MicroRNA as a molecular target for gastrointestinal cancers
Introduction
MicroRNAs (miRNAs) are small non-coding RNAs that consist of approximately 22 nucleotides, and they post-transcriptionally suppress the expression of many target genes by pairing with complementary nucleotide sequences in the 3’-untranslated regions of the target mRNA (1). A number of reports have demonstrated that many kinds of miRNAs regulate diverse cell fates in tumor biology, including cell proliferation (2), cell cycle arrest (3), apoptosis (4), senescence (5), the epithelial-mesenchymal transition (6), invasion and metastasis (7) in human cancer cells, suggesting the potential role of miRNAs in tumor initiation, progression and metastasis. Indeed, aberrant regulation of miRNAs has been frequently reported in a variety of cancers including gastrointestinal (GI) cancers (8,9). Interestingly, a previous report has suggested that GI tumors can be strictly distinguished from non-GI tumors by analysis of global miRNA expression profiles (8). There are two types of miRNAs, oncogenic and tumor-suppressive miRNAs, which are involved in the pathogenesis of GI cancers (10-14). In this review, we have focused on the functional role of two types of cancer-related miRNAs, oncogenic miR-21 (15) (Figure 1A,B) and the tumor-suppressive miR-34 family (16) (Figure 2A,B). These miRNAs are commonly and frequently deregulated in GI cancers (9,17) and their aberrant expression is associated with the development and progression of GI cancers. Moreover, the potential clinical application of cancer-related miRNAs as novel molecular biomarkers for cancer diagnosis and as novel molecular targets for anticancer therapy of GI cancers is discussed.
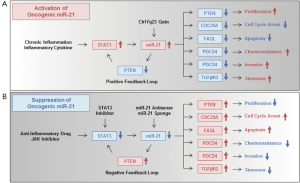
miRNAs commonly deregulated in GI cancers
Recent advances in tumor biology have revealed the aberrant expression of many miRNAs in a variety of human cancers including GI cancers, suggesting a potential role of miRNAs in tumor initiation, progression and metastasis. Indeed a number of reports have indicated that miRNAs can regulate diverse cell fates in human cancer cells. In various cancer tissues, deregulation of miRNAs has been shown to be highly associated with transcriptional deregulation, mutations, epigenetic methylations, DNA copy number abnormalities and defects in the miRNA biogenesis machinery (18). Among the many kinds of miRNAs, two cancer-related miRNAs, miR-21 and miR-34, have been shown to be commonly and frequently deregulated in GI cancer tissues (9,12,17). Oncogenic miR-21 is upregulated and tumor-suppressive miR-34 is downregulated by genetic and epigenetic alterations and by an inflammatory microenvironment in human GI cancers.
Expression, regulation and oncogenic function of miR-21 in GI cancers
miR-21 is frequently upregulated in tumor tissues of a variety of human GI cancers compared to normal tissues (Table 1). Human esophageal cancers including squamous cell carcinomas and adenocarcinomas showed significantly increased miR-21 expression (19-23) in association with clinical stage (20,21) and lymph node metastasis (21). In human gastric cancers, miR-21 was upregulated (24-28) and its overexpression was significantly associated with tumor size (27), clinical stage (27), and poor relapse-free and overall survival (28). Human colon cancers showed miR-21 upregulation (29-34), which was associated with clinical stage (29,31), metastatic activity (29,30) and poor survival (31-34). Colorectal adenoma also exhibited significant miR-21 upregulation (31). In human pancreatic cancers, miR-21 expression was significantly higher than normal controls (35-41) and miR-21 upregulation was significantly correlated with metastatic behavior (38,40), recurrence (41) and poor survival (38,39,41). Human liver cancers showed significantly higher miR-21 expression (42-46), which was significantly associated with liver cirrhosis (45), clinical stage (45,46) and poor prognosis (45,46). Recent evidences also support the potential of miR-21 overexpression as a prognostic marker in other types of cancers (51). More interestingly, some recent reports have further focused on miR-21 upregulation in tumor stromal fibroblasts in human GI cancers (47-50). Stromal miR-21 overexpression was associated with tumor size (47), clinical stage (47), lymph node metastasis (47,49), shorter disease-free survival (48), and overall survival (50). These findings suggest a functional role for miR-21 overexpression in both tumor cells and stromal fibroblasts in the development and progression of GI cancers.
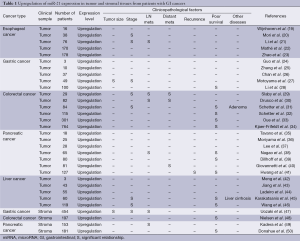
Full table
Two possible molecular mechanisms have been proposed for the upregulation of miR-21 in human GI cancer cells: (I) an inflammatory environment; and (II) chromosomal amplification (Figure 1A). Examples of (I) are the significantly higher miR-21 expression in Helicobacter pylori-infected gastric mucosa (25) and the up-regulation of miR-21 expression by the hepatitis B virus X protein (52). Moreover, several inflammatory cytokines have recently been shown to be responsible for miR-21 upregulation (32). miR-21 is upregulated by interleukin-6 (IL-6)-dependent induction of signal transducer and activator of transcription 3 (STAT3) in human colon cancer cells (53). These results strongly suggest a relationship between miR-21 upregulation and an inflammatory microenvironment. With regards to (II), chromosomal instability has also been suggested to be associated with high miR-21 expression. For example, miR-21 is located on human chromosome 17q23, which is frequently amplified in GI cancers (54). These combined findings demonstrate that miR-21 is frequently overexpressed through inflammatory stimuli and chromosomal instability in GI cancers.
Regarding the molecular mechanism of the oncogenic function of miR-21 (Figure 1A), miR-21 overexpression promotes cell proliferation in human GI cancers through suppression of the phosphatase and tensin homologue (PTEN) gene (42,55,56). miR-21-mediated suppression of PTEN upregulates STAT3 expression through activation of the IL-6 signaling pathway (53) as a positive feedback loop. miR-21 induces cell cycle progression by suppressing cell division cycle 25A (CDC25A) in human colon cancer cells (57). miR-21 further functions as an anti-apoptotic factor by suppressing the pro-apoptotic Fas ligand (FASL) (58). miR-21-mediated chemoresistance (59) and invasion ability (60,61) are induced by suppressing the tumor-suppressive programmed cell death 4 (PDCD4) gene, whose expression is significantly downregulated in human GI cancers (62). miR-21 overexpression also induces stemness properties by suppressing transforming growth factor beta receptor 2 (TGFβR2) in human colon cancer cells (63). Thus, miR-21 overexpression is highly associated with the development and progression of human GI cancers through suppression of multiple tumor-suppressive signaling pathways.
Expression, regulation and tumor-suppressive function of the miR-34 family in GI cancers
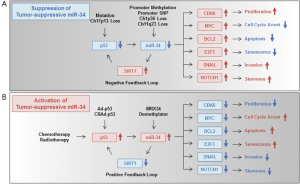
The expression levels of the miR-34 family in various types of cancers including GI cancers have been summarized in a recent review (64). This review indicates that the miR-34 family can be upregulated or downregulated in human cancer tissues. We previously reported that miR-34a expression was downregulated in 9 (36%) out of 25 human colon cancer tissues compared with the corresponding normal tissues (5). Since the miR-34 family (miR-34a, -34b and -34c) is a family of tumor suppressive miRNAs that are mainly induced by the tumor suppressor p53 gene (5,17,65-68), the miR-34 family may be upregulated by DNA damage in p53-activated tumor cells and downregulated by genetic and epigenetic alterations in p53-inactivated tumor cells (12). Three possible molecular mechanisms have been proposed for miR-34 miRNA regulation, especially for the downregulation of miR-34 family members, in human GI cancer tissues (Figure 2A): (I) p53 dysfunction; (II) methylation and single nucleotide polymorphisms (SNPs) in the promoter region; (III) chromosomal deletion. Regarding (I), dysfunction of the tumor suppressor p53 is frequently observed by mutation (69-71) or by deletions of chromosome 17p13Jeny (72-74), on which the p53 gene is located, in more than 50% of human GI cancers. Regarding (II), a variety of human cancer cells including gastric cancers exhibit frequent hypermethylation of the miR-34a promoter (75). The expression of miR-34b/c is also downregulated through promoter hypermethylation in human colon cancer tissues and cell lines, although normal colon tissues show no methylation (76). Moreover, some recent reports have suggested a possible relationship between the SNP rs4938723 of the miR-34b/c promoter region and the risk of colorectal cancers (77) and liver cancers (78-80). Regarding (III), the location of miRNA on human chromosomes has been reported to be associated with the fragile chromosomal sites that have been detected in a variety of human cancers (81). In fact, miR-34a is located on human chromosome 1p36, which is frequently deleted in GI cancers (82). miR-34b/c is located on human chromosome 11q23, which is a fragile site that is associated with breast and lung cancers (81) and which has been identified as a colorectal cancer susceptibility locus in a genome-wide association study (83). These accumulating evidences strongly suggest that the expression of the miR-34 family is frequently downregulated through p53 dysfunction, genetic and epigenetic alterations of the promoter region and chromosomal instability in GI cancers.
As for the molecular mechanism of the tumor-suppressive function of the miR-34 family (Figure 2B), miR-34 suppresses cyclin-dependent kinase 6 (CDK6) expression resulting in inhibition of proliferation (84), MYC expression, resulting in cell cycle arrest (85), BCL2 (B-cell CLL/lymphoma 2) expression, resulting in apoptosis induction (86), E2F3 (E2F transcription factor 3) expression, resulting in senescence induction (5), SNAIL (snail family zinc finger 1) expression, resulting in inhibition of invasion (87), and NOTCH1 expression, resulting in inhibition of stemness properties (88) in human cancer cells. We previously showed that miR-34a overexpression causes not only downregulation of E2F-related genes, but also upregulation of p53-related genes in human colon cancer cells (5), suggesting that miR-34 overexpression can activate the p53-signaling pathway probably through a positive feedback loop. In fact, it has been shown that suppression of SIRT1 (sirtuin 1) expression by miR-34a induces p53 activation and subsequent upregulation of p53-downstream target genes including p21 as a positive feedback loop (89) (Figure 2B). Thus, the p53-miR-34 regulatory network suppresses multiple oncogenic signaling pathways and the development and progression of GI cancers. In contrast, downregulation of the miR-34 family contributes to the activation of many miR-34-target oncogenes through SIRT1-mediated negative feedback loop (Figure 2A).
miRNAs as diagnostic and predictive biomarkers for GI cancers
Blood is a very useful sample as a non-invasive liquid biopsy for cancer patients. Circulating nucleic acids, including DNA, mRNA and miRNA in blood samples have emerged as having great potential as novel molecular biomarkers for cancer patients (90). In particular, it has recently been shown that detection of oncogenic miRNA overexpression in blood samples such as plasma and serum, is likely to be a useful method for the early diagnosis and prognostic prediction of various types of cancers (91). In contrast, tumor-suppressive miRNAs are often downregulated due to promoter hypermethylation during the pathogenesis of cancer development. The detection of promoter methylation status and the downregulation of tumor-suppressive miRNAs in tumor tissues is a promising biomarker for the diagnosis and prognosis of GI cancers. Moreover, stool samples also have potential as a non-invasive sample for detection of the DNA methylation status of GI cancer patients (92).
Upregulation of oncogenic miR-21 in blood and stool samples
Recent accumulating evidences have suggested the diagnostic and prognostic potential of circulating oncogenic miR-21 in blood samples from cancer patients (93). The expression levels of oncogenic miR-21 in plasma and serum were significantly higher in patients with esophageal cancers (94-97), gastric cancers (96,98-102), colorectal cancers (96,103-107), pancreatic cancers (108-114), and liver cancers (115-117) compared to healthy controls (Table 2). High miR-21 expression in plasma and serum is significantly associated with tumor size (95,98,102,105), clinical stage (97,98,100-102,104,105,108), metastatic activity (98,99,101,105,106,108), recurrence (94) and poor survival (105,108,110,113). Recently, the isolation of exosomes has emerged as a useful method for the detection of high miR-21 expression in serum from GI cancer patients (97,107,114,117). Some reports have shown the downregulation of high miR-21 expression after operation or chemotherapy, strongly supporting the possibility of tumor-derived circulating miR-21. miR-21 expression was also high in blood samples from patients with precancerous diseases, such as colorectal adenoma (105), chronic hepatitis B (116) and liver cirrhosis (117). In contrast, in stool samples, the expression of miR-21 was higher in patients with colorectal cancers compared to healthy controls (118-121), although pancreatic cancer patients did not show significant increases in miR-21 expression (122,123). These evidences suggest that detection of oncogenic miR-21 overexpression in blood samples is a promising screening system for the early diagnosis and prognostic prediction of GI cancers. Moreover, the isolation of miRNA using stool samples would be a useful method for the detection of miR-21 overexpression, especially in colorectal cancer patients.
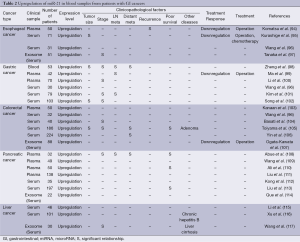
Full table
Detection of the downregulation of the tumor-suppressive miR-34 family by promoter hypermethylation in tumor and stool samples
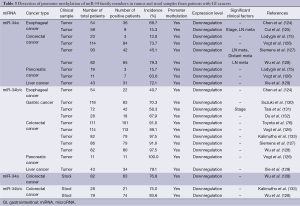
Hypermethylation of the promoters of tumor-suppressor miR-34 family members has been frequently observed in GI tumor tissues (Table 3). The incidence of miR-34a promoter methylation is moderately high (13.0-79.3%) in GI cancers (75,124-129), although the incidence of miR-34a promoter methylation in gastric cancers remains unclear. In contrast, the incidence of miR-34b/c promoter methylation was quite high (40.7-100.0%) in GI cancers (76,124,126-133). Moreover, the incidence of promoter methylation in the miR-34 family was also high (75.0-93.6%) in stool samples from colorectal cancer patients (128,133) and was similar to the methylation in tumor tissues. The promoter hypermethylation of miR-34 family members was associated with their downregulation, which significantly correlated with clinical stage (125,131), lymph node metastasis (125,127,128) and distant metastasis (127). Recent accumulating evidences strongly suggest that promoter hypermethylation of the miR-34 family is a frequent epigenetic alteration during the development and progression of GI cancers, and that tumor and stool samples are useful for detection of the expression and methylation status of the miR-34 family.
Full table
miRNAs as novel molecular targets for GI cancers
Since the expression of miR-21 and the miR-34 family is commonly and frequently dysregulated in human GI cancer tissues (Tables 1,3), these findings suggest that miR-21 and the miR-34 family are promising molecular targets for the treatment of patients with GI cancers. Human GI cancers show miR-21 upregulation, which is induced by inflammatory stimuli, STAT3 activation and chromosomal instability. miR-21 overexpression functions as oncogenic miRNA during tumor development and progression. In contrast, the miR-34 family members are downregulated by p53 dysfunction, promoter hypermethylation and chromosomal instability in human GI cancers. Downregulation of the miR-34 family contributes to cell proliferation, cell cycle progression, invasion and metastasis. Based on the molecular mechanism of the regulation of oncogenic miR-21 and the tumor-suppressive miR-34 family, several kinds of therapeutic options for miR-21-suppressing therapy and miR-34-activating therapy could be developed.
Therapeutic potential of miR-21-suppressing therapy
Since a variety of human cancer cells including GI cancers have been shown to overexpress miR-21 (24-26,28), the development of miR-21-target therapy that suppresses oncogenic miR-21 overexpression is a promising antitumor therapy against miR-21-overexpressing human cancers. There are several strategies for the suppression of oncogenic miR-21 upregulation in human cancer cells, such as anti-inflammatory drugs, a STAT3 inhibitor, miR-21 antisense oligonucleotides and miR-21 sponges (Figure 1B). To suppress inflammation-mediated miR-21 upregulation, anti-inflammatory drugs may be a useful option. For example, the anti-inflammatory drug, curcumin, has been shown to downregulate miR-21 expression in human pancreatic cancer cells (134). Curcumin inhibits IL-6-mediated STAT3 activation (135), which probably leads to miR-21 upregulation in human colon cancer cells (53). Curcumin treatment may downregulate inflammation-induced miR-21 upregulation in GI cancers. To suppress STAT3-mediated miR-21 upregulation more strongly than curcumin, inhibitors of STAT3 and JAK, which is a STAT3-activating kinase, may be useful reagents (136-140). In contrast, to suppress miR-21 upregulation due to chromosome 17q23 gain, miR-21 antisense oligonucleotides or a miR-21 sponge may be effective. miRNA antisense oligonucleotides have been frequently used to directly and specifically suppress the expression of oncogenic miRNAs in preclinical experiments. In fact, a miR-21 antisense oligonucleotide has been shown to suppress miR-21 expression in human gastric cancer cells, resulting in suppression of cell proliferation and induction of apoptotic cell death (25). Recently, a biopharmaceutical company Regulus Therapeutics Inc. is planning a clinical trial of RG-012, which is an anti-miR targeting miR-21, for the treatment of renal dysfunction in Alport syndrome patients (Table 4). Moreover, a miRNA sponge, which contains multiple binding sites for a specific miRNA, is also expected to downregulate the inhibitory effect of endogenous miRNAs against many target genes (141). It has recently been shown that a miRNA sponge for oncogenic miR-10b, whose expression is significantly associated with metastasis of breast cancers, can suppress miR-10b as efficiently as an antisense oligonucleotide (142). However, the therapeutic potential of a miRNA sponge for oncogenic miR-21 in GI cancers remains unclear. These reports suggest that the use of anti-inflammatory drugs, STAT3/JAK inhibitors, miR-21 antisense oligonucleotides or a miR-21 sponge are promising anticancer strategies for the suppression of oncogenic miR-21 overexpression in GI cancers.

Full table
Therapeutic potential of miR-34-activating therapy
For activation of the miR-34 family in human GI cancers, the status of p53 and miR-34 abnormalities should be considered (12). In both p53- and miR-34-intact human cancer cells, conventional anticancer therapy, such as chemotherapy and radiotherapy, efficiently induces miR-34 expression through activation of endogenous p53 (Figure 2B) (5,65-68). However, since more than 50% of human GI cancers lack normal p53 function and are therefore deficient in p53-induced miR-34 expression, novel anticancer strategies that can induce miR-34 expression in p53-inactivated tumors should be considered. For induction of miR-34 expression in human cancer cells in which p53 is inactivated due to mutation or to chromosome 17p13 loss, infection with exogenous p53-expressing adenovirus (Ad-p53) vectors would be a useful method (Figure 2B). Preclinical studies have shown that a replication-deficient Ad-p53 vector suppresses cell proliferation and tumor growth through p53-mediated induction of apoptotic cell death in human gastric cancer cells (143,144). We previously reported that Ad-p53-mediated wild-type p53 transfer efficiently suppressed cell proliferation, tumor growth and angiogenesis in human colon cancer cells (145,146). A phase I clinical trial has shown that treatment with Ad-p53 was well tolerated in patients with advanced esophageal cancers (147). However, the low transduction rate of p53 gene transfer by the replication deficient Ad-p53 is a major problem that needs to be overcome in order to improve the clinical outcome in patients with advanced GI cancers. We recently reported that combination therapy of Ad-p53 with a replication-competent oncolytic adenovirus enhances and sustains the expression level of the p53 protein, leading to enhanced apoptotic cell death of human colon cancer cells (148). Furthermore, a tumor-specific conditionally replicating Ad-p53 (CRAd-p53) has been shown to enhance and sustain p53 gene expression more efficiently than Ad-p53Jeny (149-151), which probably contributed to strong miR-34 induction in the infected human cancer cells. However, in order to induce miR-34 expression in human cancer cells in which miR-34 is inactivated due to miR-34 family promoter methylation and/or loss of chromosomes 1p36 and 11q23, direct miR-34 upregulation by miR-34 mimics rather than p53 replacement therapy should be attempted (Figure 2B). We previously reported that ectopic expression of miR-34a suppressed cell viability and induced subsequent senescence-like growth arrest in human colon cancer cells with either wild-type or mutant p53 protein (5). Interestingly, miR-34a overexpression was recently reported to suppress the stemness properties of p53-mutant human gastric cancer cells (86), human colon cancer stem cells (88), and human pancreatic cancer stem cells (152). These findings strongly suggest that miR-34-based anticancer therapy can target cancer stem cells within GI cancer tissues (153). Recently, a phase I clinical trial of MRX34, which is an miR-34 mimic that induces miR-34 expression following introduction into cells by a liposome delivery system, has been conducted by Mirna Therapeutics, Inc. as a monotherapy in patients with advanced liver cancers (154) (Table 4). In the future, exploration of the antitumor effect of miR-34-based anticancer therapy will shed light on the development of novel anticancer strategies against GI cancers. Moreover, human cancer cells in which miR-34 is inactivated by promoter methylation of the miR-34 family would be further sensitive to demethylating agents, although other kinds of miRNAs may be also re-activated after demethylating therapy.
Future direction of research on miR-21 and miR-34 family
Genetic and epigenetic analyses using clinical samples have shown that oncogenic miR-21 is upregulated and tumor-suppressive miR-34 family is downregulated in most GI cancers (Tables 1-3). More understanding of the precise molecular mechanism underlying miR-21 upregulation and miR-34 downregulation would be needed to develop the miRNA-based anticancer therapy. Recently, clinical studies of miR-21-suppressing and miR-34-activating therapies have been conducted to confirm the safe and feasibility of miRNA-targeting strategy (Table 4). For the clinical application of miRNA-targeting therapy, we should confirm the therapeutic potential of miRNA normalization therapy in combination with conventional anticancer therapy, such as chemotherapy and radiotherapy. Moreover, the identification of drugs that suppress miR-21 and/or activate miR-34 would provide novel insights in developing the multimodal antitumor strategy targeting miRNA expression.
Conclusions
Previous studies by many cancer researchers have revealed that diverse genetic and epigenetic alterations in protein-coding genes play central roles in the pathogenesis of GI cancers. However, since non-coding miRNAs have been shown to be deregulated in a variety of human cancers including GI cancers (8,9), it is now also necessary to understand the miRNA-based pathogenesis of GI cancers and the molecular mechanism of the interaction between protein-coding genes and non-coding miRNA genes (155). The development of an early detection system for oncogenic miR-21 and promoter methylation of the tumor-suppressive miR-34 family using clinical samples, such as tumor, blood and stool, would improve the clinical outcome of patients with GI cancers. p53 replacement therapy using Ad-p53 and CRAd-p53 is a promising anticancer therapy against GI cancers with p53 dysfunction. Adenovirus-mediated overexpression of tumor suppressor p53 may further suppress oncogenic miR-21 expression through suppression of STAT3 expression (156,157). In contrast, in human GI cancers with miR-34 dysfunction, direct restoration of miR-34 using miR-34 mimics would be a more effective strategy than p53 replacement therapy for efficient induction of miR-34 expression. As a recent report suggested that combination therapy with miR-34 mimics and KRAS siRNA, which may suppress miR-21 expression (158), has great therapeutic potential in human lung cancer cells in both in vitro and in vivo settings (159), the combination of miR-34-activating therapy and miR-21-suppressing therapy may be a promising antitumor strategy. Thus, an understanding at the molecular level of miRNA-mediated cancer progression would provide a novel platform for the development of miRNA-based cancer diagnosis and anticancer therapy for the treatment of patients with GI cancers.
Acknowledgements
This study was supported by grants from the Ministry of Health, Labour, and Welfare of Japan and from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Disclosure: The authors declare no conflict of interest.
References
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281-97. [PubMed]
- Si ML, Zhu S, Wu H, et al. miR-21-mediated tumor growth. Oncogene 2007;26:2799-803. [PubMed]
- Carleton M, Cleary MA, Linsley PS. MicroRNAs and cell cycle regulation. Cell Cycle 2007;6:2127-32. [PubMed]
- Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A 2005;102:13944-9. [PubMed]
- Tazawa H, Tsuchiya N, Izumiya M, et al. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc Natl Acad Sci U S A 2007;104:15472-7. [PubMed]
- Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 2008;10:593-601. [PubMed]
- Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature 2007;449:682-8. [PubMed]
- Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature 2005;435:834-8. [PubMed]
- Volinia S, Calin GA, Liu CG, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A 2006;103:2257-61. [PubMed]
- Saito Y, Suzuki H, Hibi T. The role of microRNAs in gastrointestinal cancers. J Gastroenterol 2009;44 Suppl 19:18-22. [PubMed]
- Song B, Ju J. Impact of miRNAs in gastrointestinal cancer diagnosis and prognosis. Expert Rev Mol Med 2010;12:e33. [PubMed]
- Tazawa H, Kagawa S, Fujiwara T. MicroRNAs as potential target gene in cancer gene therapy of gastrointestinal tumors. Expert Opin Biol Ther 2011;11:145-55. [PubMed]
- Song JH, Meltzer SJ. MicroRNAs in pathogenesis, diagnosis, and treatment of gastroesophageal cancers. Gastroenterology 2012;143:35-47. [PubMed]
- Song S, Ajani JA. The role of microRNAs in cancers of the upper gastrointestinal tract. Nat Rev Gastroenterol Hepatol 2013;10:109-18. [PubMed]
- Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med 2009;13:39-53. [PubMed]
- Hermeking H. The miR-34 family in cancer and apoptosis. Cell Death Differ 2010;17:193-9. [PubMed]
- Rokavec M, Li H, Jiang L, et al. The p53/microRNA connection in gastrointestinal cancer. Clin Exp Gastroenterol 2014;7:395-413. [PubMed]
- Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet 2009;10:704-14. [PubMed]
- Wijnhoven BP, Hussey DJ, Watson DI, et al. MicroRNA profiling of Barrett's oesophagus and oesophageal adenocarcinoma. Br J Surg 2010;97:853-61. [PubMed]
- Mori Y, Ishiguro H, Kuwabara Y, et al. MicroRNA-21 induces cell proliferation and invasion in esophageal squamous cell carcinoma. Mol Med Rep 2009;2:235-9. [PubMed]
- Li P, Mao WM, Zheng ZG, et al. Down-regulation of PTEN expression modulated by dysregulated miR-21 contributes to the progression of esophageal cancer. Dig Dis Sci 2013;58:3483-93. [PubMed]
- Mathé EA, Nguyen GH, Bowman ED, et al. MicroRNA expression in squamous cell carcinoma and adenocarcinoma of the esophagus: associations with survival. Clin Cancer Res 2009;15:6192-200. [PubMed]
- Zhao Y, Schetter AJ, Yang GB, et al. microRNA and inflammatory gene expression as prognostic marker for overall survival in esophageal squamous cell carcinoma. Int J Cancer 2013;132:2901-9. [PubMed]
- Guo J, Miao Y, Xiao B, et al. Differential expression of microRNA species in human gastric cancer versus non-tumorous tissues. J Gastroenterol Hepatol 2009;24:652-7. [PubMed]
- Zhang Z, Li Z, Gao C, et al. miR-21 plays a pivotal role in gastric cancer pathogenesis and progression. Lab Invest 2008;88:1358-66. [PubMed]
- Chan SH, Wu CW, Li AF, et al. miR-21 microRNA expression in human gastric carcinomas and its clinical association. Anticancer Res 2008;28:907-11. [PubMed]
- Motoyama K, Inoue H, Mimori K, et al. Clinicopathological and prognostic significance of PDCD4 and microRNA-21 in human gastric cancer. Int J Oncol 2010;36:1089-95. [PubMed]
- Li X, Zhang Y, Ding J, et al. Survival prediction of gastric cancer by a seven-microRNA signature. Gut 2010;59:579-85. [PubMed]
- Slaby O, Svoboda M, Fabian P, et al. Altered expression of miR-21, miR-31, miR-143 and miR-145 is related to clinicopathologic features of colorectal cancer. Oncology 2007;72:397-402. [PubMed]
- Drusco A, Nuovo GJ, Zanesi N, et al. MicroRNA profiles discriminate among colon cancer metastasis. PLoS One 2014;9:e96670. [PubMed]
- Schetter AJ, Leung SY, Sohn JJ, et al. MicroRNA expression profiles associated with prognosis and therapeutic outcome in colon adenocarcinoma. Jama 2008;299:425-36. [PubMed]
- Schetter AJ, Nguyen GH, Bowman ED, et al. Association of inflammation-related and microRNA gene expression with cancer-specific mortality of colon adenocarcinoma. Clin Cancer Res 2009;15:5878-87. [PubMed]
- Oue N, Anami K, Schetter AJ, et al. High miR-21 expression from FFPE tissues is associated with poor survival and response to adjuvant chemotherapy in colon cancer. Int J Cancer 2014;134:1926-34. [PubMed]
- Kjaer-Frifeldt S, Hansen TF, Nielsen BS, et al. The prognostic importance of miR-21 in stage II colon cancer: a population-based study. Br J Cancer 2012;107:1169-74. [PubMed]
- Tavano F, di Mola FF, Piepoli A, et al. Changes in miR-143 and miR-21 expression and clinicopathological correlations in pancreatic cancers. Pancreas 2012;41:1280-4. [PubMed]
- Moriyama T, Ohuchida K, Mizumoto K, et al. MicroRNA-21 modulates biological functions of pancreatic cancer cells including their proliferation, invasion, and chemoresistance. Mol Cancer Ther 2009;8:1067-74. [PubMed]
- Lee EJ, Gusev Y, Jiang J, et al. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer 2007;120:1046-54. [PubMed]
- Nagao Y, Hisaoka M, Matsuyama A, et al. Association of microRNA-21 expression with its targets, PDCD4 and TIMP3, in pancreatic ductal adenocarcinoma. Mod Pathol 2012;25:112-21. [PubMed]
- Dillhoff M, Liu J, Frankel W, et al. MicroRNA-21 is overexpressed in pancreatic cancer and a potential predictor of survival. J Gastrointest Surg 2008;12:2171-6. [PubMed]
- Giovannetti E, Funel N, Peters GJ, et al. MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res 2010;70:4528-38. [PubMed]
- Hwang JH, Voortman J, Giovannetti E, et al. Identification of microRNA-21 as a biomarker for chemoresistance and clinical outcome following adjuvant therapy in resectable pancreatic cancer. PLoS One 2010;5:e10630. [PubMed]
- Meng F, Henson R, Wehbe-Janek H, et al. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 2007;133:647-58. [PubMed]
- Jiang J, Gusev Y, Aderca I, et al. Association of MicroRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin Cancer Res 2008;14:419-27. [PubMed]
- Ladeiro Y, Couchy G, Balabaud C, et al. MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology 2008;47:1955-63. [PubMed]
- Karakatsanis A, Papaconstantinou I, Gazouli M, et al. Expression of microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c, miR-221, miR-222, and miR-223 in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma and its prognostic significance. Mol Carcinog 2013;52:297-303. [PubMed]
- Wang WY, Zhang HF, Wang L, et al. miR-21 expression predicts prognosis in hepatocellular carcinoma. Clin Res Hepatol Gastroenterol 2014;38:715-9. [PubMed]
- Uozaki H, Morita S, Kumagai A, et al. Stromal miR-21 is more important than miR-21 of tumour cells for the progression of gastric cancer. Histopathology 2014;65:775-83. [PubMed]
- Nielsen BS, Jørgensen S, Fog JU, et al. High levels of microRNA-21 in the stroma of colorectal cancers predict short disease-free survival in stage II colon cancer patients. Clin Exp Metastasis 2011;28:27-38. [PubMed]
- Kadera BE, Li L, Toste PA, et al. MicroRNA-21 in pancreatic ductal adenocarcinoma tumor-associated fibroblasts promotes metastasis. PLoS One 2013;8:e71978. [PubMed]
- Donahue TR, Nguyen AH, Moughan J, et al. Stromal MicroRNA-21 levels predict response to 5-fluorouracil in patients with pancreatic cancer. J Surg Oncol 2014;110:952-9. [PubMed]
- Zhou X, Wang X, Huang Z, et al. Prognostic value of miR-21 in various cancers: an updating meta-analysis. PLoS One 2014;9:e102413. [PubMed]
- Qiu X, Dong S, Qiao F, et al. HBx-mediated miR-21 upregulation represses tumor-suppressor function of PDCD4 in hepatocellular carcinoma. Oncogene 2013;32:3296-305. [PubMed]
- Iliopoulos D, Jaeger SA, Hirsch HA, et al. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell 2010;39:493-506. [PubMed]
- Andersen CL, Monni O, Wagner U, et al. High-throughput copy number analysis of 17q23 in 3520 tissue specimens by fluorescence in situ hybridization to tissue microarrays. Am J Pathol 2002;161:73-9. [PubMed]
- Zhang BG, Li JF, Yu BQ, et al. microRNA-21 promotes tumor proliferation and invasion in gastric cancer by targeting PTEN. Oncol Rep 2012;27:1019-26. [PubMed]
- Xiong B, Cheng Y, Ma L, et al. MiR-21 regulates biological behavior through the PTEN/PI-3 K/Akt signaling pathway in human colorectal cancer cells. Int J Oncol 2013;42:219-28. [PubMed]
- Wang P, Zou F, Zhang X, et al. microRNA-21 negatively regulates Cdc25A and cell cycle progression in colon cancer cells. Cancer Res 2009;69:8157-65. [PubMed]
- Sayed D, He M, Hong C, et al. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J Biol Chem 2010;285:20281-90. [PubMed]
- Paik WH, Kim HR, Park JK, et al. Chemosensitivity induced by down-regulation of microRNA-21 in gemcitabine-resistant pancreatic cancer cells by indole-3-carbinol. Anticancer Res 2013;33:1473-81. [PubMed]
- Asangani IA, Rasheed SA, Nikolova DA, et al. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 2008;27:2128-36. [PubMed]
- Zhu Q, Wang Z, Hu Y, et al. miR-21 promotes migration and invasion by the miR-21-PDCD4-AP-1 feedback loop in human hepatocellular carcinoma. Oncol Rep 2012;27:1660-8. [PubMed]
- Ma G, Zhang H, Dong M, et al. Downregulation of programmed cell death 4 (PDCD4) in tumorigenesis and progression of human digestive tract cancers. Tumour Biol 2013;34:3879-85. [PubMed]
- Yu Y, Kanwar SS, Patel BB, et al. MicroRNA-21 induces stemness by downregulating transforming growth factor beta receptor 2 (TGFbetaR2) in colon cancer cells. Carcinogenesis 2012;33:68-76. [PubMed]
- Wang L, Yu J, Xu J, et al. The analysis of microRNA-34 family expression in human cancer studies comparing cancer tissues with corresponding pericarcinous tissues. Gene 2015;554:1-8. [PubMed]
- He L, He X, Lim LP, et al. A microRNA component of the p53 tumour suppressor network. Nature 2007;447:1130-4. [PubMed]
- Raver-Shapira N, Marciano E, Meiri E, et al. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol Cell 2007;26:731-43. [PubMed]
- Chang TC, Wentzel EA, Kent OA, et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol Cell 2007;26:745-52. [PubMed]
- Bommer GT, Gerin I, Feng Y, et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes. Curr Biol 2007;17:1298-307. [PubMed]
- Tamura G, Kihana T, Nomura K, et al. Detection of frequent p53 gene mutations in primary gastric cancer by cell sorting and polymerase chain reaction single-strand conformation polymorphism analysis. Cancer Res 1991;51:3056-8. [PubMed]
- Renault B, van den Broek M, Fodde R, et al. Base transitions are the most frequent genetic changes at P53 in gastric cancer. Cancer Res 1993;53:2614-7. [PubMed]
- Kastrinakis WV, Ramchurren N, Rieger KM, et al. Increased incidence of p53 mutations is associated with hepatic metastasis in colorectal neoplastic progression. Oncogene 1995;11:647-52. [PubMed]
- Sano T, Tsujino T, Yoshida K, et al. Frequent loss of heterozygosity on chromosomes 1q, 5q, and 17p in human gastric carcinomas. Cancer Res 1991;51:2926-31. [PubMed]
- Khine K, Smith DR, Goh HS. High frequency of allelic deletion on chromosome 17p in advanced colorectal cancer. Cancer 1994;73:28-35. [PubMed]
- Risio M, Casorzo L, Chiecchio L, et al. Deletions of 17p are associated with transition from early to advanced colorectal cancer. Cancer Genet Cytogenet 2003;147:44-9. [PubMed]
- Lodygin D, Tarasov V, Epanchintsev A, et al. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle 2008;7:2591-600. [PubMed]
- Toyota M, Suzuki H, Sasaki Y, et al. Epigenetic silencing of microRNA-34b/c and B-cell translocation gene 4 is associated with CpG island methylation in colorectal cancer. Cancer Res 2008;68:4123-32. [PubMed]
- Oh J, Kim JW, Lee BE, et al. Polymorphisms of the pri-miR-34b/c promoter and TP53 codon 72 are associated with risk of colorectal cancer. Oncol Rep 2014;31:995-1002. [PubMed]
- Xu Y, Liu L, Liu J, et al. A potentially functional polymorphism in the promoter region of miR-34b/c is associated with an increased risk for primary hepatocellular carcinoma. Int J Cancer 2011;128:412-7. [PubMed]
- Son MS, Jang MJ, Jeon YJ, et al. Promoter polymorphisms of pri-miR-34b/c are associated with hepatocellular carcinoma. Gene 2013;524:156-60. [PubMed]
- Han Y, Pu R, Han X, et al. Associations of pri-miR-34b/c and pre-miR-196a2 polymorphisms and their multiplicative interactions with hepatitis B virus mutations with hepatocellular carcinoma risk. PLoS One 2013;8:e58564. [PubMed]
- Calin GA, Sevignani C, Dumitru CD, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci U S A 2004;101:2999-3004. [PubMed]
- Ragnarsson G, Eiriksdottir G, Johannsdottir JT, et al. Loss of heterozygosity at chromosome 1p in different solid human tumours: association with survival. Br J Cancer 1999;79:1468-74. [PubMed]
- Tenesa A, Farrington SM, Prendergast JG, et al. Genome-wide association scan identifies a colorectal cancer susceptibility locus on 11q23 and replicates risk loci at 8q24 and 18q21. Nat Genet 2008;40:631-7. [PubMed]
- Sun F, Fu H, Liu Q, et al. Downregulation of CCND1 and CDK6 by miR-34a induces cell cycle arrest. FEBS Lett 2008;582:1564-8. [PubMed]
- Wei JS, Song YK, Durinck S, et al. The MYCN oncogene is a direct target of miR-34a. Oncogene 2008;27:5204-13. [PubMed]
- Ji Q, Hao X, Meng Y, et al. Restoration of tumor suppressor miR-34 inhibits human p53-mutant gastric cancer tumorspheres. BMC cancer 2008;8:266. [PubMed]
- Siemens H, Jackstadt R, Hunten S, et al. miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions. Cell Cycle 2011;10:4256-71. [PubMed]
- Bu P, Chen KY, Chen JH, et al. A microRNA miR-34a-regulated bimodal switch targets Notch in colon cancer stem cells. Cell Stem Cell 2013;12:602-15. [PubMed]
- Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc Natl Acad Sci U S A 2008;105:13421-6. [PubMed]
- Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer 2011;11:426-37. [PubMed]
- Cortez MA, Calin GA. MicroRNA identification in plasma and serum: a new tool to diagnose and monitor diseases. Expert Opin Biol Ther 2009;9:703-11. [PubMed]
- Nagasaka T, Tanaka N, Cullings HM, et al. Analysis of fecal DNA methylation to detect gastrointestinal neoplasia. J Natl Cancer Inst 2009;101:1244-58. [PubMed]
- Wang Y, Gao X, Wei F, et al. Diagnostic and prognostic value of circulating miR-21 for cancer: a systematic review and meta-analysis. Gene 2014;533:389-97. [PubMed]
- Komatsu S, Ichikawa D, Takeshita H, et al. Circulating microRNAs in plasma of patients with oesophageal squamous cell carcinoma. Br J Cancer 2011;105:104-11. [PubMed]
- Kurashige J, Kamohara H, Watanabe M, et al. Serum microRNA-21 is a novel biomarker in patients with esophageal squamous cell carcinoma. J Surg Oncol 2012;106:188-92. [PubMed]
- Wang B, Zhang Q. The expression and clinical significance of circulating microRNA-21 in serum of five solid tumors. J Cancer Res Clin Oncol 2012;138:1659-66. [PubMed]
- Tanaka Y, Kamohara H, Kinoshita K, et al. Clinical impact of serum exosomal microRNA-21 as a clinical biomarker in human esophageal squamous cell carcinoma. Cancer 2013;119:1159-67. [PubMed]
- Zheng Y, Cui L, Sun W, et al. MicroRNA-21 is a new marker of circulating tumor cells in gastric cancer patients. Cancer Biomark 2011-2012;10:71-7. [PubMed]
- Ma GJ, Gu RM, Zhu M, et al. Plasma post-operative miR-21 expression in the prognosis of gastric cancers. Asian Pac J Cancer Prev 2013;14:7551-4. [PubMed]
- Li BS, Zhao YL, Guo G, et al. Plasma microRNAs, miR-223, miR-21 and miR-218, as novel potential biomarkers for gastric cancer detection. PLoS One 2012;7:e41629. [PubMed]
- Kim SY, Jeon TY, Choi CI, et al. Validation of circulating miRNA biomarkers for predicting lymph node metastasis in gastric cancer. J Mol Diagn 2013;15:661-9. [PubMed]
- Song J, Bai Z, Zhang J, et al. Serum microRNA-21 levels are related to tumor size in gastric cancer patients but cannot predict prognosis. Oncol Lett 2013;6:1733-1737. [PubMed]
- Kanaan Z, Rai SN, Eichenberger MR, et al. Plasma miR-21: a potential diagnostic marker of colorectal cancer. Ann Surg 2012;256:544-51. [PubMed]
- Basati G, Emami Razavi A, Abdi S, et al. Elevated level of microRNA-21 in the serum of patients with colorectal cancer. Med Oncol 2014;31:205. [PubMed]
- Toiyama Y, Takahashi M, Hur K, et al. Serum miR-21 as a diagnostic and prognostic biomarker in colorectal cancer. J Natl Cancer Inst 2013;105:849-59. [PubMed]
- Yin J, Bai Z, Song J, et al. Differential expression of serum miR-126, miR-141 and miR-21 as novel biomarkers for early detection of liver metastasis in colorectal cancer. Chin J Cancer Res 2014;26:95-103. [PubMed]
- Ogata-Kawata H, Izumiya M, Kurioka D, et al. Circulating exosomal microRNAs as biomarkers of colon cancer. PLoS One 2014;9:e92921. [PubMed]
- Abue M, Yokoyama M, Shibuya R, et al. Circulating miR-483-3p and miR-21 is highly expressed in plasma of pancreatic cancer. Int J Oncol 2015;46:539-47. [PubMed]
- Wang J, Chen J, Chang P, et al. MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev Res (Phila) 2009;2:807-13. [PubMed]
- Ali S, Almhanna K, Chen W, et al. Differentially expressed miRNAs in the plasma may provide a molecular signature for aggressive pancreatic cancer. Am J Transl Res 2010;3:28-47. [PubMed]
- Liu J, Gao J, Du Y, et al. Combination of plasma microRNAs with serum CA19-9 for early detection of pancreatic cancer. Int J Cancer 2012;131:683-91. [PubMed]
- Kong X, Du Y, Wang G, et al. Detection of differentially expressed microRNAs in serum of pancreatic ductal adenocarcinoma patients: miR-196a could be a potential marker for poor prognosis. Dig Dis Sci 2011;56:602-9. [PubMed]
- Liu R, Chen X, Du Y, et al. Serum microRNA expression profile as a biomarker in the diagnosis and prognosis of pancreatic cancer. Clin Chem 2012;58:610-8. [PubMed]
- Que R, Ding G, Chen J, et al. Analysis of serum exosomal microRNAs and clinicopathologic features of patients with pancreatic adenocarcinoma. World J Surg Oncol 2013;11:219. [PubMed]
- Li J, Wang Y, Yu W, et al. Expression of serum miR-221 in human hepatocellular carcinoma and its prognostic significance. Biochem Biophys Res Commun 2011;406:70-3. [PubMed]
- Xu J, Wu C, Che X, et al. Circulating microRNAs, miR-21, miR-122, and miR-223, in patients with hepatocellular carcinoma or chronic hepatitis. Mol Carcinog 2011;50:136-42. [PubMed]
- Wang H, Hou L, Li A, et al. Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed Res Int 2014;2014:864894.
- Link A, Balaguer F, Shen Y, et al. Fecal MicroRNAs as novel biomarkers for colon cancer screening. Cancer Epidemiol Biomarkers Prev 2010;19:1766-74. [PubMed]
- Koga Y, Yasunaga M, Takahashi A, et al. MicroRNA expression profiling of exfoliated colonocytes isolated from feces for colorectal cancer screening. Cancer Prev Res (Phila) 2010;3:1435-42. [PubMed]
- Wu CW, Ng SS, Dong YJ, et al. Detection of miR-92a and miR-21 in stool samples as potential screening biomarkers for colorectal cancer and polyps. Gut 2012;61:739-45. [PubMed]
- Ahmed FE, Ahmed NC, Vos PW, et al. Diagnostic microRNA markers to screen for sporadic human colon cancer in stool: I. Proof of principle. Cancer Genomics Proteomics 2013;10:93-113. [PubMed]
- Link A, Becker V, Goel A, et al. Feasibility of fecal microRNAs as novel biomarkers for pancreatic cancer. PLoS One 2012;7:e42933. [PubMed]
- Ren Y, Gao J, Liu JQ, et al. Differential signature of fecal microRNAs in patients with pancreatic cancer. Mol Med Rep 2012;6:201-9. [PubMed]
- Chen X, Hu H, Guan X, et al. CpG island methylation status of miRNAs in esophageal squamous cell carcinoma. Int J Cancer 2012;130:1607-13. [PubMed]
- Cui X, Zhao Z, Liu D, et al. Inactivation of miR-34a by aberrant CpG methylation in Kazakh patients with esophageal carcinoma. J Exp Clin Cancer Res 2014;33:20. [PubMed]
- Vogt M, Munding J, Grüner M, et al. Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG methylation in colorectal, pancreatic, mammary, ovarian, urothelial, and renal cell carcinomas and soft tissue sarcomas. Virchows Arch 2011;458:313-22. [PubMed]
- Siemens H, Neumann J, Jackstadt R, et al. Detection of miR-34a promoter methylation in combination with elevated expression of c-Met and beta-catenin predicts distant metastasis of colon cancer. Clin Cancer Res 2013;19:710-20. [PubMed]
- Wu XD, Song YC, Cao PL, et al. Detection of miR-34a and miR-34b/c in stool sample as potential screening biomarkers for noninvasive diagnosis of colorectal cancer. Med Oncol 2014;31:894. [PubMed]
- Xie K, Liu J, Chen J, et al. Methylation-associated silencing of microRNA-34b in hepatocellular carcinoma cancer. Gene 2014;543:101-7. [PubMed]
- Suzuki H, Yamamoto E, Nojima M, et al. Methylation-associated silencing of microRNA-34b/c in gastric cancer and its involvement in an epigenetic field defect. Carcinogenesis 2010;31:2066-73. [PubMed]
- Tsai KW, Wu CW, Hu LY, et al. Epigenetic regulation of miR-34b and miR-129 expression in gastric cancer. Int J Cancer 2011;129:2600-10. [PubMed]
- Du Y, Liu Z, Gu L, et al. Characterization of human gastric carcinoma-related methylation of 9 miR CpG islands and repression of their expressions in vitro and in vivo. BMC Cancer 2012;12:249. [PubMed]
- Kalimutho M, Di Cecilia S, Del Vecchio Blanco G, et al. Epigenetically silenced miR-34b/c as a novel faecal-based screening marker for colorectal cancer. Br J Cancer 2011;104:1770-8. [PubMed]
- Ali S, Ahmad A, Banerjee S, et al. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res 2010;70:3606-17. [PubMed]
- Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol 2003;171:3863-71. [PubMed]
- Toyonaga T, Nakano K, Nagano M, et al. Blockade of constitutively activated Janus kinase/signal transducer and activator of transcription-3 pathway inhibits growth of human pancreatic cancer. Cancer Lett 2003;201:107-16. [PubMed]
- Liu Y, Li PK, Li C, et al. Inhibition of STAT3 signaling blocks the anti-apoptotic activity of IL-6 in human liver cancer cells. J Biol Chem 2010;285:27429-39. [PubMed]
- Du W, Hong J, Wang YC, et al. Inhibition of JAK2/STAT3 signalling induces colorectal cancer cell apoptosis via mitochondrial pathway. J Cell Mol Med 2012;16:1878-88. [PubMed]
- Judd LM, Menheniott TR, Ling H, et al. Inhibition of the JAK2/STAT3 pathway reduces gastric cancer growth in vitro and in vivo. PLoS One 2014;9:e95993. [PubMed]
- Fang J, Chu L, Li C, et al. JAK2 inhibitor blocks the inflammation and growth of esophageal squamous cell carcinoma in vitro through the JAK/STAT3 pathway. Oncol Rep 2015;33:494-502. [PubMed]
- Ebert MS, Neilson JR, Sharp PA. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods 2007;4:721-6. [PubMed]
- Ma L, Reinhardt F, Pan E, et al. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat Biotechnol 2010;28:341-7. [PubMed]
- Ohashi M, Kanai F, Ueno H, et al. Adenovirus mediated p53 tumour suppressor gene therapy for human gastric cancer cells in vitro and in vivo. Gut 1999;44:366-71. [PubMed]
- Tatebe S, Matsuura T, Endo K, et al. Adenovirus-mediated transfer of wild-type p53 gene results in apoptosis or growth arrest in human cultured gastric carcinoma cells. Int J Oncol 1999;15:229-35. [PubMed]
- Spitz FR, Nguyen D, Skibber JM, et al. In vivo adenovirus-mediated p53 tumor suppressor gene therapy for colorectal cancer. Anticancer Res 1996;16:3415-22. [PubMed]
- Bouvet M, Ellis LM, Nishizaki M, et al. Adenovirus-mediated wild-type p53 gene transfer down-regulates vascular endothelial growth factor expression and inhibits angiogenesis in human colon cancer. Cancer Res 1998;58:2288-92. [PubMed]
- Shimada H, Matsubara H, Shiratori T, et al. Phase I/II adenoviral p53 gene therapy for chemoradiation resistant advanced esophageal squamous cell carcinoma. Cancer Sci 2006;97:554-61. [PubMed]
- Sakai R, Kagawa S, Yamasaki Y, et al. Preclinical evaluation of differentially targeting dual virotherapy for human solid cancer. Mol Cancer Ther 2010;9:1884-93. [PubMed]
- van Beusechem VW, van den Doel PB, Grill J, et al. Conditionally replicative adenovirus expressing p53 exhibits enhanced oncolytic potency. Cancer Res 2002;62:6165-71. [PubMed]
- Wang X, Su C, Cao H, et al. A novel triple-regulated oncolytic adenovirus carrying p53 gene exerts potent antitumor efficacy on common human solid cancers. Mol Cancer Ther 2008;7:1598-603. [PubMed]
- Yamasaki Y, Tazawa H, Hashimoto Y, et al. A novel apoptotic mechanism of genetically engineered adenovirus-mediated tumour-specific p53 overexpression through E1A-dependent p21 and MDM2 suppression. Eur J Cancer 2012;48:2282-91. [PubMed]
- Ji Q, Hao X, Zhang M, et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS One 2009;4:e6816. [PubMed]
- Agostini M, Knight RA. miR-34: from bench to bedside. Oncotarget 2014;5:872-81. [PubMed]
- Bader AG. miR-34 - a microRNA replacement therapy is headed to the clinic. Front Genet 2012;3:120. [PubMed]
- Slaby O, Svoboda M, Michalek J, et al. MicroRNAs in colorectal cancer: translation of molecular biology into clinical application. Mol Cancer 2009;8:102. [PubMed]
- Lin J, Tang H, Jin X, et al. p53 regulates Stat3 phosphorylation and DNA binding activity in human prostate cancer cells expressing constitutively active Stat3. Oncogene 2002;21:3082-8. [PubMed]
- Lin J, Jin X, Rothman K, et al. Modulation of signal transducer and activator of transcription 3 activities by p53 tumor suppressor in breast cancer cells. Cancer Res 2002;62:376-80. [PubMed]
- Kern HB, Niemeyer BF, Parrish JK, et al. Control of MicroRNA-21 expression in colorectal cancer cells by oncogenic epidermal growth factor/Ras signaling and Ets transcription factors. DNA Cell Biol 2012;31:1403-11. [PubMed]
- Xue W, Dahlman JE, Tammela T, et al. Small RNA combination therapy for lung cancer. Proc Natl Acad Sci U S A 2014;111:E3553-61. [PubMed]


