Atrophic gastritis and pre-malignant gastric lesions
Atrophic gastritis: a precancerous condition
Atrophic gastritis is an inflammatory condition characterized by the loss of gastric glandular structures which are replaced by connective tissue (non-metaplastic atrophy) or by glandular structures inappropriate for location (metaplastic atrophy) (1). Gastric mucosal atrophy and intestinal metaplasia confer a high risk for the development of gastric cancer as they constitute the background in which dysplasia and intestinal-type gastric adenocarcinoma may develop (2,3). For this reason, atrophic gastritis and intestinal metaplasia are considered to be precancerous conditions.
In many cases, the development of the intestinal-type gastric adenocarcinoma represents the end step of an inflammation-metaplasia-dysplasia-carcinoma sequence, also called the Correa cascade of multistep gastric carcinogenesis. According to this cascade, gastric cancer develops as a consequence of a gradual progression from normal gastric mucosa through chronic Helicobacter pylori (H. pylori)-related non-atrophic gastritis, atrophic gastritis, and intestinal metaplasia, to dysplasia and carcinoma (4,5). Longitudinal studies have confirmed this model (6,7).
Different phenotypes of atrophic gastritis may develop due to different environmental exposure and genetic profiles. The intra-gastric distribution of premalignant changes of the gastric mucosa is one determinant of gastric cancer risk: cases of oxyntic gland atrophy and/or intestinal metaplasia distributed in a multifocal pattern including the lesser curvature of the corpus and fundus, are called multifocal atrophic gastritis, and this phenotype, described as “extensive”, has been associated with a higher risk of gastric cancer. Similarly, the concept of ‘gastritis of the carcinoma phenotype’ proposes that the corpus-predominant gastritis increases the risk of gastric cancer, likely due to changes of the intra-gastric milieu as increased pH, reduced ascorbic acid (AA) and scavenging of nitrites and other potential carcinogenic substances (8-10).
Intestinal metaplasia may be classified as “complete” or “incomplete”, where complete intestinal metaplasia displays goblet and absorptive cells, decreased expression of certain gastric mucins as MUC1, MUC5AC, and MUC6, but expression of the intestinal mucin MUC2. In contrast, incomplete intestinal metaplasia displays goblet and columnar non-absorptive cells co-expressing gastric and intestinal mucins (11-13). Some studies indicate a positive correlation between the extent of intestinal metaplasia and the degree of incomplete intestinal metaplasia, but in routine diagnostics, subtyping of intestinal metaplasia is not widespread.
More recently, a third pattern of intestinal metaplasia has been described, known as the spasmolytic polypeptide-expressing metaplasia (SPEM), which is characterized by the expression of the TFF2 spasmolytic polypeptide, associated with oxyntic atrophy. This pattern of intestinal metaplasia, observed in the gastric body, has been reported to be strongly associated with H. pylori infection and gastric cancer, and may possibly represent another pathway to gastric neoplasia (14). A very recent study conducted in Taiwan showed that genomic single nucleotide polymorphisms (ITGA5-1160/ITGB1-1949/ITGB1 + 31804 as T/A/C carriers and COX-2-1195/IL-10-592 as G-carrier/AA) in the offspring of gastric cancer patients predispose to SPEM after H. pylori infection, and may serve as marker to identify high-risk subjects for H. pylori eradication (15).
Gastric dysplasia represents the penultimate stage of the sequence of gastric carcinogenesis. This lesion is histologically defined as unequivocal neoplastic epithelium without evidence of tissue invasion (16), which is characterized by cellular atypia reflective of abnormal differentiation and disorganized glandular architecture. Gastric dysplasia thus, is to be considered a direct neoplastic precancerous lesion. The Padova, Vienna, and WHO classifications are proposals to standardize the terminology for the morphological spectrum of gastric dysplastic lesions (17-20).
Epidemiological data suggest that atrophic gastritis is associated not only with intestinal-type gastric cancer, but also with type 1 gastric carcinoid. The pathophysiological mechanisms which lead to the development of these gastric tumors are profoundly different. As mentioned above, gastric cancer develops as the final result of a multistep process initiating from H. pylori-related gastritis to atrophic gastritis, intestinal metaplasia and dysplasia (4). In contrast, type 1 gastric carcinoids are gastrin-dependent tumors. These tumors are well-differentiated with low proliferative index and a generally benign behavior, and constitute up to 80% of all gastric carcinoids (21). A major pathogenetic factor for type 1 gastric carcinoids is hypergastrinemia due to atrophic gastritis. Gastrin acts as a growth factor for enterochromaffin-like cells, which in atrophic gastritis are chronically induced to proliferate, and, through a multistep process passing from hyperplasia to dysplasia, carcinoid may develop (21-23).
Several events occur in the gastric mucosa before the development of gastric cancer or type 1 gastric carcinoid, and these events may take several years. Thus, the knowledge of atrophic gastritis prevalence in different clinical settings, its clinical features and possible risk factors associated with the progression of this condition to gastric neoplasms are important issues.
Atrophic gastritis: epidemiology and clinical features
Atrophic gastritis is a chronic disorder occurring in up to 8% of the general population, mainly characterized by atrophy of the oxyntic glands with consequent lack of gastric acid and, in a late stage, lack of intrinsic factor production. Often, the positivity of autoantibodies against parietal cells and/or intrinsic factor, the compresence of autoimmune diseases as thyroid autoimmune disease or type 1 diabetes are observed (24-27). In a study conducted in 2008 (27), of the 319 atrophic gastritis patients investigated, 169 (53%) had an associated thyroid disorder, and 89 (52.7%) of these were unaware of it. The thyroid disease was autoimmune in 128 patients (75.7%) and non-autoimmune in 41 patients. Logistic regression showed that risk factors for having autoimmune thyroid disease in atrophic gastritis patients were female gender (odds ratio 5.6), presence of parietal cell antibodies (odds ratio 2.5), and presence of metaplastic atrophy (odds ratio 2.2). These data show that autoimmune thyroid disease and atrophic gastritis occur in a closely linked fashion, and they suggest that atrophic gastritis patients should be investigated for an occult autoimmune thyroid disease, in particular women and those with positive parietal cell antibodies (27).
A frequent clinical presentation of atrophic gastritis is pernicious anemia, a megaloblastic anemia arising from vitamin B12 malabsorption as a consequence of intrinsic factor deficiency (28,29). Atrophic gastritis may present also with iron deficiency anemia due to iron malabsorption as a consequence of reduced gastric acid secretion together with normal or low cobalamin levels (30,31), and some of these patients may over time develop overt pernicious anemia (32). The reasons for these different clinical presentations of patients with atrophic gastritis harbouring similar gastric alterations virtually leading to vitamin B12 deficiency are not known and may have a genetic basis. In a recent study, in which a panel of single nucleotide polymorphisms related to cobalamin absorption was investigated in atrophic gastritis patients with and without pernicious anemia compared to healthy controls, showed that a genetic variant of transcobalamin II, related to lower vitamin B12 levels, was more frequent in pernicious anemia patients compared to controls, showing the plausibility of genetic factors determining the possible clinical manifestation of atrophic gastritis (33).
Pernicious anemia, the possible end-stage of atrophic gastritis, is considered an autoimmune disorder (28,29). To date, there are no clear universally accepted criteria to define autoimmune gastritis and to distinguish this clinical entity from chronic H. pylori-driven atrophic gastritis. In a previous work it was shown that features which should help to discriminate between autoimmune and not-autoimmune gastritis, as positivity to intrinsic factor and parietal cells antibodies, presence of enterochromaffin-like cells, pernicious anemia and active H. pylori infection, were similar in patients with corpus-restricted atrophic gastritis (the classical histological feature of autoimmune gastritis) and those with antral and corporal atrophic gastritis (mainly attributed to H. pylori infection), indicating that the specific clinical-histological features associated with autoimmune gastritis are far from being well defined (31).
A recent systematic review evaluated the atrophic gastritis incidence in patients free of atrophic gastritis at moment of inclusion in the study (34). The authors selected 14 follow-up studies in which atrophic gastritis was diagnosed by histology (12 studies) or by serum pepsinogen (two studies). The atrophic gastritis incidence rates ranged from 0 to 10.9% per year, probably explained by the particular clinical settings in which the atrophic gastritis diagnoses were made, including patients with reflux esophagitis and those successfully treated for H. pylori infection with lowest incidence rates (0%) (35,36) and patients who underwent vagotomy due to peptic ulcer with highest incidence rate (37). In a meta-analysis, the rate ratios comparing the atrophic gastritis incidence in H. pylori positive patients to that in H. pylori negative ones ranged from 2.4 to 7.6 with a summary estimate of 5 (95% CI: 3.1-8.3) (34).
In other studies, the prevalence of atrophic gastritis was evaluated by serological screening using surrogate markers of gastric function (pepsinogen I or pepsinogen I/pepsinogen II ratio) or by gastroscopy/histology. In many cases, the serological and histological screenings were both made in a general population. Serological studies reported atrophic gastritis prevalence rates between 3% and 7%, which were lower than those reported by histological ones (38-47). Higher rates of atrophic gastritis prevalence found in the Asian countries may be justified by the higher risk of gastric cancer in these areas are and the different definition of atrophic gastritis diagnosis between Western and Asian countries. In studies reporting from Asian countries, atrophic gastritis diagnosis included all atrophic lesions irrespective of the atrophy localization in the gastric mucosa (antrum and/or corpus); in the vast majority of the studies conducted in Western countries, atrophic gastritis diagnosis included only patients with a corpus atrophic involvement such as corpus-atrophic gastritis or a multifocal atrophic gastritis.
A very recent serological study on 5,284 participants in Sweden (48) documented an increase in the prevalence of atrophic gastritis among adults aged 35-44 years from 22 to 64/1,000 between 1990 and 2009, but a decrease prevalence of atrophic gastritis in participants 55-64 years old from 124 to 49/1,000 in the same observation period. The stabilizing seroprevalence of H. pylori and increasing prevalence of overweight and obesity might contribute to this unexpected trend; however, studies are needed to determine whether these changes have affected the incidence of gastric cancer (48).
Atrophic gastritis and risk of gastric neoplasms
Gastric cancer is still the fourth most common cancer worldwide and the second cause of cancer-related death (49). A varying progression rate of atrophic gastritis to gastric cancer up to 2% per year has been reported at follow-up periods ranging from 1 to 16 years (50-52). A recent systematic review showed in atrophic gastritis patients with pernicious anemia a pooled gastric cancer incidence-rate of 0.3% person-year and an estimated 7-fold relative risk of gastric cancer (53).
In patients with atrophic gastritis also type 1 gastric carcinoids may arise. Data on long-term incidence of type 1 gastric carcinoids are scanty (54-56). A recent cohort study reported an annual incidence rate for type 1 gastric carcinoid of 0.4% (57), while an older study reported an annual incidence of 2%, observing eight new cases of type 1 gastric carcinoids in 416 patient-year (55). In the above cited study, pernicious anemia was present in almost 50% of patients with type 1 gastric carcinoids (57), while previous studies included exclusively patients with this condition (55,56,58-60). In patients with atrophic gastritis, the need and cost-effectiveness of regular endoscopic follow-up for gastric cancer surveillance is not definitely established. Recent European guidelines recommend a scheduled surveillance for gastric cancer for those patients who have extensive—i.e., both antrum and gastric body—atrophic gastritis or intestinal metaplasia (61). However these guidelines are not addressed to patients with pernicious anemia, as corpus-restricted atrophic gastritis with antrum-spared, typically present in pernicious anemia patients, is not considered to be part of the precancerous cascade described by Correa (4). According to the data reported, different clinical management of atrophic gastritis patients with or without pernicious anemia does not seem to be justified, raising questions whether these recommendations should include also pernicious anemia patients.
With regard to surveillance for type 1 gastric carcinoids, indications are even more uncertain. A recent study on endoscopic management of these tumours, reported that for atrophic gastritis patients without recurring type 1 gastric carcinoids, endoscopic controls might be planned yearly in the early follow-up, but can probably become less intensive with endoscopic controls every 4 years according to atrophic gastritis screening for gastric cancer risk (62). To better evaluate the value of surveillance in atrophic gastritis patients and establish follow-up frequencies, more precise data on the occurrence of gastric neoplastic lesions, preferably obtained in large prospective studies with adequate follow-up, are needed (63).
The combined risk of gastric cancer and carcinoids together has been investigated many years ago limited to pernicious anemia patients (55,56). A recent study (64) investigated in a prospective cohort of patients with atrophic gastritis the occurrence of gastric cancer and carcinoids at long-term follow-up from 4 years upwards. In this study a total of 200 atrophic gastritis patients from a prospective cohort (67% females, median age 55 years) with a follow-up of 7.5 (range, 4-23.4) years were included. Follow-up gastroscopies at 4-years intervals were performed. The results of this study showed that, overall, 22 gastric neoplastic lesions were detected (crude incidence 11%). Gastric cancer was diagnosed in four patients at a median follow-up of 7.2 years (crude incidence 2%). Eleven type 1-gastric carcinoids were detected at a median follow-up of 5.1 years (crude incidence of 5.5%). In seven patients, six low-grade and one high-grade dysplasia were found. As shown in Table 1, the annual incidence rates person-year were 0.25%, 0.43%, and 0.68% for gastric cancer, dysplasia, and type 1-gastric carcinoids, respectively. From this study emerged that in atrophic gastritis patients at long-term follow-up an annual incidence rate of 1.36% person-year for gastric neoplastic lesions and that the incidence rates of gastric cancer and type 1 gastric carcinoid were not different (P=0.07), indicating that atrophic gastritis patients are similarly exposed to both risks (64). According to Globocan 2012, the annual incidence rate for gastric cancer in the general Italian population is estimated to be 0.004% (65). This study thus provides further evidence confirming the increased risk for gastric cancer in atrophic gastritis. As shown in Figure 1, the presence of pernicious anemia is associated with gastric carcinoids but not with gastric cancer, as survival free of carcinoids is significantly shorter in patients with pernicious anemia.
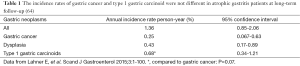
Full table

The patients’ features associated with gastric cancer and type 1 gastric carcinoids are different, keeping in step with the different pathogenetic mechanisms of these two type of tumors (4,21). The occurrence of type 1 gastric carcinoids is mainly associated with features of autoimmune gastritis as pernicious anemia and positivity to gastric autoantibodies. Gastric cancer, instead, is associated with the presence of H. pylori in the corporal mucosa (HR 8) (64) keeping in step with the concept of corpus-predominant gastritis as observed by Uemura more than ten years ago that H. pylori positive patients and those with severe gastric atrophy, corpus-predominant gastritis or intestinal metaplasia are at increased risk for gastric cancer (9). Figure 1 shows the Kaplan curves of gastric cancer and carcinoids with respect to pernicious anemia: The presence of pernicious anemia is associated with gastric carcinoids but not with gastric cancer, as survival free of carcinoids is significantly shorter in patients with pernicious anemia.
Atrophic patients are exposed to a double risk of gastric neoplastic lesions, gastric cancer and type 1 gastric carcinoids. In a retrospective case-series (66), the occurrence of gastric cancer in patients with type 1 gastric carcinoids was described in 23% (4 out of 17) of patients with type 1 gastric carcinoids over a median follow-up period of 6 years. Three cases were intestinal-type adenocarcinomas and one a signet-ring cells diffuse gastric cancer, localized in three cases in the antrum. Thus, it seems to be worthwhile to monitor type 1 gastric carcinoids patients by a long-term surveillance programme, including an accurate bioptic sampling of antral mucosa. The effects of long-standing hypergastrinemia may be a possible explanation why patients with type 1 gastric carcinoids might develop more frequently gastric cancer. Hypergastrinemia has been proposed in many models of gastric carcinogenesis and seems to be a common causative factor in otherwise different circumstances; in all species where long-term hypergastrinemia has been induced, an increased risk of gastric malignancy, with adenocarcinoma phenotype and even the signet-ring cells phenotype, was observed (67,68). Moreover, the long-term conservative management of type 1 gastric carcinoids exposes these patients to a higher risk of gastric cancer. This risk is basically present in atrophic gastritis, due to the pathophysiological changes related to gastric body atrophy, such as increased pH, reduced AA and scavenging of nitrites and other potential carcinogenic substances (69).
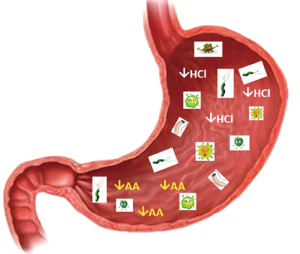
It has become apparent that besides H. pylori, other bacteria may be involved in gastric carcinogenesis; it has been shown that the gastric cancer microbiota was dominated by species of the genera Streptococcus, Lactobacillus, Veillonella and Prevotella, albeit the roles of these species in the development of gastric cancer needs to be determined (70). Figure 2 gives a schematic overview of changes of the intragastric milieu possibly involved in the higher risk of gastric neoplasms in patients with atrophic gastritis.
Detection of atrophic gastritis and premalignant changes
Although the gastric cancer incidence has declined over the past decades, especially in Western countries, the mortality rate due to gastric cancer remains high (71). Detection and surveillance of patients with premalignant conditions, as atrophic gastritis and intestinal metaplasia, could potentially lead to detection and treatment of advanced lesions—i.e., dysplastic lesions and gastric cancer—in an early stage (72-74). In patients with premalignant conditions, the risk of developing gastric cancer may be further stratified by the location, severity, and extent of gastric atrophy and/or metaplasia (75,76).
Several histological classifications have been developed for atrophic gastritis and preneoplastic changes. To date, in clinical practice and in research, the updated Sydney System is mainly used. This system combines topographic, morphological, and etiological information to standardize histological reporting (77). More recently, the systems known as OLGA (operative link for gastritis assessment), and OLGIM (operative link on gastric intestinal metaplasia) assessment have been proposed for staging of gastritis (78). Unfortunately, classifications are still difficult to use in clinical practice, and often present the disadvantage of important inter- and intra-observer variation (79).
As mandatory conditions to correctly adopt premalignant gastric lesions as reliable indicators for gastric cancer development, a standardized biopsy sampling protocol and an uniform, reproducible histological grading system need to be applied in clinical practice (61). A recent nationwide survey investigated in a community-based endoscopic setting what really happens in clinical practice with regard to the detection of gastric atrophy and intestinal metaplasia in dyspeptic patients (80). In detail, a nationwide survey was conducted on 979 consecutive patients (50-65 years old) with dyspeptic symptoms, who were examined at 24 gastrointestinal endoscopy units throughout Italy. Clinical information was collected from questionnaires; a standard bioptic mapping was performed in each unit, biopsies from each patient were analyzed by histopathology performed according to daily clinical practice in each local pathology centre. The results showed that separate descriptions of antral and corporal biopsies were included in 679 pathology reports (69%), whereas the standardized Sydney system was applied in 324 reports (33%). Gastric atrophy without intestinal metaplasia and gastric atrophy with intestinal metaplasia were detected in 322 (33%) patients. The full adherence to Sydney system significantly increased the probability of detecting gastric atrophy with intestinal metaplasia (OR =9.6; 95% CI: 5.5-16.7), gastric atrophy without intestinal metaplasia (OR =1.92; 95% CI: 1.07-3.44), and either of the conditions (OR =6.67; 95% CI: 4.36-10.19). Thus, according to these findings, in daily routine practice only one third of histology reports were worked out adhering to Sydney system showing that international guidelines are poorly observed in clinical practice (80). This may represent a critical element for surveillance strategies for gastric cancer.
Conclusions
Atrophic gastritis and intestinal metaplasia are premalignant changes, which, in a large part of patients fortunately will never progress to intestinal-type adenocarcinoma or type 1 gastric carcinoid. Many efforts have been done till now to obtain knowledge about these conditions and to optimize the management and surveillance of patients harbouring atrophic gastritis and/or intestinal metaplasia. Recently, it has been reported that nanoarray analysis is able to detect precancerous gastric lesions and gastric cancer through exhaled breath, and that possibly it could provide the missing non-invasive screening tool for gastric cancer and related precancerous lesions as well as for surveillance of the latter (81). While awaiting the validation of these or other innovative tools, important issues need to be better addressed, as the inclusion of patients with autoimmune gastritis and pernicious anemia amongst patients at higher risk for gastric cancer and carcinoids and the optimal time interval and cost-effectiveness of endoscopic-histological follow-up in patients with atrophic gastritis and intestinal metaplasia.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rugge M, Correa P, Dixon MF, et al. Gastric mucosal atrophy: interobserver consistency using new criteria for classification and grading. Aliment Pharmacol Ther 2002;16:1249-59. [PubMed]
- Kapadia CR. Gastric atrophy, metaplasia, and dysplasia: a clinical perspective. J Clin Gastroenterol 2003;36:S29-36; discussion S61-2.
- Genta RM. Review article: Gastric atrophy and atrophic gastritis--nebulous concepts in search of a definition. Aliment Pharmacol Ther 1998;12 Suppl 1:17-23. [PubMed]
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process – First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992;52:6735-40. [PubMed]
- Carneiro F, Machado JC, David L, et al. Current thoughts on the histopathogenesis of gastric cancer. Eur J Cancer Prev 2001;10:101-2. [PubMed]
- Ihamäki T, Saukkonen M, Siurala M. Long term observation of subjects with normal mucosa and with superficial gastritis: results of 23–27 years follow-up examination. Scand J Gastroenterol 1978;13:771-5. [PubMed]
- Ormiston MC, Gear MW, Codling BW. Five year follow-up study of gastritis. J Clin Pathol 1982;35:757-60. [PubMed]
- Meining A, Morgner A, Miehlke S, et al. Atrophy-metaplasia-dysplasia-carcinoma sequence in the stomach: a reality or merely an hypothesis? Best Pract Res Clin Gastroenterol 2001;15:983-98. [PubMed]
- Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001;345:784-9. [PubMed]
- Miehlke S, Hackelsberger A, Meining A, et al. Severe expression of corpus gastritis is characteristic in gastric cancer patients infected with Helicobacter pylori. Br J Cancer 1998;78:263-6. [PubMed]
- Filipe MI, Barbatis C, Sandey A, et al. Expression of intestinal mucin antigens in the gastric epithelium and its relationship with malignancy. Hum Pathol 1988;19:19-26. [PubMed]
- Silva S, Filipe MI, Pinho A. Variants of intestinal metaplasia in the evolution of chronic atrophic gastritis and gastric alcer. A follow up study. Gut 1990;31:1097-4. [PubMed]
- Reis CA, David L, Correa P, et al. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC and MUC6) expression. Cancer Res 1999;59:1003-7. [PubMed]
- Gutiérrez-González L, Wright NA. Biology of intestinal metaplasia in 2008: more than a simple phenotypic alteration. Dig Liver Dis 2008;40:510-22. [PubMed]
- Tsai YC, Hsiao WH, Lin SH, et al. Genomic single nucleotide polymorphisms in the offspring of gastric cancer patients predispose to spasmolytic polypeptide-expressing metaplasia after H. pylori infection. J Biomed Sci 2015;22:16. [PubMed]
- Correa P. Clinical implications of recent developments in gastric cancer pathology and epidemiology. Semin Oncol 1985;12:2-10. [PubMed]
- Genta RM, Rugge M. Gastric precancerous lesions: heading for an international consensus. Gut 1999;45 Suppl 1:I5-8. [PubMed]
- Guindi M, Riddell RH. The pathology of epithelial pre-malignancy of the gastrointestinal tract. Best Pract Res Clin Gastroenterol 2001;15:191-210. [PubMed]
- Riddell RH. Premalignant and early malignant lesions in the gastrointestinal tract: definitions, terminology and problems. Am J Gastroenterol 1996;91:864-72. [PubMed]
- Lauwers GY, Carneiro F, Graham DY, et al. Gastric carcinoma. In: Bosman FT, Carneiro F, Hruban RH, et al. editors. WHO Classification of tumours of the digestive system. 4: edn. Lyon: IARC Press, 2010:48-58.
- Delle Fave G, Kwekkeboom DJ, Van Cutsem E, et al. ENETS Consensus Guidelines for the management of patients with gastroduodenal neoplasms. Neuroendocrinology 2012;95:74-87. [PubMed]
- Dockray GJ, Varro A, Dimaline R, et al. The gastrins: their production and biological activities. Annu Rev Physiol 2001;63:119-39. [PubMed]
- Bordi C, D’Adda T, Azzoni C, et al. Hypergastrinemia and gastric enterochromaffin-like cells. Am J Surg Pathol 1995;19 Suppl 1:S8-19. [PubMed]
- Jacobson DL, Gange SJ, Rose NR, et al. Epidemiology and estimated population burden of selected autoimmune diseases in the United States. Clin Immunol Immunopathol 1997;84:223-43. [PubMed]
- Carmel R. Prevalence of undiagnosed pernicious anemia in the elderly. Arch Intern Med 1996;156:1097-100. [PubMed]
- De Block CE, De Leeuw IH, Van Gaal LF. Autoimmune gastritis in type 1 diabetes: a clinically oriented review. J Clin Endocrinol Metab 2008;93:363-71. [PubMed]
- Lahner E, Centanni M, Agnello G, et al. Occurrence and risk factors for autoimmune thyroid disease in patients with atrophic body gastritis. Am J Med 2008;121:136-41. [PubMed]
- Toh BH, Alderuccio F. Pernicious anemia. Autoimmunity 2004;37:357-61. [PubMed]
- Lahner E, Annibale B. Pernicious anemia: new insights from a gastroenterological point of view. World J Gastroenterol 2009;15:5121-8. [PubMed]
- Marignani M, Delle Fave G, Mecarocci S, et al. High prevalence of atrophic body gastritis in patients with unexplained microcytic and macrocytic anemia: a prospective screening study. Am J Gastroenterol 1999;94:766-72. [PubMed]
- Lahner E, Norman GL, Severi C, et al. Reassessment of intrinsic factor and parietal cell autoantibodies in atrophic gastritis with respect to cobalamin deficiency. Am J Gastroenterol 2009;104:2071-9. [PubMed]
- Hershko C, Ronson A, Souroujon M, et al. Variable hematologic presentation of autoimmune gastritis: age-related progression from iron deficiency to cobalamin depletion. Blood 2006;107:1673-9. [PubMed]
- Lahner E, Gentile G, Purchiaroni F, et al. Single nucleotide polymorphisms related to vitamin B12 serum levels in autoimmune gastritis patients with or without pernicious anaemia. Dig Liver Dis 2015;47:285-90. [PubMed]
- Adamu MA, Weck MN, Gao L, et al. Incidence of chronic atrophic gastritis: systematic review and meta-analysis of follow-up studies. Eur J Epidemiol 2010;25:439-48. [PubMed]
- Kuipers EJ, Lundell L, Klinkenberg-Knol EC, et al. Atrophic gastritis and Helicobacter pylori infection in patients with reflux esophagitis treated with omeprazole or fundoplication. N Engl J Med 1996;334:1018-22. [PubMed]
- Tepes B, Kavcic B, Zaletel LK, et al. Two- to four-year histological follow-up of gastric mucosa after Helicobacter pylori eradication. J Pathol 1999;188:24-9. [PubMed]
- Jönsson KA, Ström M, Bodemar G, et al. Histologic changes in the gastroduodenal mucosa after long-term medical treatment with cimetidine or parietal cell vagotomy in patients with juxtapyloric ulcer disease. Scand J Gastroenterol 1988;23:433-41. [PubMed]
- Sipponen P, Laxén F, Huotari K, et al. Prevalence of low vitamin B12 and high homocysteine in serum in an elderly male population: association with atrophic gastritis and Helicobacter pylori infection. Scand J Gastroenterol 2003;38:1209-16. [PubMed]
- Green TJ, Venn BJ, Skeaff CM, et al. Serum vitamin B12 concentrations and atrophic gastritis in older New Zealanders. Eur J Clin Nutr 2005;59:205-10. [PubMed]
- Weck MN, Stegmaier C, Rothenbacher D, et al. Epidemiology of chronic atrophic gastritis: population-based study among 9444 older adults from Germany. Aliment Pharmacol Ther 2007;26:879-87. [PubMed]
- Telaranta-Keerie A, Kara R, Paloheimo L, et al. Prevalence of undiagnosed advanced atrophic corpus gastritis in Finland: an observational study among 4,256 volunteers without specific complaints. Scand J Gastroenterol 2010;45:1036-41. [PubMed]
- Oksanen A, Sipponen P, Miettinen A, et al. Evaluation of blood tests to predict normal gastric mucosa. Scand J Gastroenterol 2000;35:791-5. [PubMed]
- Borch K, Jönsson KA, Petersson F, et al. Prevalence of gastroduodenitis and Helicobacter pylori infection in a general population sample: relations to symptomatology and life-style. Dig Dis Sci 2000;45:1322-9. [PubMed]
- Asaka M, Sugiyama T, Nobuta A, et al. Atrophic gastritis and intestinal metaplasia in Japan: results of a large multicenter study. Helicobacter 2001;6:294-9. [PubMed]
- Redéen S, Petersson F, Jönsson KA, et al. Relationship of gastroscopic features to histological findings in gastritis and Helicobacter pylori infection in a general population sample. Endoscopy 2003;35:946-50. [PubMed]
- Storskrubb T, Aro P, Ronkainen J, et al. Serum biomarkers provide an accurate method for diagnosis of atrophic gastritis in a general population: The Kalixanda study. Scand J Gastroenterol 2008;43:1448-55. [PubMed]
- Zou D, He J, Ma X, et al. Helicobacter pylori infection and gastritis: the Systematic Investigation of gastrointestinaL diseases in China (SILC). J Gastroenterol Hepatol 2011;26:908-15. [PubMed]
- Song H, Held M, Sandin S, et al. Increase in the Prevalence of Atrophic Gastritis Among Adults Age 35 to 44 Years Old in Northern Sweden Between 1990 and 2009. Clin Gastroenterol Hepatol 2015. [Epub ahead of print]. [PubMed]
- Ferlay J, Bray F, Pisani P, et al. GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide. IARC Cancer Base n.5 version 2.0. Lyon: IARC Press, 2004.
- Whiting JL, Sigurdsson A, Rowlands DC, et al. The long term results of endoscopic surveillance of premalignant gastric lesions. Gut 2002;50:378-81. [PubMed]
- Dinis-Ribeiro M, Lopes C, da Costa-Pereira A, et al. A follow up model for patients with atrophic chronic gastritis and intestinal metaplasia. J Clin Pathol 2004;57:177-82. [PubMed]
- Vannella L, Lahner E, Osborn J, et al. Risk factors for progression to gastric neoplastic lesions in patients with atrophic gastritis. Aliment Pharmacol Ther 2010;31:1042-50. [PubMed]
- Vannella L, Lahner E, Osborn J, et al. Systematic review: gastric cancer incidence in pernicious anaemia. Aliment Pharmacol Ther 2013;37:375-82. [PubMed]
- Annibale B, Azzoni C, Corleto VD, et al. Atrophic body gastritis patients with enterochromaffin-like cell dysplasia are at increased risk for the development of type I gastric carcinoid. Eur J Gastroenterol Hepatol 2001;13:1449-56. [PubMed]
- Kokkola A, Sjöblom SM, Haapiainen R, et al. The risk of gastric carcinoma and carcinoid tumours in patients with pernicious anaemia. A prospective follow-up study. Scand J Gastroenterol 1998;33:88-92. [PubMed]
- Sjöblom SM, Sipponen P, Miettinen M, et al. Gastroscopic screening for gastric carcinoids and carcinoma in pernicious anemia. Endoscopy 1988;20:52-6. [PubMed]
- Vannella L, Sbrozzi-Vanni A, Lahner E, et al. Development of type I gastric carcinoid in patients with chronic atrophic gastritis. Aliment Pharmacol Ther 2011;33:1361-9. [PubMed]
- Sjöblom SM, Sipponen P, Järvinen H. Gastroscopic follow up of pernicious anaemia patients. Gut 1993;34:28-32. [PubMed]
- Armbrecht U, Stockbrügger RW, Rode J, et al. Development of gastric dysplasia in pernicious anaemia: a clinical and endoscopic follow up study of 80 patients. Gut 1990;31:1105-9. [PubMed]
- Stockbrügger RW, Menon GG, Beilby JO, et al. Gastroscopic screening in 80 patients with pernicious anaemia. Gut 1983;24:1141-7. [PubMed]
- Dinis-Ribeiro M, Areia M, de Vries AC, et al. Management of precancerous conditions and lesions in the stomach (MAPS): guideline from the European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter Study Group (EHSG), European Society of Pathology (ESP), and the Sociedade Portuguesa de Endoscopia Digestiva (SPE). Endoscopy 2012;44:74-94. [PubMed]
- Merola E, Sbrozzi-Vanni A, Panzuto F, et al. Type I gastric carcinoids: a prospective study on endoscopic management and recurrence rate. Neuroendocrinology 2012;95:207-13. [PubMed]
- de Vries AC, Haringsma J, Kuipers EJ. The detection, surveillance and treatment of premalignant gastric lesions related to Helicobacter pylori infection. Helicobacter 2007;12:1-15. [PubMed]
- Lahner E, Esposito G, Pilozzi E, et al. Occurrence of gastric cancer and carcinoids in atrophic gastritis during prospective long-term follow up. Scand J Gastroenterol 2015;50:856-65. [PubMed]
- GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. International Agency for Research on Cancer, World Health Organization. Available online: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- Lahner E, Esposito G, Pilozzi E, et al. Gastric cancer in patients with type I gastric carcinoids. Gastric Cancer 2015;18:564-70. [PubMed]
- Waldum HL, Hauso Ø, Fossmark R. The regulation of gastric acid secretion - clinical perspectives. Acta Physiol (Oxf) 2014;210:239-56. [PubMed]
- Fossmark R, Qvigstad G, Waldum HL. Gastric cancer: animal studies on the risk of hypoacidity and hypergastrinemia. World J Gastroenterol 2008;14:1646-51. [PubMed]
- Rugge M, Capelle LG, Cappellesso R, et al. Precancerous lesions in the stomach: from biology to clinical patient management. Best Pract Res Clin Gastroenterol 2013;27:205-23. [PubMed]
- Walker MM, Talley NJ. Review article: bacteria and pathogenesis of disease in the upper gastrointestinal tract--beyond the era of Helicobacter pylori. Aliment Pharmacol Ther 2014;39:767-79. [PubMed]
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374-403. [PubMed]
- de Vries AC, van Grieken NC, Looman CW, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology 2008;134:945-52. [PubMed]
- González CA, Pardo ML, Liso JM, et al. Gastric cancer occurrence in preneoplastic lesions: a long-term follow-up in a high-risk area in Spain. Int J Cancer 2010;127:2654-60. [PubMed]
- den Hoed CM, Holster IL, Capelle LG, et al. Follow-up of premalignant lesions in patients at risk for progression to gastric cancer. Endoscopy 2013;45:249-56. [PubMed]
- El-Zimaity HM. Gastric atrophy, diagnosing and staging. World J Gastroenterol 2006;12:5757-62. [PubMed]
- Leung WK, Lin SR, Ching JY, et al. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut 2004;53:1244-9. [PubMed]
- Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. The American journal of surgical pathology 1996;20:1161-81. [PubMed]
- Rugge M, Genta RM. Staging and grading of chronic gastritis. Human pathology 2005;36:228-33. [PubMed]
- Rugge M, Pennelli G, Pilozzi E, et al. Gastritis: the histology report. Dig Liver Dis 2011;43 Suppl 4:S373-84. [PubMed]
- Lahner E, Zullo A, Hassan C, et al. Detection of gastric precancerous conditions in daily clinical practice: a nationwide survey. Helicobacter 2014;19:417-24. [PubMed]
- Amal H, Leja M, Funka K, et al. Detection of precancerous gastric lesions and gastric cancer through exhaled breath. Gut 2015. [Epub ahead of print]. [PubMed]


