 5-year relapse-free survival (HR, 0.66; 95% CI: 0.53-0.81; P<0.001) and an absolute 5-year OS benefit of 13% for the perioperative chemotherapy-arm (36% vs. 23%; HR, 0.75; 95% CI: 0.60-0.93; P=0.009). The benefits of perioperative chemotherapy were not at the cost of higher surgical morbidity and mortality (60). The French FNCLCC-FFCD trial (n=224) was the second randomized controlled trial in which the role of perioperative chemotherapy in gastric cancer was investigated, although in the majority of patients the tumor was located in the lower esophagus or at the gastro-esophageal junction (58). In this trial chemotherapy consisted of 2-3 preoperative and 3-4 postoperative cycles of fluorouracil and cisplatin, to a total of 6 cycles. Again, perioperative chemotherapy significantly improved R0 resection rates (84% vs. 73%; P=0.04), 5-year DFS and 5-year OS, without increasing surgical morbidity and mortality (58). After perioperative chemotherapy both local (60) or locoregional recurrences (58) and distant metastases were decreased (58,60). Consequently, in Europe perioperative chemotherapy became the new standard of care in patients with resectable gastric cancer (35).
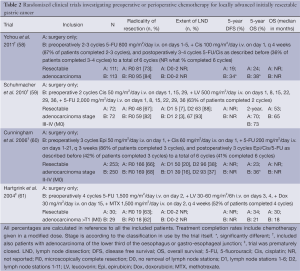
Full table
More recently, multiple phase II and III studies investigating preoperative chemotherapy have also been initiated in Asia (45,62-64). In Asia, this approach was firstly investigated in patients who are at high risk for peritoneal carcinomatosis and distant metastases, i.e., locally advanced marginally resectable Bormann type 3 and 4 (65), para-aortic/bulky nodal disease (66), and/or serosa positive/T4a (67) gastric cancer. Phase III trials are initiated following promising results of phase II trials. For example, the JCOG 0501 trial randomizes patients with resectable Bormann type 3 or 4 gastric cancer for surgery followed by postoperative S-1 for 1 year, vs. perioperative chemotherapy consisting of 2 preoperative cycles S-1 and cisplatin followed by surgery and postoperative S-1 for 1 year (ClinicalTrials.gov number NCT00252161). The results of these trials will contribute to define the role of preoperative chemotherapy in Asia.
In the MAGIC and FNCLCC-FFCD trials a significant proportion of the patients could not start and/or complete postoperative chemotherapy as planned (58,60). Therefore, the beneficial effect observed in these trials is often attributed to the preoperative chemotherapy only. Subsequently, studies that investigate the benefit of purely preoperative chemotherapy were again initiated. An example is the EORTC 40954 trial (n=144), that randomized patients for surgery-only or preoperative chemotherapy followed by surgery. This trial was closed prematurely due to a low accrual rate, and failed to demonstrate an OS benefit despite the significant higher R0 resection rate in the preoperative chemotherapy group (59). Today, it remains difficult to acknowledge the beneficial effect of preoperative and postoperative chemotherapy separately.
Toxicity of and treatment compliance with chemotherapy
The most common adverse events of preoperative and/or postoperative chemotherapy in gastric cancer patients were hematological and gastrointestinal (40,41,58,60). Less severe toxicity, grade 1 and 2, was very common in this patient population (40,41,58,60). Especially with combination chemotherapy, grade 1 and 2 side effects can be present in up to 99% of patients (40,58,60). The common occurrence of adverse events is reflected in the high percentages of chemotherapy dose modifications up to 42-90% (40,41).
More severe toxicity, grade 3 and 4, was present in up to 27% of patients per scored item (Table 3). In comparison to the patients who were treated with surgery-only, several severe grade 3-4 adverse events were more common in the patients treated with chemotherapy (40,41). Unfortunately not all before mentioned trials reported adverse events for the surgery-only group, hampering comparison (58,60). Interestingly, no significant differences in preoperatively and postoperatively occurring adverse events was found in the MAGIC trial (60), which could be explained by the selection of patients that started postoperative chemotherapy. Severe side effects depend on the chemotherapy regimen and were reported more frequently for combination chemotherapy compared to for example S-1 monotherapy with the exception of anorexia (40,41,58,60). This finding was also observed in the SAMIT trial with the exception of anorexia, nausea and vomiting (39). The reported percentages of deceased patients related to the treatment with chemotherapy were between 0-3% (39-41,43,44,58,60).
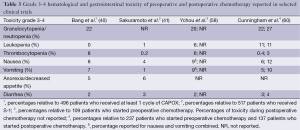
Full table
In the MAGIC trial 5% of patients stopped preoperative chemotherapy due to toxicity. Reasons for discontinuation of postoperative chemotherapy were not reported (60). In the FNCLCC-FFCD trial toxicity was the main reason to discontinue preoperative chemotherapy in 8% of patients, reasons for discontinuation of postoperative chemotherapy were again not reported (58). Discontinuation of postoperative S-1 due to adverse events or complications occurred in 14% (41) and discontinuation of CAPOX because of adverse events occurred in 10% of patients (40). However, this might be an underestimation due to the selection of patients for these trials who had to be well recovered after surgery.
In the five most recent randomized controlled trials that investigated postoperative chemotherapy (Table 1), compliance with the entire treatment regimen was 58-67% (39-41,43,44). In these trials, no information on the number of patients who were not eligible for postoperative treatment (and thus not for the trial), was provided. This limits the opportunity to discuss feasibility and treatment compliance with postoperative chemotherapy in a clinical setting. In the MAGIC and the FNCLCC-FFCD trial, compliance with chemotherapy was higher when administered before surgery than after surgery. While more than 95% and 85% (or 90% of those started) of patients could start and complete preoperative chemotherapy respectively, only around 50% and 40% (or 75% of those started) could start and complete postoperative chemotherapy respectively (58,60).
Chemoradiotherapy (CRT)
Postoperative CRT
The high rate of locoregional recurrences after potentially curative surgery for advanced gastric cancer makes CRT an attractive postoperative treatment modality (Table 4). The first randomized study that observed an OS benefit for gastric cancer patients by adding another treatment modality was the US intergroup-0116 trial (n=556) (74). This trial randomly assigned patients who had undergone an R0 resection to observation-only or postoperative CRT. Postoperative CRT, consisting of 45 Gy irradiation with concurrent fluorouracil/leucovorin on radiotherapy (RT) days 1-4 and 31-33, and preceded by 1 cycle and followed by 2 cycles of fluorouracil/leucovorin during 5 days, improved the median OS by 9 months (36 vs. 27 months; HR, 1.35; 95% CI: 1.09-1.66; P=0.005) and the median relapse-free survival by 11 months (30 vs. 19 months; HR, 1.52; 95% CI: 1.23-1.86; P<0.001) (74). In the updated analysis of this trial at a median follow-up time of 10 years for living patients, the benefit of postoperative CRT persisted equally strongly (HR for OS 1.32; 95% CI: 1.10-1.60; P=0.0046; HR for RFS 1.51; 95% CI: 1.25-1.83; P<0.001) (75). The lower rate of locoregional recurrence in the postoperative CRT group compared to the observation-only group (24% vs. 47%) confirms that the survival benefit of CRT is mainly caused by increased locoregional control. These results have led to the standard use of postoperative CRT in the United States (76,77). In Europe and Asia the administration of postoperative CRT is limited to specific indications (35,45).
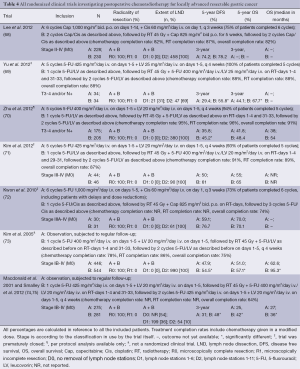
Full table
Several data indicate that postoperative CRT is especially effective in gastric cancer patients with lymph node positive disease. The ARTIST trial (n=458), in which patients were randomized to postoperative CRT including 4 cycles chemotherapy vs. chemotherapy-only after an R0 resection with a D2 LND, showed no significant difference in DFS between study arms. However, for patients with pathological tumor positive lymph nodes, DFS at 3 years was significantly better in the CRT-arm (77.5%) than in the chemotherapy-arm (72.3%) (68). In addition, a survival benefit for node positive gastric cancer patients treated with RT was reported by a meta-analysis performed by Ohri et al. (HR, 0.73; 95% CI: 0.62-0.86; P<0.001) (78). Furthermore, in the prospective trials with beneficial outcomes, the majority of patients had node positive disease (69-71,73,74), and subgroup analysis suggests a stronger benefit when more lymph nodes are affected (75).
As the majority of patients in the INT-0116 trial had undergone a D0 LND (54%) (74), it has been argued that postoperative CRT compensated for suboptimal surgery although subset analysis did not show a lack of benefit for patients with a D2 LND (75). Thereafter, several studies have investigated the efficacy of postoperative CRT after a D2 LND but thus far no conclusive evidence has been provided (68-73,78,79). The largest of these studies, an Korean observational study (n=990) with a similar CRT regimen as in the INT-0116 trial, showed a significant relapse free and OS benefit for patients treated with postoperative CRT after D2 gastric cancer surgery compared to patients who were treated with D2 surgery alone (73). In a Chinese trial (n=380) by Zhu et al. in which patients were randomized to postoperative CRT vs. chemotherapy after an R0 gastric cancer resection with a D2 LND, the 5-year local recurrence rate was significantly decreased in the CRT-arm (70), which was also observed in similarly designed other studies (69,71). In a second Chinese trial (n=68) that was prematurely closed, also a significantly increased OS for patients treated with postoperative CRT was observed (69). The abovementioned meta-analysis showed a significantly improved DFS after D2 surgery followed by CRT, but not an improved OS (78). The detection of an OS benefit was hampered by the small sample size. Also, all studies used for the analysis tested CRT against chemotherapy. Moreover, the heterogeneity of the included trials regarding RT treatment regimens was large (78). Taken together, postoperative CRT reduces locoregional recurrences (69,73,74,78,79) that results in a survival benefit (69,73,74,78), also after adequate D2 gastric cancer surgery (69,73).
As yet, postoperative CRT in gastric cancer treatment has been investigated almost invariably in patients who had undergone an R0 resection (68-74). However, as postoperative CRT increases locoregional control after surgery, patients with an R1 resection may benefit from such intensified local treatment as well. In a retrospective analysis (n=83) from Dikken et al. postoperative CRT after an R1 resection decreased the local recurrence rate (6% vs. 29% in the surgery-only R1 group, HR, 5.36; P=0.02) and improved the 2-year OS rate (66% vs. 29% in the surgery-only R1 group, HR, 2.91; P=0.002) (79). Another retrospective study (n=110) found that in patients treated with postoperative CRT after an R0 or R1 resection, an R1 resection was not associated with a higher tumor recurrence rate, nor did it lead to poorer OS (80). This finding suggests that the poor prognosis associated with an R1 resection may be offset by the use of postoperative CRT. This hypothesis was further investigated in a national Dutch cohort study (n=409). OS after an R1 resection was better in patients who were treated with postoperative CRT compared to patients who did not receive postoperative CRT (81). These results lend support to the use of postoperative CRT in patients who have undergone an R1 gastric cancer resection.
Preoperative CRT
CRT can be administered preoperatively in patients with advanced disease in order to improve R0 resection rates by downstaging and to enhance locoregional tumor control (Table 5). Major concerns of applying this strategy for gastric cancer were delay of or withdrawal from surgery due to toxicity of the CRT, and an increase in surgical morbidity and mortality. To our knowledge no randomized controlled trial applying this strategy in gastric cancer has been completed nor published. In contrast, in patients with esophageal and gastro-esophageal junction cancer, there is convincing evidence from randomized controlled trials that preoperative CRT leads to improved OS (88,94). The phase III German trial (n=62) by Stahl et al. that was prematurely closed, randomized patients with an adenocarcinoma located at the gastro-esophageal junction for induction chemotherapy followed by preoperative CRT and surgery vs. preoperative chemotherapy followed by surgery (88). CRT consisted of 2 cycles’ induction chemotherapy of fluorouracil, leucovorin and cisplatin, followed by 30 Gy irradiation in 3 weeks with concurrent cisplatin and etoposide. Chemotherapy consisted of 2.5 cycles of fluorouracil, leucovorin and cisplatin. Analysis showed a trend towards higher pathological complete response (pCR) and improved OS in the CRT-arm. The currently accruing TOP GEAR trial initiated by the Australasian Gastro-Intestinal Trials Group (ClinicalTrials.gov number NCT01924819) randomizes patients with resectable gastric or gastro-esophageal junction cancer for perioperative chemotherapy and surgery vs. induction chemotherapy, followed by preoperative CRT, surgery and postoperative chemotherapy. This trial has not completed accrual yet, and results have to be awaited.
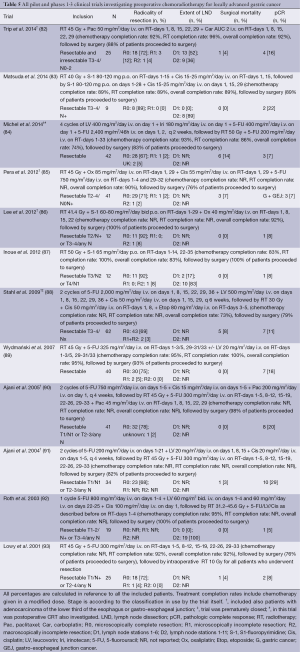
Full table
For gastric cancer specifically, several phase I and II studies have investigated the feasibility and efficacy of preoperative CRT since 2002 (Table 5) (82-87,89-93). In all of these studies preoperative CRT has been documented as a feasible treatment strategy, because toxicity of CRT was not the predominant reason of withdrawal from surgery. Indeed, 73-100% of patients could complete the preoperative CRT as planned, and 76-100% could proceed to surgery. Furthermore, surgical mortality rates (0-8%) were well within the range of reported percentages in trials investigating surgery-only (37,95). Encouraging R0 resection rates of 67-92% and pCR rates of 5-29% have been reported (82-93). Locoregional control was reported in approximately 70-80% at 5-year (96,97). Distant metastases have been frequently reported as most common site of relapse (85,87,89,97). This is also true for peritoneal carcinomatosis (82,91,96) while this was significantly decreased after preoperative CRT for esophageal cancer (98).
The high pCR rates raise the question whether preoperative CRT could also induce resectability in patients with locally advanced, but initially irresectable gastric cancer. In the phase I/II study by Trip et al., a subset of patients initially had irresectable disease without signs of peritoneal carcinomatosis confirmed by laparoscopy and without signs of distant metastases on diagnostic imaging (82). Eight out of 12 patients (67%) with initially irresectable gastric cancer underwent R0 surgery after preoperative CRT. In this study, preoperative CRT consisted of RT to a total dose of 45 Gy with concurrent weekly paclitaxel and carboplatin.
Toxicity of and treatment compliance with CRT
In general CRT for gastric cancer is an intense but feasible regimen. Several different CRT regimens were used in clinical trials, of which toxicity rates vary (Table 6). In several studies a treatment regimen according to the INT-0116 trial was administered postoperatively. Patients suffered most from hematological (7-54%) and gastrointestinal (1-33%) toxicity grade 3 or higher (69,70,73,74). Based on developments in chemotherapeutic agents, and concurrent CRT regimens in other types of cancer, Jansen et al. performed a series of phase I/II studies to optimize concurrent postoperative CRT for gastric cancer with the aim to define a less toxic regimen. The RT dose was set at 45 Gy, and the concurrent chemotherapy consisted of capecitabine with or without cisplatin (99-101). Acute toxicity was low with 7% grade 3-4 hematological, 5% grade 3-4 nausea, and 2% grade 3-4 vomiting. Similar toxicity rates were observed in other phase III trials that administered postoperative RT in combination with concurrent capecitabine only (68,72).
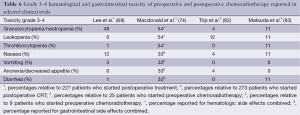
Full table
Although preoperative CRT is not yet investigated in randomized controlled phase III trials, the reported toxicity rates in phase II trials were in line with toxicity rates of postoperative CRT, and for specific regimens even lower. Nonetheless, it remains difficult to conclude that either preoperative or postoperative CRT is less toxic, because the toxicity profiles of preoperative and postoperative CRT have not yet been compared in a randomized controlled phase III trial and because of the use of different CRT regimens. Notable, however, are the low toxicity rates reported for the CRT regimen with concurrent carboplatin and paclitaxel (Tables 5,6) (82), as was also observed in the CROSS trial that administered a similar regimen preoperatively for patients with resectable esophageal cancer (94). The reported percentages of deceased patients related to the treatment with postoperative or preoperative CRT including any additional chemotherapy were between 0-1% (68-74,82,83).
Compliance rates to postoperative CRT including any induction chemotherapy were reported between 64% and 91% (68-74). This seems higher with the newer optimized CRT regimens that use concurrent oral fluoropyrimidines. For example the RT completion rate in the ARTIST trial was 87%, even after 2 courses of induction chemotherapy (68), and up to 89-97% in the phase I/II studies of Jansen et al. (99-101). Compliance rates to preoperative CRT including any induction chemotherapy were reported from 74% up to 95% as investigated in phase I and II studies (82-87,89,93). With the older CRT regimens including any induction chemotherapy, toxicity was the reason to discontinue treatment in 10-19% of patients (69,72-74), while this was around 5% with the newer CRT regimens (68,82,83,100).
Besides acute toxicity of CRT for gastric cancer, late toxicity is important as well, however, few studies reported on this. With CRT for gastric cancer, a large area of the upper abdomen is irradiated, whether this is administered preoperatively or postoperatively (102). As a result, surrounding tissues of the liver, kidneys and spleen, are irradiated as well. The most important late toxicity is radiation-induced nephrotoxicity. This is radiation dose- and volume-dependent, progressive in time, and associated with renovascular hypertension (103-105). The radiation dose to both kidneys (106) should be kept as low as possible to better preserve its function which can be accomplished by the use of highly conformal RT techniques such as Intensity Modulated Radiation Therapy (IMRT) (107) and Image Guided Radiation Therapy (IGRT). A relatively low-dose of concurrent cisplatin (20 mg/m2 i.v. weekly), a well-known nephrotoxic drug, can be administered safely with regard to nephrotoxicity (104,107). However, the consequences of the combination of concurrent cisplatin in a CRT regimen with administrating high-dose cisplatin for example as part of preoperative chemotherapy, are not yet established (107,108).
In contrast to the high amount of consideration that is placed on the kidneys, the spleen is not accounted for when administering CRT for gastric cancer, despite the fact that it is encompassed in the high dose region. However, nowadays the extremely important and unique immunological and hematological functions of the spleen are acknowledged (109-112). Following surgical splenectomy or in case of functional hyposplenia, patients are at an increased risk for fatal thromboembolic events and overwhelming postsplenectomy infections (OPSI) by encapsulated bacteria (109,110). For this matter, guidelines regarding preventive measures, including immunization against encapsulated bacteria such as Streptococcus pneumoniae and prophylactic and on-demand antibiotics have been implemented. Although radiation of the spleen has been associated with hyposplenia, it is largely unknown whether and to what extent the functions of the spleen are affected by radiation, and to what extent we can draw a parallel from radiating the spleen to surgical splenectomy (109), or hyposplenia (111). Guidelines regarding the management of patients that have received irradiation to the spleen have not yet been established.
Discussion
Based on the accumulating evidence of the past decade that multimodality treatment improves OS in locally advanced resectable gastric cancer, all patients should be discussed by multidisciplinary teams and considered for multimodality treatment. The variety on multimodality regimens creates opportunities to improve the treatment of gastric cancer patients by considering subgroups that benefit most from certain treatments.
Both chemotherapy and CRT added to the surgical resection of gastric cancer have shown to improve OS in randomized controlled trials (40,41,60,74). Only a few trials have compared chemotherapy and CRT directly, which were all conducted in Asia (68-72). The outcomes of the completed trials did not show an OS benefit for either one of the treatments, but a trend favoring CRT could often be observed. The meta-analysis by Ohri et al. including these trials, detected a significant beneficial effect of postoperative CRT over chemotherapy (78). Currently, two large-scale phase III randomized controlled trials investigate the possible superiority of CRT to chemotherapy, i.e., the Dutch CRITICS trial initiated by the Dutch Colorectal Cancer Group (ClinicalTrials.gov number NCT00407186) that randomizes patients for perioperative chemotherapy vs. preoperative chemotherapy followed by surgery and postoperative CRT, and the Australian TOP GEAR trial that randomizes patients for perioperative chemotherapy vs. preoperative CRT followed by surgery and postoperative chemotherapy.
One subgroup of gastric cancer patients is formed by patients who have undergone an R1 resection. As an R1 resection is associated with a dismal prognosis, many physicians question whether these patients should continue with a potentially curative treatment regimen. These patients were invariably excluded from randomized controlled trials, and to perform a trial exclusively with these patients is not feasible and may be unethical. Therefore, evidence for optimal treatment is and will be limited to retrospective analyses and/or subgroup analyses of large randomized trials in which no deviations from the randomized treatment arm are made after an R1 resection. Several retrospective analyses from our group have shown a clear benefit from postoperative CRT for gastric cancer patients who had undergone an R1 resection (79-81). In these articles, only a few patients received preoperative chemotherapy, and therefore questions remain on the efficacy of postoperative CRT after an R1 resection when preoperative chemotherapy has been administered. Subgroup analyses of the CRITICS trial might inform us on the efficacy of postoperative CRT under these circumstances (113).
Preferably, an R1 resection and its associated dismal prognosis should be prevented. Preoperative CRT is a promising approach to obtain an R0 resection (82,88,94). This treatment might also have the potential to induce resectability in initially irresectable gastric cancer (82). Pathologic complete response rates after preoperative CRT are independently prognostic for OS in several studies, as well as pCR rates after preoperative chemotherapy (90,114,115). Pathologic CR rates tend to be higher after preoperative CRT compared to chemotherapy alone though (60,88). Rightfully, preoperative CRT is nowadays not anymore confined to the higher located gastro-intestinal tumors such as esophageal and gastro-esophageal junction tumors, but also applied in more proximally located gastric tumors, for example within the TOP GEAR trial.
The majority of gastric cancer patients in the Western part of the world present themselves with lymph node metastases at diagnosis, forming a large subgroup of patients with node positive disease. Consequently, these patients also form the majority in clinical trials investigating the addition of chemotherapy (40,41,58,60) as well as CRT (68,74). In subgroup analyses, postoperative CRT is more beneficial when node positive disease is present than when no lymph node metastases are present (75,78). Moreover, in the subset of node positive patients in the ARTIST trial, postoperative CRT was more beneficial than chemotherapy-only (68). However, this does not mean that node negative patients do not benefit from CRT or from chemotherapy. Hopefully the ARTIST-II trial (ClinicalTrials.gov number NCT01761461) that includes only node positive patients, will further clarify the role of postoperative CRT and chemotherapy in this group of patients.
Other subgroups of patients can be based on the extent of the surgical LND. A lot of debate focusses on the efficacy of additional treatment modalities when optimal surgery, i.e., at least a D1+ LND, has been performed, because in the past the majority of patients in all large-scale clinical trials conducted in the West underwent suboptimal surgery, and outcomes between clinical trials conducted in the West or East that differ in surgical quality, are conflicting. Conceptually, the combination of multimodality treatment and a D2 LND could be overtreatment if these two modalities would both prevent the same relapses, i.e., locoregional recurrences or secondary distant metastases resulting from residual affected lymph nodes. Based on positive outcomes of multimodality treatment, both chemotherapy and CRT, after a D2 LND from all (subsets of) Eastern and Western clinical trials (40,41,60,68,73,74), we can only assume that multimodality treatment is beneficial irrespective to the extent of the LND. Future trials will give further insight in this issue as recently a D2 LND has become the standard of care in Western countries and is applied in currently ongoing trials (113), and as Asian trials are initiated that routinely apply D2 LND.
A major problem concerning the addition of extra treatment modalities to surgery in the treatment of gastric cancer is the accompanied toxicity, when this leads to non-compliance with treatment and especially to the delay of or withdrawal from potentially curative surgery. The reported outcomes of clinical trials thus far refute these concerns. The reported toxicity rates of preoperative chemotherapy and CRT are in general lower than those of postoperative treatment. Furthermore, toxicity rates of the newer optimized CRT regimens are lower than of chemotherapy, either preoperatively or postoperatively. In addition, compliance with preoperative chemotherapy and CRT regimens is higher than with postoperative treatment. Importantly, this higher compliance offers the chance to administer more intensified, combination chemotherapy or CRT. Moreover, preoperative regimens improve pathology-related surgical results without increasing surgical morbidity and mortality. Taken together, preoperative chemotherapy and/or CRT are preferable to postoperative regimens. However this has to be further confirmed in phase III studies.
To conclude, future randomized controlled trials for locally advanced resectable gastric cancer should include preoperative multimodality treatment.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE--5-a population-based study. Lancet Oncol 2014;15:23-34. [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [PubMed]
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137-50. [PubMed]
- Wu H, Rusiecki JA, Zhu K, et al. Stomach carcinoma incidence patterns in the United States by histologic type and anatomic site. Cancer Epidemiol Biomarkers Prev 2009;18:1945-52. [PubMed]
- Akoh JA, Macintyre IM. Improving survival in gastric cancer: review of 5-year survival rates in English language publications from 1970. Br J Surg 1992;79:293-9. [PubMed]
- Macintyre IM, Akoh JA. Improving survival in gastric cancer: review of operative mortality in English language publications from 1970. Br J Surg 1991;78:771-6. [PubMed]
- Bunt AM, Hermans J, Smit VT, et al. Surgical/pathologic-stage migration confounds comparisons of gastric cancer survival rates between Japan and Western countries. J Clin Oncol 1995;13:19-25. [PubMed]
- Wu CW, Hsiung CA, Lo SS, et al. Stage migration influences on stage-specific survival comparison between D1 and D3 gastric cancer surgeries. Eur J Surg Oncol 2005;31:153-7. [PubMed]
- Yoshikawa T, Sasako M, Sano T, et al. Stage migration caused by D2 dissection with para-aortic lymphadenectomy for gastric cancer from the results of a prospective randomized controlled trial. Br J Surg 2006;93:1526-9. [PubMed]
- Gunderson LL. Gastric cancer--patterns of relapse after surgical resection. Semin Radiat Oncol 2002;12:150-61. [PubMed]
- Chang JS, Lim JS, Noh SH, et al. Patterns of regional recurrence after curative D2 resection for stage III (N3) gastric cancer: implications for postoperative radiotherapy. Radiother Oncol 2012;104:367-73. [PubMed]
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [PubMed]
- Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 2008;359:453-62. [PubMed]
- Wu CW, Hsiung CA, Lo SS, et al. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol 2006;7:309-15. [PubMed]
- Roviello F, Marrelli D, de Manzoni G, et al. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J Surg 2003;90:1113-9. [PubMed]
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965;64:31-49. [PubMed]
- Rausei S, Dionigi G, Sano T, et al. Updates on surgical management of advanced gastric cancer: new evidence and trends. Insights from the First International Course on Upper Gastrointestinal Surgery--Varese (Italy), December 2, 2011. Ann Surg Oncol 2013;20:3942-7. [PubMed]
- Bickenbach KA, Gonen M, Strong V, et al. Association of positive transection margins with gastric cancer survival and local recurrence. Ann Surg Oncol 2013;20:2663-8. [PubMed]
- Cunningham SC, Kamangar F, Kim MP, et al. Survival after gastric adenocarcinoma resection: eighteen-year experience at a single institution. J Gastrointest Surg 2005;9:718-25. [PubMed]
- Songun I, Bonenkamp JJ, Hermans J, et al. Prognostic value of resection-line involvement in patients undergoing curative resections for gastric cancer. Eur J Cancer 1996;32A:433-7. [PubMed]
- Stiekema J, Cats A, Kuijpers A, et al. Surgical treatment results of intestinal and diffuse type gastric cancer. Implications for a differentiated therapeutic approach? Eur J Surg Oncol 2013;39:686-93. [PubMed]
- Wang SY, Yeh CN, Lee HL, et al. Clinical impact of positive surgical margin status on gastric cancer patients undergoing gastrectomy. Ann Surg Oncol 2009;16:2738-43. [PubMed]
- Kim SH, Karpeh MS, Klimstra DS, et al. Effect of microscopic resection line disease on gastric cancer survival. J Gastrointest Surg 1999;3:24-33. [PubMed]
- Morgagni P, Garcea D, Marrelli D, et al. Resection line involvement after gastric cancer surgery: clinical outcome in nonsurgically retreated patients. World J Surg 2008;32:2661-7. [PubMed]
- de Steur WO, Dikken JL, Hartgrink HH. Lymph node dissection in resectable advanced gastric cancer. Dig Surg 2013;30:96-103. [PubMed]
- Lee JH, Ahn SH. Clinical impact of tumor infiltration at the transected surgical margin during gastric cancer surgery. J Surg Oncol 2012;106:772-6. [PubMed]
- Cheng CT, Tsai CY, Hsu JT, et al. Aggressive surgical approach for patients with T4 gastric carcinoma: promise or myth? Ann Surg Oncol 2011;18:1606-14. [PubMed]
- Papachristou DN, Agnanti N, D'Agostino H, et al. Histologically positive esophageal margin in the surgical treatment of gastric cancer. Am J Surg 1980;139:711-3. [PubMed]
- Chen JD, Yang XP, Shen JG, et al. Prognostic improvement of reexcision for positive resection margins in patients with advanced gastric cancer. Eur J Surg Oncol 2013;39:229-34. [PubMed]
- Gouzi JL, Huguier M, Fagniez PL, et al. Total versus subtotal gastrectomy for adenocarcinoma of the gastric antrum. A French prospective controlled study. Ann Surg 1989;209:162-6. [PubMed]
- Bozzetti F, Marubini E, Bonfanti G, et al. Subtotal versus total gastrectomy for gastric cancer: five-year survival rates in a multicenter randomized Italian trial. Italian Gastrointestinal Tumor Study Group. Ann Surg 1999;230:170-8. [PubMed]
- Bonenkamp JJ, Songun I, Hermans J, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet 1995;345:745-8. [PubMed]
- Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 1999;79:1522-30. [PubMed]
- Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer 1998;1:10-24. [PubMed]
- Waddell T, Verheij M, Allum W, et al. Gastric cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Eur J Surg Oncol 2014;40:584-91. [PubMed]
- Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999;340:908-14. [PubMed]
- Hartgrink HH, van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 2004;22:2069-77. [PubMed]
- Degiuli M, Sasako M, Ponti A, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg 2014;101:23-31. [PubMed]
- Tsuburaya A, Yoshida K, Kobayashi M, et al. Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): a phase 3 factorial randomised controlled trial. Lancet Oncol 2014;15:886-93. [PubMed]
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [PubMed]
- Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 2007;357:1810-20. [PubMed]
- Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011;29:4387-93. [PubMed]
- Kulig J, Kolodziejczyk P, Sierzega M, et al. Adjuvant chemotherapy with etoposide, adriamycin and cisplatin compared with surgery alone in the treatment of gastric cancer: a phase III randomized, multicenter, clinical trial. Oncology 2010;78:54-61. [PubMed]
- Di Costanzo F, Gasperoni S, Manzione L, et al. Adjuvant chemotherapy in completely resected gastric cancer: a randomized phase III trial conducted by GOIRC. J Natl Cancer Inst 2008;100:388-98. [PubMed]
- Fujitani K. Overview of adjuvant and neoadjuvant therapy for resectable gastric cancer in the East. Dig Surg 2013;30:119-29. [PubMed]
- Tsavaris N, Tentas K, Kosmidis P, et al. A randomized trial comparing adjuvant fluorouracil, epirubicin, and mitomycin with no treatment in operable gastric cancer. Chemotherapy 1996;42:220-6. [PubMed]
- Macdonald JS, Fleming TR, Peterson RF, et al. Adjuvant chemotherapy with 5-FU, adriamycin, and mitomycin-C (FAM) versus surgery alone for patients with locally advanced gastric adenocarcinoma: A Southwest Oncology Group study. Ann Surg Oncol 1995;2:488-94. [PubMed]
- Bouché O, Ychou M, Burtin P, et al. Adjuvant chemotherapy with 5-fluorouracil and cisplatin compared with surgery alone for gastric cancer: 7-year results of the FFCD randomized phase III trial (8801). Ann Oncol 2005;16:1488-97. [PubMed]
- Chipponi J, Huguier M, Pezet D, et al. Randomized trial of adjuvant chemotherapy after curative resection for gastric cancer. Am J Surg 2004;187:440-5. [PubMed]
- Ma BB, Hui EP, Mok TS. Population-based differences in treatment outcome following anticancer drug therapies. Lancet Oncol 2010;11:75-84. [PubMed]
- Shimoyama S. Pharmacogenetics of fluoropyrimidine and cisplatin. A future application to gastric cancer treatment. J Gastroenterol Hepatol 2009;24:970-81. [PubMed]
- Diaz-Nieto R, Orti-Rodríguez R, Winslet M. Post-surgical chemotherapy versus surgery alone for resectable gastric cancer. Cochrane Database Syst Rev 2013;9:CD008415. [PubMed]
- Zhao SL, Fang JY. The role of postoperative adjuvant chemotherapy following curative resection for gastric cancer: a meta-analysis. Cancer Invest 2008;26:317-25. [PubMed]
- Paoletti X, Oba K, Bang YJ, et al. Progression-free survival as a surrogate for overall survival in advanced/recurrent gastric cancer trials: a meta-analysis. J Natl Cancer Inst 2013;105:1667-70. [PubMed]
- Earle CC, Maroun JA. Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: revisiting a meta-analysis of randomised trials. Eur J Cancer 1999;35:1059-64. [PubMed]
- Hermans J, Bonenkamp JJ, Boon MC, et al. Adjuvant therapy after curative resection for gastric cancer: meta-analysis of randomized trials. J Clin Oncol 1993;11:1441-7. [PubMed]
- Janunger KG, Hafström L, Glimelius B. Chemotherapy in gastric cancer: a review and updated meta-analysis. Eur J Surg 2002;168:597-608. [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [PubMed]
- Schuhmacher C, Gretschel S, Lordick F, et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J Clin Oncol 2010;28:5210-8. [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [PubMed]
- Hartgrink HH, van de Velde CJ, Putter H, et al. Neoadjuvant chemotherapy for operable gastric cancer: long term results of the Dutch randomised FAMTX trial. Eur J Surg Oncol 2004;30:643-9. [PubMed]
- Yoshikawa T, Sasako M, Yamamoto S, et al. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg 2009;96:1015-22. [PubMed]
- Yoshikawa T, Taguri M, Sakuramoto S, et al. A comparison of multimodality treatment: two and four courses of neoadjuvant chemotherapy using S-1/CDDP or S-1/CDDP/docetaxel followed by surgery and S-1 adjuvant chemotherapy for macroscopically resectable serosa-positive gastric cancer: a randomized phase II trial (COMPASS-D trial). Jpn J Clin Oncol 2012;42:74-7. [PubMed]
- Yoshikawa T, Rino Y, Yukawa N, et al. Neoadjuvant chemotherapy for gastric cancer in Japan: a standing position by comparing with adjuvant chemotherapy. Surg Today 2014;44:11-21. [PubMed]
- Iwasaki Y, Sasako M, Yamamoto S, et al. Phase II study of preoperative chemotherapy with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210). J Surg Oncol 2013;107:741-5. [PubMed]
- Katayama H, Ito S, Sano T, et al. A Phase II study of systemic chemotherapy with docetaxel, cisplatin, and S-1 (DCS) followed by surgery in gastric cancer patients with extensive lymph node metastasis: Japan Clinical Oncology Group study JCOG1002. Jpn J Clin Oncol 2012;42:556-9. [PubMed]
- Miyashiro I, Furukawa H, Sasako M, et al. Randomized clinical trial of adjuvant chemotherapy with intraperitoneal and intravenous cisplatin followed by oral fluorouracil (UFT) in serosa-positive gastric cancer versus curative resection alone: final results of the Japan Clinical Oncology Group trial JCOG9206-2. Gastric Cancer 2011;14:212-8. [PubMed]
- Lee J. Phase III trial comparing capecitabine plus cisplatin versus capecitabine plus cisplatin with concurrent capecitabine radiotherapy in completely resected gastric cancer with D2 lymph node dissection: the ARTIST trial. J Clin Oncol 2012;30:268-73. [PubMed]
- Yu C, Yu R, Zhu W, et al. Intensity-modulated radiotherapy combined with chemotherapy for the treatment of gastric cancer patients after standard D1/D2 surgery. J Cancer Res Clin Oncol 2012;138:255-9. [PubMed]
- Zhu WG, Xua DF, Pu J, et al. A randomized, controlled, multicenter study comparing intensity-modulated radiotherapy plus concurrent chemotherapy with chemotherapy alone in gastric cancer patients with D2 resection. Radiother Oncol 2012;104:361-6. [PubMed]
- Kim TH, Park SR, Ryu KW, et al. Phase 3 trial of postoperative chemotherapy alone versus chemoradiation therapy in stage III-IV gastric cancer treated with R0 gastrectomy and D2 lymph node dissection. Int J Radiat Oncol Biol Phys 2012;84:e585-92. [PubMed]
- Kwon HC, Kim MC, Kim KH, et al. Adjuvant chemoradiation versus chemotherapy in completely resected advanced gastric cancer with D2 nodal dissection. Asia Pac J Clin Oncol 2010;6:278-85. [PubMed]
- Kim S, Lim DH, Lee J, et al. An observational study suggesting clinical benefit for adjuvant postoperative chemoradiation in a population of over 500 cases after gastric resection with D2 nodal dissection for adenocarcinoma of the stomach. Int J Radiat Oncol Biol Phys 2005;63:1279-85. [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-30. [PubMed]
- Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol 2012;30:2327-33. [PubMed]
- Coburn NG, Guller U, Baxter NN, et al. Adjuvant therapy for resected gastric cancer--rapid, yet incomplete adoption following results of intergroup 0116 trial. Int J Radiat Oncol Biol Phys 2008;70:1073-80. [PubMed]
- Sherman KL, Merkow RP, Bilimoria KY, et al. Treatment trends and predictors of adjuvant and neoadjuvant therapy for gastric adenocarcinoma in the United States. Ann Surg Oncol 2013;20:362-70. [PubMed]
- Ohri N, Garg MK, Aparo S, et al. Who benefits from adjuvant radiation therapy for gastric cancer? A meta-analysis. Int J Radiat Oncol Biol Phys 2013;86:330-5. [PubMed]
- Dikken JL, Jansen EP, Cats A, et al. Impact of the extent of surgery and postoperative chemoradiotherapy on recurrence patterns in gastric cancer. J Clin Oncol 2010;28:2430-6. [PubMed]
- Stiekema J, Trip AK, Jansen EP, et al. The prognostic significance of an R1 resection in gastric cancer patients treated with adjuvant chemoradiotherapy. Ann Surg Oncol 2014;21:1107-14. [PubMed]
- Stiekema J, Trip AK, Jansen EP, et al. Does adjuvant chemoradiotherapy improve the prognosis of gastric cancer after an R1 resection? Results from a dutch cohort study. Ann Surg Oncol 2015;22:581-8. [PubMed]
- Trip AK, Poppema BJ, van Berge Henegouwen MI, et al. Preoperative chemoradiotherapy in locally advanced gastric cancer, a phase I/II feasibility and efficacy study. Radiother Oncol 2014;112:284-8. [PubMed]
- Matsuda S, Takahashi T, Fukada J, et al. Phase I study of neoadjuvant chemoradiotherapy with S-1 plus biweekly cisplatin for advanced gastric cancer patients with lymph node metastasis: -KOGC04-. Radiat Oncol 2014;9:9. [PubMed]
- Michel P, Breysacher G, Mornex F, et al. Feasibility of preoperative and postoperative chemoradiotherapy in gastric adenocarcinoma. Two phase II studies done in parallel. Fédération Francophone de Cancérologie Digestive 0308. Eur J Cancer 2014;50:1076-83. [PubMed]
- Pera M, Gallego R, Montagut C, et al. Phase II trial of preoperative chemoradiotherapy with oxaliplatin, cisplatin, and 5-FU in locally advanced esophageal and gastric cancer. Ann Oncol 2012;23:664-70. [PubMed]
- Lee DJ, Sohn TS. Phase I study of neoadjuvant chemoradiotherapy with S-1 and oxaliplatin in patients with locally advanced gastric cancer. Cancer Chemother Pharmacol 2012;69:1333-8. [PubMed]
- Inoue T, Yachida S, Usuki H, et al. Pilot feasibility study of neoadjuvant chemoradiotherapy with S-1 in patients with locally advanced gastric cancer featuring adjacent tissue invasion or JGCA bulky N2 lymph node metastases. Ann Surg Oncol 2012;19:2937-45. [PubMed]
- Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851-6. [PubMed]
- Wydmański J, Suwinski R, Poltorak S, et al. The tolerance and efficacy of preoperative chemoradiotherapy followed by gastrectomy in operable gastric cancer, a phase II study. Radiother Oncol 2007;82:132-6. [PubMed]
- Ajani JA, Mansfield PF, Crane CH, et al. Paclitaxel-based chemoradiotherapy in localized gastric carcinoma: degree of pathologic response and not clinical parameters dictated patient outcome. J Clin Oncol 2005;23:1237-44. [PubMed]
- Ajani JA, Mansfield PF, Janjan N, et al. Multi-institutional trial of preoperative chemoradiotherapy in patients with potentially resectable gastric carcinoma. J Clin Oncol 2004;22:2774-80. [PubMed]
- Roth AD, Allal AS, Bründler MA, et al. Neoadjuvant radiochemotherapy for locally advanced gastric cancer: a phase I-II study. Ann Oncol 2003;14:110-5. [PubMed]
- Lowy AM, Feig BW, Janjan N, et al. A pilot study of preoperative chemoradiotherapy for resectable gastric cancer. Ann Surg Oncol 2001;8:519-24. [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [PubMed]
- Degiuli M, Sasako M, Ponti A, et al. Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg 2010;97:643-9. [PubMed]
- Allal AS, Zwahlen D, Bründler MA, et al. Neoadjuvant radiochemotherapy for locally advanced gastric cancer: long-term results of a phase I trial. Int J Radiat Oncol Biol Phys 2005;63:1286-9. [PubMed]
- Reed VK, Krishnan S, Mansfield PF, et al. Incidence, natural history, and patterns of locoregional recurrence in gastric cancer patients treated with preoperative chemoradiotherapy. Int J Radiat Oncol Biol Phys 2008;71:741-7. [PubMed]
- Oppedijk V, van der Gaast A, van Lanschot JJ, et al. Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J Clin Oncol 2014;32:385-91. [PubMed]
- Jansen EP, Boot H, Dubbelman R, et al. Postoperative chemoradiotherapy in gastric cancer -- a Phase I/II dose-finding study of radiotherapy with dose escalation of cisplatin and capecitabine chemotherapy. Br J Cancer 2007;97:712-6. [PubMed]
- Jansen EP, Boot H, Dubbelman R, et al. Postoperative chemoradiotherapy in gastric cancer--a phase I-II study of radiotherapy with dose escalation of weekly cisplatin and daily capecitabine chemotherapy. Ann Oncol 2010;21:530-4. [PubMed]
- Jansen EP, Boot H, Saunders MP, et al. A phase I-II study of postoperative capecitabine-based chemoradiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys 2007;69:1424-8. [PubMed]
- Jansen EP, Nijkamp J, Gubanski M, et al. Interobserver variation of clinical target volume delineation in gastric cancer. Int J Radiat Oncol Biol Phys 2010;77:1166-70. [PubMed]
- Dawson LA, Kavanagh BD, Paulino AC, et al. Radiation-associated kidney injury. Int J Radiat Oncol Biol Phys 2010;76:S108-15. [PubMed]
- Jansen EP, Saunders MP, Boot H, et al. Prospective study on late renal toxicity following postoperative chemoradiotherapy in gastric cancer. Int J Radiat Oncol Biol Phys 2007;67:781-5. [PubMed]
- Verheij M, Dewit LG, Valdés Olmos RA, et al. Evidence for a renovascular component in hypertensive patients with late radiation nephropathy. Int J Radiat Oncol Biol Phys 1994;30:677-83. [PubMed]
- Dewit L, Verheij M, Valdés Olmos RA, et al. Compensatory renal response after unilateral partial and whole volume high-dose irradiation of the human kidney. Eur J Cancer 1993;29A:2239-43. [PubMed]
- Trip AK, Nijkamp J, van Tinteren H, et al. IMRT limits nephrotoxicity after chemoradiotherapy for gastric cancer. Radiother Oncol 2014;112:289-94. [PubMed]
- Welz S, Hehr T, Kollmannsberger C, et al. Renal toxicity of adjuvant chemoradiotherapy with cisplatin in gastric cancer. Int J Radiat Oncol Biol Phys 2007;69:1429-35. [PubMed]
- Rubin LG, Schaffner W. Clinical practice. Care of the asplenic patient. N Engl J Med 2014;371:349-56. [PubMed]
- de Porto AP, Lammers AJ, Bennink RJ, et al. Assessment of splenic function. Eur J Clin Microbiol Infect Dis 2010;29:1465-73. [PubMed]
- Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states. Lancet 2011;378:86-97. [PubMed]
- Crary SE, Buchanan GR. Vascular complications after splenectomy for hematologic disorders. Blood 2009;114:2861-8. [PubMed]
- Dikken JL, van Sandick JW, Maurits Swellengrebel HA, et al. Neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy for patients with resectable gastric cancer (CRITICS). BMC Cancer 2011;11:329. [PubMed]
- Lowy AM, Mansfield PF, Leach SD, et al. Response to neoadjuvant chemotherapy best predicts survival after curative resection of gastric cancer. Ann Surg 1999;229:303-8. [PubMed]
- Becker K, Langer R, Reim D, et al. Significance of histopathological tumor regression after neoadjuvant chemotherapy in gastric adenocarcinomas: a summary of 480 cases. Ann Surg 2011;253:934-9. [PubMed]


