Can Milan criteria be expanded effectively for liver transplantation in patients with HCC?
In the study by Xu et al., a large retrospective analysis from the China Liver Transplant Registry compares post-transplant outcomes based on different levels of hepatocellular carcinoma (HCC) burden (1).
In a cohort of 6,012 patients, the standard HCC liver transplant Milan criteria are compared with a number of validated expanded criteria including the Valencia, University of California San Francisco, University Clinic of Navarra and Hangzhou criteria (Table 1).
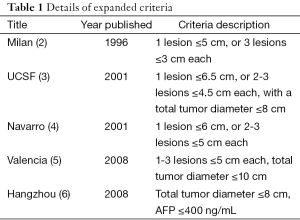
Full table
According to the authors, when compared to the Milan criteria the expanded criteria provided a significant expansion of the applicability of liver transplantation with the largest benefit corresponding to the Hangzhou criteria (51.5%).
When fulfilling but not exceeding the above mentioned different expanded criteria, long term disease free survival rates were comparable to those of the Milan criteria.
Since the Hangzhou criteria correlated with the broadest applicability of liver transplantation without detrimental impact in outcome, the authors focused the analysis further.
The 1-, 3-, 5- and 10-year disease free survival rates for the patients exceeding the Milan criteria, but fulfilling the Hangzhou criteria versus those exceeding the Hangzhou criteria were 81.6%, 64.3%, 56.5% and 37.2% vs. 58.2%, 35.1%, 28.2% and 16.3%, respectively (P<0.001).
Exceeding the Hangzhou criteria is pointed out by the authors as an independent risk factor for tumor recurrence in patients exceeding the Milan criteria. Moreover, univariate and multivariate analyses revealed AFP >100 ng/dL and tumor size >8 cm as independent risk factors for tumor recurrence in patients outside Milan criteria but within Hangzhou criteria.
The authors point out that in China about 40% of liver transplants are performed in HCC recipients, and if strictly adhered to the Milan criteria only about 43% of the cohort study would have the opportunity of transplantation. Regarding the subgroup of patients exceeding the Milan criteria, more than 60% did not experience tumor recurrence at 5 years post-transplant.
In sum, the authors conclude that the Milan criteria can be expanded without significant detriment in outcome, and advocate for the Hangzhou criteria to do so.
Once again as with others, this analysis puts transplant clinicians in the conundrum of rethinking the right balance of organ distribution policy for HCC patients in an era of persistent organ shortage (7). It is a point of agreement within a substantial portion of the transplant scientific community that the Milan criteria (single tumor ≤5 cm or 3 tumors each <3 cm) provide excellent oncological outcomes comparable to patients without HCC (2), but at the same time can be too strict (8).
The dilemma continues to be twofold; the large discrepancy between organ demand and availability and lack of accurate predictors of tumor recurrence. Relaying solely on measurement of tumor size and number appears to be rudimentary as it leaves out of the predictive equation a big deal regarding tumor biology (9). Similarly, including AFP level as a predictor factor falls short as it is a poor specific biomarker (10).
Pre-transplant tumor biopsy has been proposed to identify predictive biomarkers, however due to tumor multifocality or heterogeneity and risk of tumor cell spread through the biopsy needle track, this approach can be impractical (11,12).
In an effort to overcome difficulty of unveiling tumor behavior, it has been proposed to evaluate tumor response and stability after locoregional therapy in patients outside Milan criteria before being selected for liver transplantation. This strategy appears to aid transplant selection in patients beyond the standard criteria (13).
In conclusion, since we do not have accurate predictors of tumor relapse, rather than to generalize transplant inclusion criteria based on specific expanded morphometric criteria, we should consider pre-transplant response to treatment as a better indicator to define transplant eligibility. Additionally, weighting on live donor liver transplantation for expanded criteria HCC patients could serve as an option to prevent unfair balance in the use of the cadaveric organ pool.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Xu X, Lu D, Ling Q, et al. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Gut 2015. [Epub ahead of print]. [PubMed]
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-9. [PubMed]
- Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001;33:1394-403. [PubMed]
- Herrero JI, Sangro B, Quiroga J, et al. Influence of tumor characteristics on the outcome of liver transplantation among patients with liver cirrhosis and hepatocellular carcinoma. Liver Transpl 2001;7:631-6. [PubMed]
- Silva M, Moya A, Berenguer M, et al. Expanded criteria for liver transplantation in patients with cirrhosis and hepatocellular carcinoma. Liver Transpl 2008;14:1449-60. [PubMed]
- Zheng SS, Xu X, Wu J, et al. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation 2008;85:1726-32. [PubMed]
- Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2013 Annual Data Report: liver. Am J Transplant 2015;15 Suppl 2:1-28. [PubMed]
- Prasad KR, Young RS, Burra P, et al. Summary of candidate selection and expanded criteria for liver transplantation for hepatocellular carcinoma: a review and consensus statement. Liver Transpl 2011;17 Suppl 2:S81-9. [PubMed]
- Agopian VG, Harlander-Locke M, Zarrinpar A, et al. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: analysis of 865 consecutive liver transplant recipients. J Am Coll Surg 2015;220:416-27. [PubMed]
- Waghray A, Murali AR, Menon KN. Hepatocellular carcinoma: From diagnosis to treatment. World J Hepatol 2015;7:1020-9. [PubMed]
- Silva MA, Hegab B, Hyde C, et al. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut 2008;57:1592-6. [PubMed]
- Lopez KT, Kuwada SK, Wong LL. Consequences of needle tract seeding of hepatocellular cancer after liver transplant. Clin Transplant 2013;27:E400-6. [PubMed]
- Roberts JP, Venook A, Kerlan R, et al. Hepatocellular carcinoma: Ablate and wait versus rapid transplantation. Liver Transpl 2010;16:925-9. [PubMed]


