Excision repair cross-complementary group for chemotherapy efficacy in gastric cancer
Introduction
Gastric cancer is the most common carcinoma in Asia and the third leading cause of death in men and the fourth leading cause of death in women around the world (1). Detection of gastric cancer at an early stage increases the chances of accomplishing a complete surgical resection and contributes to a long survival. About 35 percent of advanced gastric cancer patients show local or distal recurrence after surgery (2). A standard treatment for patients with unresectable or recurrent gastric cancer is systemic chemotherapy, and the recent development of new anti-cancer drugs has improved survival time in these population (3-5). On the other hand, the frequency of somatic gene mutations in advanced gastric cancer was low (6), thus indicating that further reverse translational research is required in order to identify predictive and prognostic factors that might help to individualize anti-cancer treatment.
A combination of fluoropyrimidine and platinum is the most commonly accepted first-line chemotherapy in patients with unresectable or recurrent gastric cancer (7-10). The objective response rate (ORR) of the combined chemotherapy is almost 50-60% according to previous clinical trials. About 50% of patients cannot obtain a response to first-line chemotherapy, so new biological markers to select responders efficiently before treatment are required in order for individual treatment in advanced gastric cancer patients to succeed.
Excision repair cross-complementary group 1 (ERCC1) is a significant protein in the nucleotide excision repair (NER) pathway (11,12). ERCC1 and ERCC2 proteins are major components in the NER complex and act as rate-limiting enzymes in the NER pathway. The ERCC1 gene is located on chromosome 19q13.2-q13.3 and encodes different isoforms by alternative splicing. The ERCC1/xeroderma pigmentosum complementation group F (XPF) plays significant roles in several DNA repair pathways. ERCC1/XPF is a heterodimeric DNA structure—specific endonuclease that is able to cleave the sugar-phosphate backbone at the double-strand—single-strand junction of any branched DNA and at 3’-protruding single-strand ends. This makes ERCC1/XPF essential in several different pathways associated with DNA repair, such as NER, interstrand cross-link repair (ICL-R), ROS-induced single-strand break repair (SSB-R), and two sub-pathways associated with double-strand break repair (DSB-R), which are called single-strand annealing (SSA) and microhomology-mediated end joining (MMEJ), respectively.
ERCC1 activity has been previously reported as a significant biomarker for the efficacy of platinum-based chemotherapy in solid tumors, such as ovarian (13,14), lung (15,16), gastric (17) and colorectal tumors (18). These studies indicated that a low expression of ERCC1 was associated with higher chemotherapeutic sensitivity.
The roles of ERCC1 in platinum-based chemotherapy in non-small cell lung cancer (NSCLC) were evaluated in two prospective multicenter randomized trials: GECP/98-02 trial and the International Adjuvant Lung Cancer Trial (IALT). Rosell et al. (GECP/98-02) evaluated the association between the outcome of gemcitabine plus cisplatin treatment and the mRNA level of ERCC1 in 56 patients with advanced (stage IIIb or IV) NSCLC (15). In this study, there were no significant associations between ERCC1 expression and the response to chemotherapy. The median overall survival (OS) was significantly longer in patients with low ERCC1 expression tumors compared to that of patients with high expression tumors. Multivariate analyses indicated that ERCC1 expression was an independent prognostic factor in advanced NSCLC.
The biology part of the IALT was an immunohistochemical (IHC) biomarker analysis of the ERCC1 expression in 761 paraffin-embedded tumor samples. IALT was a randomized phase III trial to evaluate the ability of adjuvant chemotherapy to improve survival after complete resection in 1,867 patients in stage I-III of NSCLC (16). Adjuvant chemotherapy, as compared with observation, significantly improved OS in patients with ERCC1-negative tumors [adjusted hazard ratio (HR) 0.65; 95% confidence interval (CI) 0.50-0.86; P=0.002] but not in patients with ERCC1-positive tumors (adjusted HR 1.14; 95% CI 0.84-1.55; P=0.40). Among patients who did not receive adjuvant chemotherapy, OS in those with ERCC1-positive tumors was significantly longer than that in those with ERCC1-negative tumors (adjusted HR 0.66; 95% CI 0.49-0.90; P=0.009). Accordingly, the clinical benefit from cisplatin-based adjuvant chemotherapy in NSCLC patients was associated with ERCC1 negativity (test for interaction, P=0.009) in this trial.
The Gynecologic Oncology Group (GOG)-0158 and GOG-172 trials evaluated the roles of ERCC1 expression in patients who received platinum-based chemotherapy. The GOG-0158 trial was a randomized phase III trial that compared the efficacy and safety of paclitaxel plus carboplatin with paclitaxel plus cisplatin in stage III of epithelial ovarian cancer (EOC). Translational analyses of this phase III trial investigated platinum-DNA adducts and expression of mRNA for ERCC1 as biomarkers for taxane plus platinum (carboplatin and cisplatin) efficacy in 170 EOC patients (13). This study indicated that there was no difference in PFS and OS related to ERCC1 expression in patients who were treated with taxane-platinum chemotherapy (PFS: HR 0.978, 95% CI 0.655-1.461, P=0.915; OS: HR 1.026, 95% CI 0.648-1.626, P=0.912).
The GOG-172 trial was a randomized phase III trial of intravenous versus intraperitoneal cisplatin and paclitaxel administration in patients with optimally resected, stage III EOC or primary peritoneal carcinoma. A translational analysis of the GOG-172 trial investigated the association between the polymorphism of the ERCC1 gene and outcomes of platinum-based chemotherapy. This study revealed that ERCC1 codon 118 polymorphism was not associated with clinical outcome, but that the C8092A polymorphism, in contrast, was an independent predictor of PFS and OS in women with optimally resected EOC (14).
According to these translational analyses of ERCC1 expression in large-scale prospective clinical trials of NSCLC and EOC, the role of ERCC1 as a biomarker in platinum-based chemotherapy varies by type of carcinoma and the diagnostic methods used for detection of ERCC1 in tumor tissues. In this review, we mainly describe the prognostic role of ERCC1 in chemotherapy of advanced gastric cancer patients. First, we show the results of small-scale previous studies on the role of ERCC1 as a biomarker in platinum-based chemotherapy and the problems of evaluation of ERCC1expression in advanced gastric cancer patients. Then, we describe the results of the JCOG 9912 trial, which is a randomized phase III trial that investigated the superiority of irinotecan plus cisplatin (IP) and the non-inferiority of S-1 compared with 5-FU continuous venous-infusion and the concept of JCOG 1103 trial in unresectable or recurrent gastric cancer patients in Japan (17,19).
Previous small studies on the clinical roles of ERCC1 in platinum-based chemotherapy in patients with advanced gastric cancer
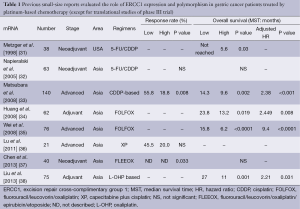
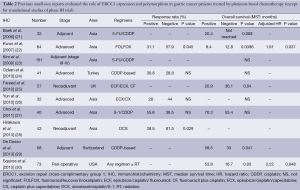
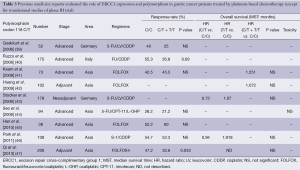
There are many previous reports published on studies that were mainly retrospective in individual institutions and only included small populations. No translational research has been published on ERCC1 in large-scale prospective clinical trials on advanced gastric cancer patients who received systemic chemotherapy, except for those on mRNA levels of ERCC1 in the JCOG 9912 trial (17) and polymorphism of ERCC1 in a phase III of the Arbeitsgemeinschaft Internistische Onkologie (AIO) group (20). Except for these two large-scale prospective trials, ERCC1 expression or polymorphism as a biomarker of platinum-based chemotherapy in solid tumors were evaluated in blood samples by intensity of IHC (21-30), mRNA levels in tumor tissues and polymorphism of ERCC1 (39-47). Previous reports on the clinical role of ERCC1 in small studies on patients with gastric cancer are summarized in Tables 1-3. Biomarker analyses of ERCC1 were mainly carried out in Asian countries such as China, Korea and Japan (21-23,26-28,32-38,41,42,44-47), but several studies were performed in American and European populations (24,25,29-31,39,40,43). Cisplatin or oxaliplatin was administered as platinum-based chemotherapy in advanced gastric cancer patients in all studies.
Full table
Full table
Full table
We identified eight previous reports that evaluated the ERCC1 mRNA levels in gastric cancer patients who received the platinum-based chemotherapy. Most of these biomarker analyses were carried out in Asian countries. In all of these studies, the mRNA levels of ERCC1 in tumor tissues were measured by the quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR), and the patients in these studies received systemic chemotherapy for advanced gastric cancer (33,37,38) or adjuvant chemotherapy before or after surgery (31,32,34,37,38). Some of the studies found that low levels of ERCC1 mRNA were significantly associated with better RR (33,37) and OS (33-35,38) compared with high levels of ERCC1 mRNA. On the other hand, three studies found no significant difference in terms of RR and OS irrespective of ERCC1 mRNA levels (32,36).
Ten studies evaluated the expression of ERCC1 by IHC in gastric cancer patients who received the platinum-based chemotherapy (21-30). Kwon et al. and Hirakawa et al. described that IHC negativity of ERCC1 was significantly associated with a better response to platinum-based chemotherapy compared with IHC positivity of ERCC1 (22,28). Four studies described no significant difference in terms of RR in spite of ERCC1 staining by IHC (24-27). In terms of OS, three studies described that patients with negative IHC status had longer OS compared with those with positive IHC status (21,22,25). On the other hand, De Dosso et al. and Squires et al. reported that patients with negative IHC status of ERCC1 had shorter OS compared with those with positive IHC status (29,30). Four studies indicated no association between IHC status of ERCC1 and OS (23,24,26,27).
We identified nine small retrospective analyses that evaluated the ERCC1 codon 118 C/T polymorphism in gastric cancer patients who received the platinum-based chemotherapy in advanced disease or adjuvant setting (39-48). In one report, the genotype of C/C was significantly associated with a better response to platinum-based chemotherapy compared with the genotypes of C/T or T/T in patients who received fluorouracil, leucovorin and oxaliplatin (FOLFOX4) regimen as adjuvant chemotherapy (47). On the other hand, seven studies found no significant difference in ORR between the genotypes of C/T or T/T and that of C/C (39-41,43-47). Five studies evaluated the value of ERCC1 codon 118 polymorphism in terms of OS in patients who received platinum-based chemotherapy (41-43,46,47), but there were no significant difference in OS associated with any of the genotypes of the ERCC1 codon 118 C/T polymorphism. A translational analysis of polymorphisms within the genes of TS, MTHFR, MTR, OPRT, XPD, ERCC1, XRCC1, XPA, GSTP1, GSTT1, and GSTM1 in 134 gastro-esophageal cancer patients who were enrolled in a randomized phase III trial of the AIO group indicated that there were significant differences in ORR related to the presence of the ERCC1-118C/8092C haplotype (odds rate: 2.55, P=0.013). On the other hand, there was no association between survival benefit of platinum-based chemotherapy and polymorphism of ERCC1 (20).
According to these data, polymorphism 118C/T of ERCC1 may not be a predictive or prognostic factor in patients who received platinum-based chemotherapy in advanced gastric cancer patients.
Problems associated with evaluation of ERCC1 as a biomarker
The results of above studies on mRNA/IHC/polymorphism of ERCC1 in patients who received platinum-based chemotherapy varied among different studies. A major reason for the variations was that the definition of cut-off values of ERCC1 levels varied among the studies. The unique cut-off values of mRNA levels included the median values (31,36,38), which are considered the best way to separate patients into low and high ERCC1 expression subgroups (33-35,37). Definition of positivity by IHC of ERCC1 varied according to intensity of IHC or percentage of stained tumor cells among previous studies. Baek et al. and Ozkan et al. defined positivity of IHC as staining of 10% or more of the tumor cells (21,24). Kwon et al. and Hirakawa et al. decided each score according to intensity of IHC staining and percentage of stained tumor cells; positivity of ERCC1 was defined both by the grades of intensity and percentage of stained cells or as the grades of staining intensity plus staining of 2% or more of the tumor cells (22,28). Fareed et al. and Yun et al. defined tumor cells showing nuclear staining of ERCC1 as positive (25,26). In four studies, positivity of ERCC1 was defined by composite scores of both intensity of staining and percentage of stained tumor cells (23,27,29,30).
Soria et al. performed a validation study by IHC of the results of the IALT Biology study to confirm the predictive role of ERCC1 expression in 494 patients in the independent prospective trial of NSCLC (48). This IHC study was unable to validate the predictive effect of ERCC1 and showed that the available antibodies did not provide adequate discrimination for making therapeutic decisions regarding cisplatin. On the other hand, ERCC1-202 was detected as a unique functional isoform of ERCC1 that could predict the clinical benefit of cisplatin in this study. Development of a specific antibody and of specific primers and probes for qRT-PCR, such as the ERCC1-202 isoform, may solve the discordance of the results of translational analyses of ERCC1 expression in solid tumors.
Among other reasons for the varying results, previous reports had methodical heterogeneity in terms of (I) collecting samples such as endoscopic biopsy and surgical resection; (II) preservation of materials such as FFPE samples and frozen tissue samples; (III) patients’ characteristics such as age, gender, performance status, clinical stage and chemotherapeutic regimens and timing by each study. Some meta-analyses have been published that evaluated the association between ERCC1 expression/polymorphism and efficacy of platinum-based chemotherapy in gastric cancer patients (49,50), but these data were not based on the individual data of previous studies. Finally, biomarker analyses of large-scale prospective studies are required to truly evaluate the clinical roles of ERCC1.
Biomarker analyses of ERCC1 mRNA levels in the JCOG 9912 trial
The JCOG 9912 trial is a randomized phase III trial that investigated the superiority of IP and non-inferiority of S-1 compared with 5-FU with the primary endpoint of OS in unresectable or recurrent gastric cancer patients in Japan (19). This trial revealed non-inferiority of S-1 to 5-FU (HR 0.83; 95% CI 0.68-1.01; P<0.001) with regard to OS, but failed to show superiority of IP (HR 0.85; 95% CI 0.70-1.04; P=0.055) (19). Yamada et al. performed biomarker analyses, including ERCC1, on endoscopic biopsy specimens from primary lesions in 445 of 704 gastric cancer patients in the JCOG 9912 trial (17). In all of the patients, the ERCC1 and DPD mRNA expression in the diffuse type adenocarcinoma was significantly higher than the expression in the intestinal type. Multivariate analyses showed that high ERCC1 expression was associated with a shorter OS (HR 1.37; 95% CI 1.08-1.75; P=0.010). In a subgroup receiving IP (n=84), there was a significant difference in RR between patients with low levels and those with high levels of ERCC1 mRNA (52.5% vs. 29.6%, P=0.045). On the other hand, there were no PFS or OS differences between IP and S-1 among patients with low ERCC1.
Finally, no predictive marker for selecting 5-FU or IP rather than S-1 could be found in this study. High ERCC1 values were observed frequently in patients with diffuse-type adenocarcinoma and was an independent prognostic factors in all patients in JCOG 9912.
Next step in systemic chemotherapy in patients with metastatic gastric cancer in Japan—JCOG 1013 trial (ADOPT study)
JCOG 1013 is a randomized phase III trial that investigates the superiority of a triplet regimen of docetaxel, S-1 and cisplatin (DCS) in relation to S-1 and cisplatin (CS) in patients with unresectable or recurrent gastric cancer. The schedule of the DCS regimen is as follows: Cisplatin (60 mg/m2) plus docetaxel (40 mg/m2) intravenously on day 1 and S-1 (80 mg/m2) on days 1-14, of a 4-week cycle. In addition to the primary endpoint of OS in all patients who are enrolled in this trial, the JCOG 1013 trial also investigates differences in OS according to tumor tissue classification [differentiated carcinoma (intestinal type) vs. undifferentiated carcinoma (diffuse type)] as a key secondary endpoint. A schema of the JCOG 1013 trial is shown in Figure 1. A biomarker study of JCOG 9912 revealed that ERCC1 mRNA expression was significantly higher in the diffuse type adenocarcinoma compared with the intestinal type and that high ERCC1 was associated with a poor prognosis in unresectable or recurrent gastric cancer patients. This finding indicated that a therapeutic strategy of more active treatment of gastric cancer patients with advanced-stage diffuse-type adenocarcinoma and higher ERCC1 expression in the tumor is required. DCS regimens are expected to be effective in advanced gastric cancer patients with these poor prognostic factors. JCOG 1013 will clarify the difference in treatment selection of first-line chemotherapy between triplet and doublet regimens according to tumor tissue type (diffuse type/intestinal type) or ERCC1 levels (high/low) in unresectable and recurrent gastric cancer patients.
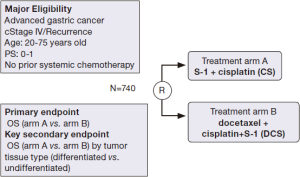
A triplet regimen as first-line chemotherapy was considered to be more active than a doublet regimen in a previous randomized phase III trial (V325) (51). In this study, the efficacy of docetaxel and cisplatin plus fluorouracil (DCF) was compared with cisplatin and fluorouracil (CF) as first-line chemotherapy in advanced gastric cancer; it revealed that the DCF regimen significantly improved ORR, time to progression and OS compared with the CF regimen. A high rate of febrile neutropenia was noted as a problem that might prevent continuation of the treatment in patients who received DCF in the V325 trial. In JCOG 1013, dose modification of S-1 and cisplatin was determined beforehand according to renal function in order to avoid a massive toxicity of the triplet regimen because renal dysfunction delays the excretion of 5-chloro-2,4-dihydroxypyridine (CDHP), which is a component of S-1, and elevates the serum level of 5-FU (52).
Adding docetaxel to the CS regimen as first-line chemotherapy may be a better strategy to improve the OS in advanced gastric cancer patients. The frequency of patients who could receive taxanes after first-line chemotherapy is lower in patients with poor prognosis compared with those with better prognosis. In Japan, a combined analysis of JCOG 9205 and JCOG 9912 indicated that the second-line chemotherapy is a significant factor to prolong the OS in advanced gastric cancer patients who received systemic chemotherapy (53).
Conclusions
According to large-scale translational analyses of JCOG 9912 a high level of ERCC1 is considered a poor prognostic factor in terms of OS in advanced gastric cancer patients who received systemic chemotherapy. As a future approach, it would be advantageous to establish strict guidelines for standard protocols regarding sample collection and preservation of samples and to develop target-specific antibodies for IHC and primers and probes for qRT-PCR of functional ERCC1 isoforms. These improvements would solve the methodological heterogeneity of ERCC1 determinations. In addition, other molecular biomarkers associated with chemo-sensitivity should be investigated in future studies in order to identify predictive markers of cytotoxic agents in advanced gastric cancer patients. Also, large-scale randomized trials to validate the roles of molecular markers, including ERCC1 expression, in advanced gastric cancer patients who receive chemotherapy are required in the future.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011;29:4387-93. [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [PubMed]
- Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224-35. [PubMed]
- Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31-9. [PubMed]
- Yamada Y. Molecular therapy for gastric cancer. Chin Clin Oncol 2013;2:5. [PubMed]
- Koizumi W, Narahara H, Hara T. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008;9:215-21. [PubMed]
- Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008;358:36-46. [PubMed]
- Okines AF, Norman AR, McCloud P, et al. Meta-analysis of the REAL-2 and ML17032 trials: evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil-based combination chemotherapy for the treatment of advanced oesophago-gastric cancer. Ann Oncol 2009;20:1529-34. [PubMed]
- Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol 2015;26:141-8. [PubMed]
- Besse B, Olaussen KA, Soria JC. ERCC1 and RRM1: ready for prime time? J Clin Oncol 2013;31:1050-60. [PubMed]
- Martin LP, Hamilton TC, Schilder RJ. Platinum resistance: the role of DNA repair pathways. Clin Cancer Res 2008;14:1291-5. [PubMed]
- Darcy KM, Tian C, Reed E. A Gynecologic Oncology Group study of platinum-DNA adducts and excision repair cross-complementation group 1 expression in optimal, stage III epithelial ovarian cancer treated with platinum-taxane chemotherapy. Cancer Res 2007;67:4474-81. [PubMed]
- Krivak TC, Darcy KM, Tian C, et al. Relationship between ERCC1 polymorphisms, disease progression, and survival in the Gynecologic Oncology Group Phase III Trial of intraperitoneal versus intravenous cisplatin and paclitaxel for stage III epithelial ovarian cancer. J Clin Oncol 2008;26:3598-606. [PubMed]
- Lord RV, Brabender J, Gandara D, et al. Low ERCC1 expression correlates with prolonged survival after cisplatin plus gemcitabine chemotherapy in non-small cell lung cancer. Clin Cancer Res 2002;8:2286-91. [PubMed]
- Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med 2006;355:983-91. [PubMed]
- Yamada Y, Boku N, Nishina T, et al. Impact of excision repair cross-complementing gene 1 (ERCC1) on the outcomes of patients with advanced gastric cancer: correlative study in Japan Clinical Oncology Group Trial JCOG9912. Ann Oncol 2013;24:2560-5. [PubMed]
- Shirota Y, Stoehlmacher J, Brabender J, et al. ERCC1 and thymidylate synthase mRNA levels predict survival for colorectal cancer patients receiving combination oxaliplatin and fluorouracil chemotherapy. J Clin Oncol 2001;19:4298-304. [PubMed]
- Boku N, Yamamoto S, Fukuda H, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 2009;10:1063-9. [PubMed]
- Goekkurt E, Al-Batran SE, Hartmann JT, et al. Pharmacogenetic analyses of a phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil and leucovorin plus either oxaliplatin or cisplatin: a study of the arbeitsgemeinschaft internistische onkologie. J Clin Oncol 2009;27:2863-73. [PubMed]
- Baek SK, Kim SY, Lee JJ, et al. Increased ERCC expression correlates with improved outcome of patients treated with cisplatin as an adjuvant therapy for curatively resected gastric cancer. Cancer Res Treat 2006;38:19-24. [PubMed]
- Kwon HC, Roh MS, Oh SY, et al. Prognostic value of expression of ERCC1, thymidylate synthase, and glutathione S-transferase P1 for 5-fluorouracil/oxaliplatin chemotherapy in advanced gastric cancer. Ann Oncol 2007;18:504-9. [PubMed]
- Kim JS, Kim MA, Kim TM, et al. Biomarker analysis in stage III-IV (M0) gastric cancer patients who received curative surgery followed by adjuvant 5-fluorouracil and cisplatin chemotherapy: epidermal growth factor receptor (EGFR) associated with favourable survival. Br J Cancer 2009;100:732-8. [PubMed]
- Ozkan M, Akbudak IH, Deniz K, et al. Prognostic value of excision repair cross-complementing gene 1 expression for cisplatin-based chemotherapy in advanced gastric cancer. Asian Pac J Cancer Prev 2010;11:181-5. [PubMed]
- Fareed KR, Al-Attar A, Soomro IN, et al. Tumour regression and ERCC1 nuclear protein expression predict clinical outcome in patients with gastro-oesophageal cancer treated with neoadjuvant chemotherapy. Br J Cancer 2010;102:1600-7. [PubMed]
- Yun J, Kim KM, Kim ST, et al. Predictive value of the ERCC1 expression for treatment response and survival in advanced gastric cancer patients receiving cisplatin-based first-line chemotherapy. Cancer Res Treat 2010;42:101-6. [PubMed]
- Choi IS, Lee HS, Lee KW, et al. Biomarker analysis in patients with advanced gastric cancer treated with S-1 plus cisplatin chemotherapy: orotate phosphoribosyltransferase expression is associated with treatment outcomes. Med Oncol 2011;28:991-8. [PubMed]
- Hirakawa M, Sato Y, Ohnuma H, et al. A phase II study of neoadjuvant combination chemotherapy with docetaxel, cisplatin, and S-1 for locally advanced resectable gastric cancer: nucleotide excision repair (NER) as potential chemoresistance marker. Cancer Chemother Pharmacol 2013;71:789-97. [PubMed]
- De Dosso S, Zanellato E, Nucifora M, et al. ERCC1 predicts outcome in patients with gastric cancer treated with adjuvant cisplatin-based chemotherapy. Cancer Chemother Pharmacol 2013;72:159-65. [PubMed]
- Squires MH 3rd, Fisher SB, Fisher KE, et al. Differential expression and prognostic value of ERCC1 and thymidylate synthase in resected gastric adenocarcinoma. Cancer 2013;119:3242-50. [PubMed]
- Metzger R, Leichman CG, Danenberg KD, et al. ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol 1998;16:309-16. [PubMed]
- Napieralski R, Ott K, Kremer M, et al. Combined GADD45A and thymidine phosphorylase expression levels predict response and survival of neoadjuvant-treated gastric cancer patients. Clin Cancer Res 2005;11:3025-31. [PubMed]
- Matsubara J, Nishina T, Yamada Y, et al. Impacts of excision repair cross-complementing gene 1 (ERCC1), dihydropyrimidine dehydrogenase, and epidermal growth factor receptor on the outcomes of patients with advanced gastric cancer. Br J Cancer 2008;98:832-9. [PubMed]
- Huang ZH, Hua D, Du X, et al. ERCC1 polymorphism, expression and clinical outcome of oxaliplatin-based adjuvant chemotherapy in gastric cancer. World J Gastroenterol 2008;14:6401-7. [PubMed]
- Wei J, Zou Z, Qian X, et al. ERCC1 mRNA levels and survival of advanced gastric cancer patients treated with a modified FOLFOX regimen. Br J Cancer 2008;98:1398-402. [PubMed]
- Lu M, Gao J, Wang XC, et al. Expressions of Thymidylate Synthase,Thymidine Phosphorylase, Class III β-tubulin, and Excision Repair Cross-complementing Group 1predict Response in Advanced Gastric Cancer Patients Receiving Capecitabine Plus Paclitaxel or Cisplatin. Chin J Cancer Res 2011;23:288-94. [PubMed]
- Chen L, Li G, Li J, et al. Correlation between expressions of ERCC1/TS mRNA and effects of gastric cancer to chemotherapy in the short term. Cancer Chemother Pharmacol 2013;71:921-8. [PubMed]
- Liu YP, Ling Y, Qi QF, et al. The effects of ERCC1 expression levels on the chemosensitivity of gastric cancer cells to platinum agents and survival in gastric cancer patients treated with oxaliplatin-based adjuvant chemotherapy. Oncol Lett 2013;5:935-942. [PubMed]
- Goekkurt E, Hoehn S, Wolschke C, et al. Polymorphisms of glutathione S-transferases (GST) and thymidylate synthase (TS)--novel predictors for response and survival in gastric cancer patients. Br J Cancer 2006;94:281-6. [PubMed]
- Ruzzo A, Graziano F, Kawakami K, et al. Pharmacogenetic profiling and clinical outcome of patients with advanced gastric cancer treated with palliative chemotherapy. J Clin Oncol 2006;24:1883-91. [PubMed]
- Keam B, Im SA, Han SW, et al. Modified FOLFOX-6 chemotherapy in advanced gastric cancer: Results of phase II study and comprehensive analysis of polymorphisms as a predictive and prognostic marker. BMC Cancer 2008;8:148. [PubMed]
- Huang ZH, Hua D, Li LH. The polymorphisms of TS and MTHFR predict survival of gastric cancer patients treated with fluorouracil-based adjuvant chemotherapy in Chinese population. Cancer Chemother Pharmacol 2009;63:911-8. [PubMed]
- Stocker G, Ott K, Henningsen N, et al. CyclinD1 and interleukin-1 receptor antagonist polymorphisms are associated with prognosis in neoadjuvant-treated gastric carcinoma. Eur J Cancer 2009;45:3326-35. [PubMed]
- Seo BG, Kwon HC, Oh SY, et al. Comprehensive analysis of excision repair complementation group 1, glutathione S-transferase, thymidylate synthase and uridine diphosphate glucuronosyl transferase 1A1 polymorphisms predictive for treatment outcome in patients with advanced gastric cancer treated with FOLFOX or FOLFIRI. Oncol Rep 2009;22:127-36. [PubMed]
- Han SW, Oh DY, Im SA, et al. Epidermal growth factor receptor intron 1 CA dinucleotide repeat polymorphism and survival of advanced gastric cancer patients treated with cetuximab plus modified FOLFOX6. Cancer Sci 2010;101:793-9. [PubMed]
- Park SR, Kong SY, Nam BH, et al. CYP2A6 and ERCC1 polymorphisms correlate with efficacy of S-1 plus cisplatin in metastatic gastric cancer patients. Br J Cancer 2011;104:1126-34. [PubMed]
- Qi YJ, Cui S, Yang YZ, et al. Excision repair cross-complementation group 1 codon 118 polymorphism, micro ribonucleic acid and protein expression, clinical outcome of the advanced gastric cancer response to first-line FOLFOX-4 in Qinghai-Tibetan plateau population. J Cancer Res Ther 2013;9:410-5. [PubMed]
- Friboulet L, Olaussen KA, Pignon JP, et al. ERCC1 isoform expression and DNA repair in non-small-cell lung cancer. N Engl J Med 2013;368:1101-10. [PubMed]
- Yao A, Wang Y, Peng X, et al. Predictive value of excision repair cross-complementation group 1 expression for platinum-based chemotherapy and survival in gastric cancer: a meta-analysis. J Cancer Res Clin Oncol 2014;140:2107-17. [PubMed]
- Wang Z, Chen JQ, Liu JL, et al. Polymorphisms in ERCC1, GSTs, TS and MTHFR predict clinical outcomes of gastric cancer patients treated with platinum/5-Fu-based chemotherapy: a systematic review. BMC Gastroenterol 2012;12:137. [PubMed]
- Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991-7. [PubMed]
- Fujita K, Yamamoto W, Endo S, et al. CYP2A6 and the plasma level of 5-chloro-2, 4-dihydroxypyridine are determinants of the pharmacokinetic variability of tegafur and 5-fluorouracil, respectively, in Japanese patients with cancer given S-1. Cancer Sci 2008;99:1049-54. [PubMed]
- Takashima A, Boku N, Kato K, et al. Survival prolongation after treatment failure of first-line chemotherapy in patients with advanced gastric cancer: combined analysis of the Japan Clinical Oncology group trials JCOG9205 and JCOG9912. Gastric Cancer 2014;17:522-8. [PubMed]


