Cells of origin and cancer stem cells in cholangiocarcinoma
Abstract
Many years of investigations and daily clinical practice suggest an alternative model of carcinogenesis where only a subset of cancer stem cells (CSCs) has the ability to proliferate extensively and form new tumours. Signalling pathways associated with oncogenesis, including the Notch, Sonic hedgehog and Wnt signalling play a major role in regulating stem cell self-renewal. Although the terms CSC and “cell-of-origin” have been used interchangeably, they are distinct concepts referring to cancer-initiating cells and cancer-propagating cells, respectively. The term “cell-of-origin” defines the normal cell that acquires the first cancer-promoting mutation(s); on the other side, the definition “cancer stem cell (CSC)” indicates the cellular subset within the tumour that uniquely sustains malignant growth. However, an unresolved question regarding liver cancers is which cell has to be considered as cell of origin. Recently, the study of the detailed immunohistochemical profile has revealed that a whole range of phenotypical traits of hepatocytes, cholangiocytes and progenitor cells can be seen in liver primitive tumors [hepatocarcinoma (HCC) and cholangiocarcinoma (CCA)] being consistent with an origin from the hepatic stem cell compartment within canals of Hering and the biliary tree stem cells located within peribiliary glands. In liver malignancies, a number of cell surface markers have proved useful for the isolation of subsets enriched for CSCs, including CD133 (Prominin-1), CD44, CD24, EpCAM, AFP, Thy-1 and ATP-binding cassette B5, as well as Hoechst33342 exclusion by the side population cells.
Key words
Cholangiocarcinoma; hepatic stem cell; peribiliary glands; classification
Introduction
Many years of investigations and daily clinical practice suggest an alternative model of carcinogenesis where only a subset of cancer stem cells (CSCs) has the ability to proliferate extensively and form new tumours (1-3). Signalling pathways associated with oncogenesis, including the Notch, Sonic hedgehog (Shh) and Wnt signalling play a major role in regulating stem cell self-renewal (3). The machinery for self-renewal is already activated in CSCs, thus fewer mutations may be required to maintain self-renewal than to activate it ectopically. Stem cells often persist for long periods of time, increasing the probability of mutations. CSCs tend to be more resistant to chemotherapeutics due to high levels of expression of multidrug resistance genes (1-3).
Primitive liver tumours are grossly classified, according to morphological and phenotypical criteria, in hepatocellular carcinoma (HCC) and cholangiocarcinoma (CCA), the former deriving from malignant transformation of cells belonging to the hepatocytic lineage, the latter to the biliary lineage (4). The increasing knowledge on cancer stem cell biology is paralleled, as far as the liver is concerned, by the new insights in liver parenchyma and biliary tree stem cell compartment organization brought from studies in adults as wells as by recent advances in developmental biology of the liver and biliary tree (5,6). The complexity of the organization of the liver stem cell compartments underlies the CCA clinicalpathological heterogeneity and well justifies the difficulties in clinical-pathological classification of primitive liver tumours (6,7). Another point of great consideration is the relationship between chronic liver damage and the development of primitive liver tumours. Indeed, the chronically injured liver and biliary tree could be considered a classic model of stem cell derived carcinogenesis where the activation and proliferation of the stem cell compartment in response to tissue injury represent the first step of the carcinogenetic process (5,8,9).
In this review, we have deal with: (I) clarif ying the nomenclature regarding cancer cell of origin and cancer stem cell; (II) describing the hepatic and biliary tree stem cell compartments underling the regenerative response under pathological conditions and involved in the carcinogenetic process; (III) discussing the relevance of the new insights in hepatic and biliary tree stem cells in the etiopathogenesis and clinical-pathological classification of CCA.
Cell of Origin of Cancer and cancer stem cell: definitions
The term “cell-of-origin” defines the normal cell that acquires the first cancer-promoting mutation(s) (10); on the other side, the definition “cancer stem cell (CSC)” indicates the cellular subset within the tumour that uniquely sustains malignant growth. Although the terms “cell-of-origin” and CSC have been used interchangeably, they are distinct concepts referring to cancerinitiating cells and cancer-propagating cells, respectively (Figure 1) (2,10). The cancer-initiating cell denotes the cell of origin. It is important to note that the cell of origin is not necessarily related to the cancer stem cell (CSC) and its phenotype may be substantially different (10).
Phenotypic and functional heterogeneity are hallmarks of cancers arising in several organs. Variability can occur between tumors arising in the same organ (intertumoral heterogeneity) but also occurs within individual tumors (intratumoral heterogeneity). Intratumoral heterogeneity is characterized by tumor cells, which have a range of functional properties and a diverse expression of markers. The CSC and clonal-evolution models can explain the intratumoral heterogeneity and intrinsic differences in tumour regenerating capacity (11).
Intertumoral heterogeneity leads to the classification of discrete tumor subtypes, which are typically characterized, by their molecular profile, their morphology and expression of specific markers. Intertumoral heterogeneity is linked to the cell of origin of the cancer and can be explained by two main mechanisms: (I) different genetic/epigenetic mutations occur within the same cell of origin resulting in different tumor subtypes or (II) different tumour subtypes arise from distinct cells within the tissue that serve as cells of origin (Figure 2). These two mechanisms can act together and/or extrinsic mechanisms may be involved in generating tumor heterogeneity (10,12).
The cancer cell of origin has great importance in tumor cell fate and pathology; the activation of the same genetic/ epigenetic mutation in different maturational phases within a cellular lineage of a given organ may have profound implication either in malignant potential either in cancer morphology and phenotype (intratumoural heterogeneity) (13). This is the case of a transgenic mouse model which showed how the mutation of Hras targeted to the hair follicle region (earlier cells within the lineage) highly predisposed mice to squamous carcinomas, whereas the targeting to interfollicular or suprabasal cells (more differentiated cells) resulted in papillomas with low malignant potential (14,15).
The identification of the cells of origin of cancers has as a prerequisite the characterization of the normal cellular hierarchy within a given tissue and the study of stem cells niches (5). The most primitive cells, stem cells, are candidates for targets of transformation because of their self-renewal and longevity, which would allow the sequential accumulation of genetic or epigenetic mutations required for oncogenesis. Nevertheless, any cell in the hierarchy with proliferative capacity could serve as a cell of origin in cancer (10).
The accurate comparison of lineage markers between normal and neoplastic cells can led to individuate the cell of origin in different tumor subtypes arising within a given organ even if tumor cells show phenotypic plasticity or dedifferentiate during neoplastic progression, then lineage markers and molecular signatures of tumor cells may not precisely reflect the true cell of origin in normal tissue.
Classification of CCA
CCA is the primary malignancy of the biliary tract (16). This cancer has been classified as either intrahepatic or extrahepatic, with the second-order bile ducts acting as the separation point (4).
Classically, extrahepatic CCA has been divided into perihilar and distal CCA. According to the American Joint Cancer Committee/Union for International Cancer Control (AJCC/ UICC), perihilar CCA is proximally separated from intrahepatic cholangiocarcinoma by the second-order bile ducts, and distally separated from distal extrahepatic cholangiocarcinoma by the insertion of the cystic duct into the extrahepatic biliary tree (17).
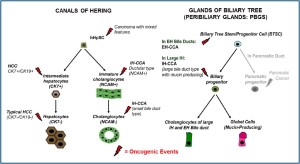
CCA arises from the lining epithelium and peribiliary glands (PBGs) of the intrahepatic (IH) and extrahepatic (EH) biliary tree (7). Grossly, IH-CCA could be classified into massforming, periductal-infiltrating, intraductal-growth type and a mixed type. IH-CCA can arise from biliary epithelia at any portion of the intrahepatic biliary system, from the segmental bile ducts (mostly lined by mucin-producing cylindrical cells) to the ductules (lined by cuboidal cholangiocytes without mucin production) and from glandular elements within the wall of larger bile ducts (PBGs) (16,18). Mucin-producing cells can give rise to mucin-producing tubular adenocarcinoma with or without micropapillary structures. On the other hand, ductularrelated intrahepatic CCA can present with mixed hepatocellular and/or cholangiocellular features, since ductules are composed of hepatic progenitor cells capable of differentiating to both hepatocytes and cholangiocytes (19,20).
Mucin-producing tubular adenocarcinoma are also observed in perihilar CCA and distal CCA, as their tumor locations are lined with a similar phenotype of cholangiocytes and contain PBGs (16).
Recently, a new classification for the IH-CCA has been proposed based on the recent studies on the pathological similarities between pancreatic and biliar y neoplasms (7,21). IH-CCA has been classified histologically into four categories: a conventional (bile duct) type, a bile ductular type (cholangiolocellular carcinoma: CLC), an intraductal neoplasm type and rare variants. Conventional IH-CCA is further classified into small and large bile duct types. The former is characterized by a mass forming tumor grossly with or without involvement of the small bile ducts and the latter by evident cancerous large bile duct(s) showing frequently involvement of PBGs and their conduits. Bile ductular IH-CCA is proposed based on morphological similarities to proliferating and reactive bile ductules with features of hepatic stem/progenitor cells (7,22).
Liver stem cell niches
In normal circumstances, liver cells hardly proliferate (6,20,23). Hepatocytes in normal adult liver have a lifespan of over a year. However, after partial hepatectomy, proliferation of the main epithelial compartments (hepatocytes and cholangiocytes) quickly restores the liver parenchyma (24,25). Serial transplantation experiments have shown that hepatocytes have a near infinite capacity to proliferate (26).
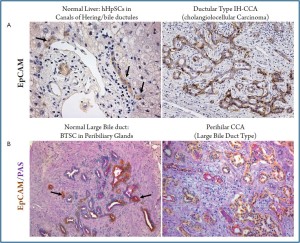
Recently, it has been shown that hepatocytes are senescent owing to telomere shortening in the cirrhotic stage of a wide variety of chronic human liver diseases (27,28). Probably this hepatocyte replicative senescence is in part the result of ongoing proliferation during 20-30 years of chronic liver disease (20). Chronic inflammation, presence of growth factors, DNAdamaging agents (such as reactive oxygen species and nitrogen species) could also play a role. Replicative senescence of hepatocytes triggers resident stem/progenitor cell activation (29). A hepatic stem cell niche has been firstly identified within the adult liver in the canals of Hering (6,23,30,31). Human Hepatic Stem Cells (hHpSCs) can differentiate into hepatocytes and cholangiocytes and have a role in the turnover of liver parenchymal cells both in normal and in pathological conditions (6,23,29,30,32,33). Actually, the role of hHpSC in normal turnover of hepatocyte and cholangiocyte is debated (34,35); by the contrast, numerous evidences indicated the activation of resident stem cell compartment in the majority of acute and chronic liver diseases. In chronic diseases, hHpSC highly proliferate and give rise to newly derived EpCAM positive hepatocytes in correlation with hepatocyte senescence (29,36).
More recently, an additional stem cell niche has been identified in the glands of biliary tree (peribiliary glands: PBGs) (37,38). PBGs are distributed along the biliary tree starting intrahepatically from the septal/segmental bile ducts and ending into the hepatopancreatic common duct near the duodenum (38). PBGs are particularly high in density at the level of cystic duct, hilum and periampular region, sites where CCAs typically emerge (39). The intrahepatic PBGs are indistinguishable from the extrahepatic ones. Within PBGs, stem/progenitor cell niche has been recently described which is composed by multipotent stem/progenitor cells of endodermal origin (Biliary Tree Stem/ progenitor Cells: BTSCs) and probably supplies the renewal of the surface epithelium of large intrahepatic bile duct and extrahepatic biliary tree (37,38). The stem/progenitor-like cell compartment within PBGs resembles the organization of the stem cell niche within intestines: the stem/progenitor-like cells and transit amplifying cells are located at the bottom of the glands; the cells with an intermediate phenotype are positioned in the middle of the gland and fully differentiated cells are in continuum with the surface epithelium (38).
The existence of two different stem cell niches, the canals of Hering and the PBGs, involved in the cell turnover of intrahepatic and extrahepatic biliary tree in adult life and activated in pathologic conditions, has been recently put in relation with CCA clinical-pathological heterogeneity as well as with differences in risk factors between IH- and EH-CCA (7,40,41). In Table 1, a comparison of markers between hHpSC in Canals of Hering and BTSC in PBGs is furnished.
Cell of Origin of Cholangiocarcinoma
In adult life, the two major primitive liver cancers are HCC and CCA. In addition, mixed forms of HCC and CCA are described (4,16,20,42). An unresolved question regarding liver cancers is which cell–hepatocytes, cholangiocytes, hepatic stem/progenitor cells or all three–has to be considered as cell of origin. As previously mentioned, mature liver parenchymal cells (hepatocytes and cholangiocytes) have the requisite to be targets of transformation because of their self-renewal and longevity, which would allow the sequential accumulation of genetic or epigenetic mutations required for oncogenesis (24). Moreover, chronic injury of hepatocytes and cholangiocytes may determine an increased proliferation rate of these mature liver parenchymal cells allowing an higher frequency of genetic or epigenetic mutations (20,24,25). This aspect is also supported by epidemiological considerations: in humans, chronic viral hepatitis B and C, alcoholic and non-alcoholic steatohepatitis, metabolic diseases and mutagens like aflatoxins (toxic metabolites of the food mould Aspergillus sp.) are the most important risk factors for the development of HCC. Chronic inflammatory biliary diseases such as primary sclerosing cholangitis, hepatolithiasis (gall stones) and liver fluke infestation (Opisstorchis viverrini and Clonorchis sinensis) are known risk factors for the development of CCAs (16,40,41).
Recently, detailed studies on immunohistochemical profile have revealed that a whole range of phenotypical traits of hepatocytes, cholangiocytes and progenitor cells can be seen in liver primitive tumors (HCC and CCA), being consistent with a origin from the hepatic stem cell compartment within canals of Hering and the BTSC located within peribiliary glands (7,9,19,20,42). Since hHpSCs are activated in most chronic liver diseases that are known risk factors for the development of HCC as well as CCA, these cells are potential target cells for carcinogenesis (5,8). As regard HCC, a substantial number (ranging from 28 to 50%) of human HCCs express markers of progenitor/biliary cells like CK7, CK19, OV6 suggesting a origin from stem/progenitor cells within canals of Hering (20,43). Morphologically, these tumors consist of cells with a very immature phenotype and a range of cells with intermediate phenotypes between progenitor cells and hepatocytes. Especially, CK19 expression in HCC has been associated with a worse prognosis and higher rates of recurrence after surgical treatment (20,43). In HCC, the presence of bile duct thrombi, especially with small or undetectable primary lesion and/or no histopathological evidence of bile duct invasion, supports the hypothesis that HCC might derive from hHpSCs residing in the canals of Hering and, possibly, some primary lesions are formed firstly within the intrahepatic biliary tree (44).
As regard CCA (Figure 3), strong evidences suggest a progenitor cell origin of cholangiolocellular carcinoma (CLC or bile ductular type CCA) (19). In the paper by Komuta and colleagues (19), the clinical-pathological features of 30 CLCs and their relationship to hHpSCs have been investigated. More than 90% of the tumor was composed of CLC areas that showed small monotonous and/or anastomosing glands, strongly positive for CK7 and CK19 (19). These areas consisted of ductular reaction-like structures, which were clearly positive for the hHpSCs markers (CK7, CK19, and NCAM) and the ABC transporters. At the tumor boundary, all cases showed HCClike trabecular area and CCA areas: HCC-like trabecular area were characterized by a phenotype similar to an intermediate hepatocytes (canalicular CD10 expression and submembranous CK7 expression) while CCA areas were constituted by papillary and/or clear glandular formation. Gene expression analysis showed an upregulation of stem cell markers such as prominin-1 (CD133), c-kit, octamer-4 transcription factor (OCT-4), and leukemia inhibitory factor (LIF). Comparison of CLCs with CK19-positive HCCs indicated a high homology.
The new classification of CCA recently proposed by Nakanuma et al. (7) stressed the concept of CCA heterogeneity and the pathological similarities between biliary and pancreatic neoplasms. Recently, Bonner-Weir and associates (45) or Tayer and associates (46) have determined that the pancreatic duct glands (PDGs) are comprised of progenitors and are sites responding to injuries and diseases. PDGs are distinct gland-like mucinous compartments with a distinct molecular signature. In response to injury, PDGs undergo a mucinous gastrointestinal metaplasia and PDG may provide a link between mucinous metaplasia, neoplasia and represent a possible cell of origin of pancreatic cancer (46).
Similar to pancreatic duct glands, we recently demonstrated that glands in biliary tree are stem cell niches in human bile duct system. These stem cells niches participate to the cell turnover of entire bile ducts distal to the interlobular bile ducts (37,38) and could be considered as vulnerable sites to oncogenic transformation (10). Several observations suggest that PBGs could be involved with the insurgence of EH-CCA with mucinous aspects: (I) PBGs contain mucin-producing cells (38), (II) their location overlaps with the sites in which the mucinous CCA typically occurs (39) and (III) CCA have been definitively associated with factors (such as primary sclerosing cholangitis, liver fluke infection) which determinate the proliferation of PBGs (21,42).
In keeping, responding to the need of classifying IH-CCA in relation to the heterogeneity of the small versus large IH bile ducts, Nakanuma et al. proposed to consider separately a small duct type (peripheral type) and a large bile duct type (or perihilar type) (7). This heterogeneity could be a consequence of the presence of two different stem cell niches within the liver (canals of Hering/hHpSCs and peribiliary glands/BTSCs).
The small duct type IH-CCA is mainly described as a tubular adenocarcinoma while the large bile duct type involves the IH large bile ducts and present mucin producing elements (7). In accordance with phenotypical differences between interlobular and large bile ducts, Aishima et al. investigated 87 cases of IHCCA smaller than 5 cm in diameter (47). They considered a perihilar type, showing IH large bile duct involvement within the tumour, and a peripheral type contained preserved architecture of the portal triad. They demonstrated that the frequency of perineural invasion, lymph node metastasis, vascular invasion, intrahepatic metastasis and extrahepatic recurrence of IHCCA from large ducts were significantly higher than that of IHCCA from small ducts. The survival of patients with IH-CCA from large ducts was worse than that of patients with IH-CCA from small ducts (47). These clinical-pathological differences observed between small bile duct type CCA and large bile duct type CCA could recognize a different cell of origin, with the former arising from a cell in the lineage of hHpSC while the latter arising from a cell descendant of the BTSC lineage. Reflecting the different cell of origin, the large bile duct type (perihilar) have similarities with the EH-CCA; by the contrast, small bile duct type (peripheral) IH-CCA has features in common with ductular type cholangiolocellular carcinoma and with CK19+ HCC (16).
Accordingly, in the study by Roskams T. et al. (Roskams T, 2010, unpublished data), the 52% of CCA were pure mucin producing whereas the 48% showed mixed differentiation features including focal hepatocytic differentiation and CLC features. CCA with mixed features (mixed-CCAs) showed peripheral location, larger tumor size, less micro-vascular invasion, less lymph node involvement compared to pure mucin producing CCAs which showed perihilar location, smaller tumour size, more microvascular invasion and more lymph node involvement. Molecular profiling showed high homology between mixed-CCAs and CK19-positive HCCs (considered of HpSCs origin). The authors concluded that mixed-CCAs and CK19-positive HCCs have a molecular profile similar to the bile ductules (containing HpSCs) while mucin-producing CCAs have a similar profile to large IH and EH bile ducts (containing peribiliary glands).
| Hepatic Stem Cells (hHpSCs) in Canals of Hering | Biliary Tree Stem/Progenitor Cells (BTSC) in Peribiliary Glands |
| / | Nanog, OCT4 |
| / | CXCR4 |
| / | FoxA 1/2 |
| / | PDX1, NGN3 |
| AFP | AFP |
| Sox 9/17 | Sox 9/17 |
| Hes1 | Hes1 |
| Prox1 | Prox1 |
| HNF 4/6 | HNF6 |
| CK7/8/18/19 | CK7/8/18/19 |
| Prominin-1 (CD133) | Prominin-1 (CD133) |
| EpCAM | EpCAM |
| NCAM | NCAM |
| Thy-1 | Not assessed |
| Abbreviations: AFP, α-fetoprotein; CD133, prominin; CK, cytokeratin; CXCR4, CXC-chemokine receptor 4; EpCAM, epithelial cell adhesion molecule; FOXa2, forkhead box a2; HES1, a member of the basic helix-loop-helix family of transcription factors and a transcriptional repressor of genes requiring an HLH protein for their transcription; HNF, hepatocyte nuclear factor; NCAM, neural cell adhesion molecule; NGN3, neurogenin 3; PDX1, Pancreatic and duodenal homeobox 1; PROX1, Prospero homeobox protein 1; SOX, Sry-related HMG box; S. | |
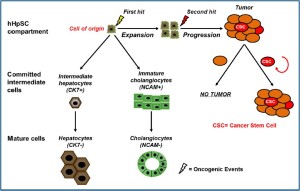
Cancer Stem Cell in Cholangiocarcinoma
The presence of stem/progenitor cell features in a tumor can be explained in two ways: either the cell of origin is a stem/ progenitor cell (maturation arrest theory) or, alternatively, tumors dedifferentiate and acquire stem/progenitor cell features during carcinogenesis (dedifferentiation theory). Two primary approaches have been used to tackle this question: (I) transgenic or conditionally targeted gene technologies to explore the effects of oncogenes and tumor suppressors in different cellular contexts; and, (II) genetic alteration of cells ex vivo before evaluating their tumorigenic capacity in mice (10).
In the paper by Chiba T et al. (48), Hepatic Stem/Progenitor Cells has been purified from fetal mice by FACS sorting of c-Kit-/CD29+/CD49f+/CD45-/Ter-119- cells. Successively, on purified hepatic stem/progenitor cell, gain-of-function and lossof- function analyses have been conducted to investigate the impact of the self-renewal signals, Bmi1 and the Wnt/β-catenin pathways. Signaling pathways that regulate the self-renewal of stem cells are important drivers of cell proliferation and survival and are frequently relevant to carcinogenesis when disrupted by mutations (1,49). Notably, both increased expression of the Bmi1 gene and activation of the Wnt/β-catenin pathway are frequently observed in liver cancers such as HCC (50,51). In keeping, the tumorigenicity of these transduced cells was assessed by transplantation into non-obese diabetic/severe combined immunodeficient mice (NOD/SCID mice). The transplantation of Bmi1- or β-catenin–transduced cells clonally expanded from single hepatic stem/progenitor cells produced tumors, which exhibited the histological features of combined HCC and CCA. These observations imply that the deregulated self-renewal of hepatic stem/progenitor cells serves as an early event in hepatocarcinogenesis and highlights the important roles of Bmi1 and the Wnt/β-catenin pathway in regulating the selfrenewal of normal or cancer stem cells in liver, further providing solid evidence of hepatocarcinogenesis that targets hepatic stem/ progenitor cells (48). Since α-fetoprotein (AFP) is one of the most common markers for hepatic progenitor cells in the fetal liver, Ishii et al. separated AFP+ cell from several human CCA cell lines (52); their single-cell culture analyses showed that only the AFP+ cell fraction had self-renewal ability and differentiation potency since it had the ability to generate in vitro and in vivo both the AFP+ cells and the AFP- cells. Moreover, the higher in vitro malignant potency of the AFP+ cells has been observed in the MTS assay, anchorage-independent growth assay, and sphere-forming assay. Xenotransplantation experiments showed that the AFP-producing cells had a markedly greater tumorigenicity than the AFP-non-producing cells did; these data indicated that the AFP-producing cells were cancer-initiating cells both in vitro and in vivo and showed similarities to lineagecommitted progenitor cells. Moreover, the Notch pathway was specifically expressed only in the AFP+ cell fraction, thus suggesting the Notch signaling pathway to be activated in the CCA cancer-initiating cells (52).
Recently, in the study by Wang et al., the molecular markers CD24, CD44, CD34, and EpCAM have been used to identify a subpopulation of cells in EH-CCA with cancer stem/progenitor cell-like properties (53). CD24+/CD44+/EpCAMhigh cells account for the 0.39–2.27% of cells in human EH-CCA tissues. These tumorigenic EH-CCA cells exhibited self-renewal properties and the ability to produce heterogeneous progeny. Moreover, this subpopulation could be duplicated during serial in vivo passaging in NOD/SCID mice and showed higher tumorigenic potential compared with CD24-/CD44-/EpCAMlow cells (53). EpCAM has been identified as a direct transcriptional target of Wnt/β-catenin signalling in HCC (54,55). A number of EpCAM-regulated target genes have been identified including c-myc and cyclins, and additional genes involved in cell growth and proliferation, cell cycle, and cell death (56). These findings indicated that expression of EpCAM is strongly linked with proliferation of stem cells and that cancer development from CSCs may occur after aberrant EpCAM re-expression (57).
Shi et al. investigated the role of CD133 (prominin-1) as surface marker for the CSC of gallbladder cancer (58,59). CD133+ cells possessed higher clonogenicity than their antigennegative counterparts. Subsequent in vivo tumorigenesis experiments demonstrated that CD133+ cells possessed higher tumorigenicity than the CD133- subpopulation. Furthermore, the tumors generated in nude mice displayed the same phenotype as the primary gallbladder tissue. Taken together, these results firmly suggest that CD133+ cells possess the potentials for self-renewal and high tumorigenicity, exhibiting cancer stem-cell-like characteristics in human gallbladder cancer representing a potential CSC marker in this tumor (59).
When specific cell surface markers are unknown, the Side Population fraction is a useful tool for cancer stem cell studies in solid tumors. The Side population (SP) phenotype due to the Hoechst33342 efflux pump present on the plasma membrane in diverse cell types, conferred by the ABC transporter ABCG2 (2). In many gastrointestinal cancers and HCC cell lines, SP fraction cells have been identified and characterized by their capacity for self-renewal and their high tumorgenicity (60). A recent paper suggested that Liver CSCs may have a distinct miRNA expression fingerprint during hepatocarcinogenesis (61); this study allowed to identify miRNAs whose deregulation was closely correlated with the malignant phenotype of liver cancer stem cells, as distinguished from normal hepatic stem cells and from oncogene and tumor suppressor gene mutations (61).
Concluding Remarks
In liver malignancies, a number of cell surface markers have been proved to be useful for the isolation of CSC enriched fractions, including CD133 (also known as Prominin-1), CD44, CD24, EpCAM, AFP, Thy-1 and ATP-binding cassette B5 (ABCB5), as well as Hoechst33342 exclusion by the side population cells (2).
However, an unresolved question regarding liver cancers is which cells have to be considered as cells of origin. The cancer cell of origin has great importance in tumor cell fate and pathology; the activation of the same genetic/epigenetic mutation in different maturational phases within a cellular lineage of a given organ may have profound implication in malignant potential (10).
On the basis of recent observations on glands of the biliary tree (peribiliary glands), we hypothesize that cells within peribiliary glands could be the cells of origin of large duct type IH-CCA as well as EH-CCA which are associated with typical chronic diseases or pathologic conditions of the large IH and of the EH bile ducts. These observations are supported by the similarities between the neoplastic pathologies of biliary tract and pancreas (7). Most biliary and pancreatic neoplasia are of ductal lineage, characterized by tubule (gland), papilla formation, or mucin production and expression of mucin-related glycoproteins. Some neoplastic diseases of the bile duct closely resemble those of the pancreas, clinicopathologically. Advanced CCA and preneoplastic or early intraepithelial neoplasms of the biliary tract show similar morphological or genetic features with respect to their pancreatic counterparts (21). These findings suggest that some biliary tract and pancreatic diseases develop via the same process and show similar morphology and phenotypes. Accordingly, pancreatic duct glands have been recently indicated as a possible cell of origin of pancreatic cancer.
On the other side, small bile duct type IH-CCA, cholangiolocellular carcinoma and CK19 positive HCC could arise from the cells deriving from hHpSC located in the finer branches of biliary tree; these tumor subtypes being associated with parenchymal liver diseases including, chronic viral and non-viral liver diseases, and especially liver cirrhosis.
Further studies are needed to corroborate the hypotheses, which could explain the large different epidemiologic profile of IH- and EH-CCA. Given the differences in biology and management (62), IH-CCA with mixed features (mixed-CCAs) and mucin producing IH-CCA should be considered separately (Figure 2). The molecular profile shows high homology between mixed-CCAs and CK19-positive HCCs (considered of hHpSCs origin) while mucin producing IH-CCAs have a similar profile with respect to hilar, extrahepatic CCA.
Acknowledgments
E. Gaudio was supported by research project grant from the University “Sapienza” of Rome and FIRB grant # RBAP10Z7FS_001 and by PRIN grant # 2009X84L84_001. D. Alvaro was supported by FIRB grant # RBAP10Z7FS_004 and by PRIN grant # 2009X84L84_002. The study was also supported by Consorzio Interuniversitario Trapianti d'Organo, Rome, Italy.
References
- Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature 2001;414:105-11.[LinkOut]
- Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer 2008;8:755-68.[LinkOut]
- Oishi N, Wang XW. Novel therapeutic strategies for targeting liver cancer stem cells. Int J Biol Sci 2011;7:517-35.[LinkOut]
- Nakanuma Y, Leong A, Sripa B, et al. Intrahepatic cholangiocarcinoma. In: Hamilton SR, Aaltonen LA, eds. Pathology and Genetics of Tumours of the Digestive System. World Health Organization Classification of Tumours. Lyon: IARC Press, 2000.
- Alison MR. Liver stem cells: implications for hepatocarcinogenesis. Stem Cell Rev 2005;1:253-60.[LinkOut]
- Turner R, Lozoya O, Wang Y, et al. Human hepatic stem cell and maturational liver lineage biology. Hepatology 2011;53:1035-45.[LinkOut]
- Nakanuma Y, Sato Y, Harada K, et al. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol 2010;2:419-27.[LinkOut]
- Alison MR. Liver cancer: a disease of stem cells? Panminerva Med 2006;48:165-74.[LinkOut]
- Alison MR, Islam S, Lim S. Stem cells in liver regeneration, fibrosis and cancer: the good, the bad and the ugly. J Pathol 2009;217:282-98.[LinkOut]
- Visvader JE. Cells of origin in cancer. Nature 2011;469:314-22.[LinkOut]
- Marusyk A, Polyak K. Tumor heterogeneity: causes and consequences. Biochim Biophys Acta 2010;1805:105-17.[LinkOut]
- Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol 2006;1:119-50.[LinkOut]
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997;3:730-7.[LinkOut]
- Bailleul B, Surani MA, White S, et al. Skin hyperkeratosis and papilloma formation in transgenic mice expressing a ras oncogene from a suprabasal keratin promoter. Cell 1990;62:697-708.[LinkOut]
- Brown K, Strathdee D, Bryson S, et al. The malignant capacity of skin tumours induced by expression of a mutant H-ras transgene depends on the cell type targeted. Curr Biol 1998;8:516-24.[LinkOut]
- Blechacz B, Komuta M, Roskams T, et al. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2011;8:512-22.[LinkOut]
- Farges O, Fuks D, Le Treut YP, et al. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: By the AFC-IHCC-2009 study group. Cancer 2011;117:2170-7.[LinkOut]
- Nakanuma Y, Hoso M, Sanzen T, et al. Microstructure and development of the normal and pathologic biliary tract in humans, including blood supply. Microsc Res Tech 1997;38:552-70.[LinkOut]
- Komuta M, Spee B, Vander Borght S, et al. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology 2008;47:1544-56.[LinkOut]
- Roskams T. Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene 2006;25:3818-22.[LinkOut]
- Nakanuma Y. A novel approach to biliary tract pathology based on similarities to pancreatic counterparts: is the biliary tract an incomplete pancreas? Pathol Int 2010;60:419-29.[LinkOut]
- Kozaka K, Sasaki M, Fujii T, et al. A subgroup of intrahepatic cholangiocarcinoma with an infiltrating replacement growth pattern and a resemblance to reactive proliferating bile ductules: ‘bile ductular carcinoma’. Histopathology 2007;51:390-400.[LinkOut]
- Roskams T, Katoonizadeh A, Komuta M. Hepatic progenitor cells: an update. Clin Liver Dis 2010;14:705-18.[LinkOut]
- Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology 2004;39:1477-87.[LinkOut]
- Michalopoulos GK, DeFrances MC. Liver regeneration. Science 1997;276:60-6.[LinkOut]
- Overturf K, al-Dhalimy M, Ou CN, et al. Serial transplantation reveals the stem-cell-like regenerative potential of adult mouse hepatocytes. Am J Patho 1997;151:1273-80.[LinkOut]
- Marshall A, Rushbrook S, Davies SE, et al. Relation between hepatocyte G1 arrest, impaired hepatic regeneration, and fibrosis in chronic hepatitis C virus infection. Gastroenterology 2005;128:33-42.[LinkOut]
- Wiemann SU, Satyanarayana A, Tsahuridu M, et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J 2002;16:935-42.[LinkOut]
- Yoon SM, Gerasimidou D, Kuwahara R, et al. Epithelial cell adhesion molecule (EpCAM) marks hepatocytes newly derived from stem/progenitor cells in humans. Hepatology 2011;53:964-73.[LinkOut]
- Gaudio E, Carpino G, Cardinale V, et al. New insights into liver stem cells. Dig Liver Dis 2009;41:455-62.[LinkOut]
- Kon J, Ichinohe N, Ooe H, et al. Thy1-positive cells have bipotential ability to differentiate into hepatocytes and biliary epithelial cells in galactosamine-induced rat liver regeneration. Am J Pathol 2009;175:2362- 71.[LinkOut]
- Libbrecht L, Roskams T. Hepatic progenitor cells in human liver diseases. Semin Cell Dev Biol 2002;13:389-96.[LinkOut]
- Spee B, Carpino G, Schotanus BA, et al. Characterisation of the liver progenitor cell niche in liver diseases: potential involvement of Wnt and Notch signalling. Gut 2010;59:247-57.[LinkOut]
- Carpentier R, Suñer RE, van Hul N, et al. Embryonic ductal plate cells give rise to cholangiocytes, periportal hepatocytes, and adult liver progenitor cells. Gastroenterology 2011;141:1432-8.[LinkOut]
- Furuyama K, Kawaguchi Y, Akiyama H, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 2011;43:34-41.[LinkOut]
- Heo J, Factor VM, Uren T, et al. Hepatic precursors derived from murine embryonic stem cells contribute to regeneration of injured liver. Hepatology 2006;44:1478-86.[LinkOut]
- Cardinale V, Wang Y, Carpino G, et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes and pancreatic islets. Hepatology 2011[Epub ahead of print].[LinkOut]
- Carpino G, Cardinale V, Onori P, et al. Biliary tree stem/progenitor cells in glands of extrahepatic and intraheptic bile ducts: an anatomical in situ study yielding evidence of maturational lineages. J Anat 2011;in press.
- Kimura W, Futakawa N, Zhao B. Neoplastic diseases of the papilla of Vater. J Hepatobiliary Pancreat Surg 2004;11:223-31.[LinkOut]
- Cardinale V, Semeraro R, Torrice A, et al. Intra-hepatic and extra-hepatic cholangiocarcinoma: New insight into epidemiology and risk factors. World J Gastrointest Oncol 2010;2:407-16.[LinkOut]
- Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology 2011;54:173-84.[LinkOut]
- Zhang F, Chen XP, Zhang W, et al. Combined hepatocellular cholangiocarcinoma originating from hepatic progenitor cells: immunohistochemical and double-fluorescence immunostaining evidence. Histopathology 2008;52:224-32.[LinkOut]
- Durnez A, Verslype C, Nevens F, et al. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology 2006;49:138- 51.[LinkOut]
- Peng N, Li L, Cai X, et al. Liver stem/progenitor cells in the canals of Hering: cellular origin of hepatocellular carcinoma with bile duct tumor thrombi? Stem Cell Rev 2010;6:579-84.[LinkOut]
- Li WC, Rukstalis JM, Nishimura W, et al. Activation of pancreatic-ductderived progenitor cells during pancreas regeneration in adult rats. J Cell Sci 2010;123:2792-802.[LinkOut]
- Strobel O, Rosow DE, Rakhlin EY, et al. Pancreatic duct glands are distinct ductal compartments that react to chronic injury and mediate Shh-induced metaplasia. Gastroenterology 2010;138:1166-77.[LinkOut]
- Aishima S, Fujita N, Mano Y, et al. Different roles of S100P overexpression in intrahepatic cholangiocarcinoma: carcinogenesis of perihilar type and aggressive behavior of peripheral type. Am J Surg Pathol 2011;35:590-8.[LinkOut]
- Chiba T, Zheng YW, Kita K, et al. Enhanced self-renewal capability in hepatic stem/progenitor cells drives cancer initiation. Gastroenterology 2007;133:937-50.[LinkOut]
- Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 2003;1653:1-24.[LinkOut]
- Neo SY, Leow CK, Vega VB, et al. Identification of discriminators of hepatoma by gene expression profiling using a minimal dataset approach. Hepatology 2004;39:944-53.[LinkOut]
- Taniguchi K, Roberts LR, Aderca IN, et al. Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene 2002;21:4863-71.[LinkOut]
- Ishii T, Yasuchika K, Suemori H, et al. Alpha-fetoprotein producing cells act as cancer progenitor cells in human cholangiocarcinoma. Cancer Lett 2010;294:25-34.[LinkOut]
- Wang M, Xiao J, Shen M, et al. Isolation and characterization of tumorigenic extrahepatic cholangiocarcinoma cells with stem cell-like properties. Int J Cancer 2011;128:72-81.[LinkOut]
- Terris B, Cavard C, Perret C. EpCAM, a new marker for cancer stem cells in hepatocellular carcinoma. J Hepatol 2010;52:280-1.[LinkOut]
- Yamashita T, Ji J, Budhu A, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology 2009;136:1012-24.[LinkOut]
- Maetzel D, Denzel S, Mack B, et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat Cell Biol 2009;11:162-71.[LinkOut]
- Carpenter G, Red Brewer M. EpCAM: another surface-to-nucleus missile. Cancer Cell 2009;15:165-6.[LinkOut]
- Shi C, Tian R, Wang M, et al. CD44+ CD133+ population exhibits cancer stem cell-like characteristics in human gallbladder carcinoma. Cancer Biol Ther 2010;10:1182-90.[LinkOut]
- Shi CJ, Gao J, Wang M, et al. CD133(+) gallbladder carcinoma cells exhibit self-renewal ability and tumorigenicity. World J Gastroenterol 2011;17:2965-71.[LinkOut]
- Haraguchi N, Utsunomiya T, Inoue H, et al. Characterization of a side population of cancer cells from human gastrointestinal system. Stem Cells 2006;24:506-13.[LinkOut]
- Li R, Qian N, Tao K, et al. MicroRNAs involved in neoplastic transformation of liver cancer stem cells. J Exp Clin Cancer Res 2010;29:169.[LinkOut]
- Fava G, Marzioni M, Benedetti A, et al. Molecular pathology of biliary tract cancers. Cancer Lett 2007;250:155-67.[LinkOut]


