Lynch syndrome in the gynecologic population
Introduction
Increased public awareness on cancer prevention has focused efforts on inherited cancer predispositions. Risk assessment and mitigation are integral components in this prevention strategy. Most inherited cancer syndromes cross multiple disciplines and require a collaborative approach to management. In the gynecologic cancer arena, hereditary breast and ovarian cancer (HBOC) and Lynch syndrome (LS) have garnered the most attention. Inherited disorders that are associated with both colorectal cancer and gynecologic malignancies include LS and less commonly, Peutz-Jeghers syndrome, Li-Fraumeni syndrome, and Cowden syndrome. Significantly, approximately half of women with LS will have an endometrial cancer as their sentinel malignancy with a median of 11 years prior to a colon cancer diagnosis (1). Therefore, an understanding of the associated cancers and recommended screening and management guidelines are critical to optimize patient care and ultimately outcomes. In this review, we will specifically focus on LS and its implications for the gynecologist.
Lynch syndrome (LS)
LS is named after Dr. Henry Lynch who expanded research on family “G” first identified by Dr. Aldred Warthin in 1913. This family had a cluster of inherited uterine and gastrointestinal malignancies, and in 2005, the cancer history of 929 descendants were available for review (2-4). LS is inherited in an autosomal dominant pattern and is highly penetrant. DNA mismatch repair genes are affected to include MLH1, MSH2, MSH6, and PMS2 (5). The population prevalence is estimated at 1:660 to 1:2,000 individuals (6). Neoplasms most commonly associated with LS include colorectal cancer, endometrial cancer, and ovarian cancer. Gastric cancer, small bowel cancer, hepatobiliary cancer, renal cancer, and ureteral cancer are also frequently encountered. Women with LS carry a 25-50% lifetime risk of colorectal cancer. The risks for endometrial cancer and ovarian cancer vary depending on the specific mismatch repair gene affected (see Table 1) (7).
LS is characterized by genomic instability affecting the entire genome, including both coding and noncoding regions. Mismatch repair defects lead to microsatellite instability from the insertion or deletion of additional nucleotides into nucleotide and dinucleotide repeats in noncoding regions. Microsatellite instability is the hallmark finding in Lynch-associated malignancies (5).
Endometrial cancer
Endometrial cancer is the most common gynecologic malignancy in the United States, affecting 1 in 37 women in their lifetime. It is also one of the few cancers whose incidence has been increasing. In 2015, an estimated 54,870 new cases will be diagnosed. Fortunately, most women present early with abnormal bleeding and are diagnosed with local disease. Still, 10,170 deaths are expected this year. The overall 5-year relative survival rate is 82%. Most women with endometrial cancer are diagnosed after age 55, and it is rare in women younger than age 45 (8).
The obesity epidemic is likely the major risk factor leading to rising new endometrial cancer cases. From 2007 to 2011, the incidence increased 2.4% per year (8). In women younger than 45 who have a BMI greater than 35, a 22-fold increased risk in endometrial cancer has been shown (9). Obesity leads to increased circulating estrogens from the peripheral conversion of androgens by aromatase in adipose cells. Other common risk factors include late menopause, nulliparity, and poly-cystic ovarian syndrome, all of which cause increased unopposed estrogen levels. Tamoxifen use for the prevention and treatment of breast cancer also increases risk. Estrogen directly and indirectly regulates gene transcription, promoting the growth of at-risk endometrial cells. Under normal conditions in the premenstrual phase, progesterone counters estrogen effects, leading to glandular differentiation and stromal decidualization. In states of prolonged progesterone deficiency and estrogen excess, the risk for endometrial hyperplasia and ultimately endometrial cancer is increased (10).
Approximately 3-5% of endometrial cancer cases are attributed to a hereditary cause with LS being the most common etiology (5). In women younger than 50, almost 10% are found to have LS (9). The average age of endometrial cancer patients with LS is 47-49 years. The most common histologic type of endometrial cancer is endometrioid adenocarcinoma. While some studies have suggested LS is associated with more aggressive subtypes, others have failed to show a significant difference (5).
Endometrial cancer typically presents with either abnormal uterine bleeding or postmenopausal bleeding. In most cases, this is an early finding, and the diagnosis can be easily obtained with office endometrial sampling. Over 70% of cases are diagnosed with stage I disease (9). Advanced disease may present with abdominal pain or bloating in addition to changes in bowel and/or bladder function.
The initial management of endometrial cancer includes comprehensive surgical staging with complete hysterectomy, removal of the fallopian tubes and ovaries, and pelvic and para-aortic lymphadenectomy. The extent of surgical staging has come under increased scrutiny in recent years, but exceptions should only be made in close consultation with providers specializing in the treatment of endometrial cancer. Patients with early stages at high risk for recurrence are usually treated with adjuvant radiation. Patients with advanced stages typically receive a combination of adjuvant chemotherapy and radiation following surgical resection (9).
Ovarian cancer
Ovarian cancer is the most lethal gynecologic cancer in the United States. It is estimated that there will be 21,290 new cases diagnosed in 2015, with 14,180 deaths (8). While the death rate and incidence have decreased slightly in recent years, there has been no significant improvement in early detection. Therefore, ovarian cancer remains an elusive malignant diagnosis with the majority of cases diagnosed as stage III or IV. The overall 5-year survival rate is 45%. Approximately 8-13% of ovarian cancers are hereditary, a number that will likely increase as we identify and test for more deleterious mutations in the genome. LS is the second most common hereditary cause of ovarian malignancy, after BRCA mutations. 5-10% of patients with LS will develop ovarian cancer by age 70 (5).
LS-related ovarian cancer is diagnosed at a younger age than sporadic cases, and is more likely to be early stage. Endometrioid and clear cell histologies are more common in the Lynch cases. In a histopathologic study of cases with nonserous ovarian cancer, 21% were determined to have loss of mismatch repair on immunohistochemistry, and in their cohort of Lynch-associated ovarian cancers, there were no serous or mucinous cancers (11). There is very limited information regarding survival comparing the sporadic and familial cases, but it appears there are no significant differences (5,12). Synchronous primary endometrial cancer is found in approximately 22% of LS-associated ovarian cancer (5). Conversely, about 7% of patients with synchronous primary endometrial and ovarian cancer at time of diagnosis are found to have LS (13).
Screening strategies for ovarian cancer have not proven to be successful or cost-effective. The current NCCN guidelines do not routinely recommend surveillance CA-125 or transvaginal ultrasound (TVUS) for patients with LS; however, they leave this to the discretion of the individual provider (14). Close monitoring for signs/symptoms associated with ovarian cancer (bloating, abdominal pain/discomfort, early satiety, urinary urgency/frequency) should be incorporated into regular gynecologic evaluations and immediate referral to gynecology or gynecologic oncology made as indicated by exam, radiologic, or lab evaluation (15).
Ovarian cancer is one of the few malignancies where routine primary cytoreductive surgery is performed on advanced stages due to the chemosensitive nature of this disease. The extent of cytoreduction directly correlates with subsequent survival with most gynecologic oncologists striving to remove all visible tumors (R0 resection). In cases where an optimal surgical resection is not expected, or if the patient has extensive medical comorbidities, neoadjuvant chemotherapy followed by interval debulking surgery is a reasonable option offering similar survival outcomes with a lower perioperative morbidity rate. All epithelial ovarian cancers except for the earliest stages are treated with adjuvant platinum-based chemotherapy regimens.
Screening for LS
Assessment of an individual’s genetic predisposition to a particular cancer is rapidly transforming. Today, multiplex testing for numerous cancer susceptibility genes is possible, and often out-paces our understanding of the subsequent test results. Given the complexity of the available tests and the difficulty in interpreting outcomes, referral of at-risk individuals to an experienced genetic counselor is warranted as a first step.
The best screening algorithm for LS remains unclear and is further dependent on local resources, clinical implementation rates, and variable penetrance of the syndrome. Guidelines for LS assessment have undergone several major revisions over the past 25 years (Table 2). The Amsterdam Criteria were modified in 1999 to include extracolonic malignancies. However, while remaining specific for LS, poor sensitivity was noted with only 13-36% of confirmed LS patients meeting criteria. The Bethesda Guidelines were developed to address the limitations in the Amsterdam Criteria in 1997 and were later revised in 2004. Unlike the Amsterdam Criteria, the Bethesda Guidelines have improved sensitivity, but lack specificity (5). Fortunately, genetic assessment for LS can be accomplished first through direct tumor testing rather than germline genetic testing. Immunohistochemical testing for the most common mismatch repair proteins is relatively inexpensive and readily available in pathology labs. Advances on this front have led to updated referral guidelines (7).
The Society of Gynecologic Oncology (SGO) recently published recommendations for genetic assessment in individuals with an increased likelihood of LS. These individuals include the following: patients with endometrial or colorectal cancer that have evidence of microsatellite instability or loss of DNA mismatch repair proteins on immunohistochemistry; patients with a first-degree relative affected with endometrial or colorectal cancer diagnosed before age 60 or those identified to be at risk for LS following a focused personal and medical history; and patients with a first- or second-degree relative with a known mismatch repair gene mutation (7). The SGO also noted situations that may warrant a lower testing threshold including families with few female relatives, hysterectomy and/or oophorectomy at a young age in multiple family members, and adoption. Testing in individuals younger than age 21 is not routinely recommended as management would only rarely be affected (7).
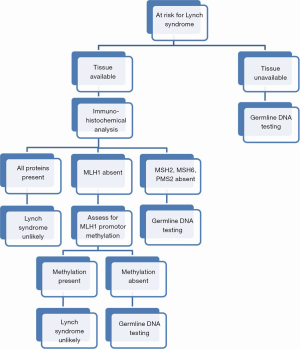
In cases where tumor tissue is available, immunohistochemical analysis for the expression of the four primary mismatch repair proteins can guide subsequent germline testing if absences are noted. In the case of absent MLH1, additional testing of the MLH1 promoter region is required as noninherited methylation can occur leading to microsatellite instability. MLH1 promoter methylation is estimated to occur in 20-30% of endometrial cancers (5). If all four mismatch repair proteins are expressed, while LS is unlikely, it may rarely still be present secondary to full production of a complete but nonfunctional protein. Therefore, in cases where LS is highly suspected, further evaluation for microsatellite instability may be warranted. Testing for microsatellite instability compares both normal and tumor tissue for insertion or deletion of nucleotides in microsatellites. If no instability is noted, then LS is excluded (see Figure 1) (5). When immunohistochemical analysis is used as a first screen, almost 20% of endometrial cancers will have abnormal findings (17). The data supporting immunohistochemical analysis for LS in ovarian cancer patients is less compelling than that for endometrial cancer. Most studies typically have small numbers, no central pathology review, and variable testing methodology. Nevertheless, accumulating evidence showing a high propensity for non-serous histologies in LS associated ovarian cancers may offer a morphologic basis for further genetic testing (18).
For at-risk patients identified through a focused review of their family history who have no tumor tissue available for immunohistochemical analysis, direct germline DNA testing can be performed. Even if a deleterious mutation is not found, LS may still be present; therefore, close coordination with a genetics expert is advisable.
The algorithm discussed above assumes universal tumor testing for LS. Although universal testing has been endorsed by many groups, this approach is not without challenges. When germline DNA testing is indicated based on tumor results, a causative mutation will not be identified in up to 15% of cases involving loss of MLH1 or PMS2 gene expression and in 40% of endometrial cancers with MSH2 or MSH6 deficiencies (5). Subsequent management in these patients and their family members remain nebulous. Universal screening may also be difficult in practice settings with limited resources. In this situation, clinical screening still remains a suitable option.
Management of high risk patients
Once the diagnosis of LS is confirmed or suspected, the gynecologist has an important role to play in screening the patient for the development of future cancers. Unfortunately, currently available screening strategies for endometrial and ovarian cancer have significant limitations primarily due to low prevalence rates and poor test specificity. For LS patients, the American College of Obstetrics and Gynecology (ACOG) recommends that women undergo a colonoscopy every 1-2 years beginning at the age of 20-25 years, or 2-5 years before the earliest diagnosis of cancer in their family, whichever is earlier. Endometrial sampling should be performed every 1-2 years starting at age 30-35 with patients instructed to keep a precise menstrual diary to evaluate for possible abnormal uterine bleeding (5). Patients who report abnormal uterine bleeding should undergo immediate endometrial biopsy. An estimated 5% of surveillance endometrial biopsies in LS patients will detect hyperplasia or carcinoma (19,20).
TVUS has also been used to screen LS patients; however, it has a low sensitivity for detecting endometrial cancer. In a study by Dove-Edwin et al., in 269 patients screened with ultrasounds, two endometrial cancers were encountered that were identified by symptoms only and not the ultrasound surveillance scans (21). Similarly, in a Finnish study published by Renkonen-Sinisalo et al., only 40% of confirmed endometrial cancers were suggested by TVUS (19).
Both TVUS and CA125 testing are commonly used to screen BRCA patients for ovarian cancer; however, its applicability to screening in LS is less clear. The histologic types and pathogenesis of ovarian cancer in LS differ from those seen in BRCA patients (22). Furthermore, in the largest LS screening study to date, no cases of ovarian cancer were identified through TVUS or CA125 testing (19).
Medical risk reducing options for endometrial and ovarian cancer are available for patients with LS. Progestin-containing contraception reduces the risk of endometrial cancer in the general population up to 50% with this risk reduction also likely to apply to patients with LS (23). While this study examined progestin containing OCPs, progestin-containing IUDs or subdermal implants are also reasonable options for these patients.
Risk-reducing surgery with hysterectomy and bilateral salpingo-oophorectomy is a very effective strategy to prevent LS associated gynecologic malignancies. Schmeler et al. found no occurrences of either endometrial or ovarian cancer following prophylactic surgery after 11 years of follow-up (24). The timing of prophylactic surgery is impacted by several factors to include age-based risk, future fertility desires, and menopausal implications. Prior to 40 years of age, endometrial cancer risk is approximately 2-4% and ovarian cancer risk is only 1-2%. Over the next decade, risk increases up to 17% for endometrial cancer and 7% for ovarian cancer. As such, women should be counselled on risk-reducing surgery by their mid-40s (5).
When looking at various mitigation strategies, quality of life and cost-effectiveness are critical to consider. Kwon et al. looked at five strategies ranging from no prevention to combined screening with prophylactic surgery. Their group found a combined approach with screening beginning at age 30 and prophylactic surgery by 40 years to have the highest net health benefit but at a cost of $194,650 per quality-adjusted life years (25). Ultimately, the mitigation strategy selected needs to be individualized.
Conclusions
Most gynecologists will encounter patients with LS during their careers. Since endometrial cancer is often the first malignancy identified in female LS patients, understanding the implications of this syndrome along with potential mitigation strategies is crucial to improving the health of not only the patient but also of their families. Patients identified with endometrial cancer or those found to have a concerning family history should be referred to a genetics expert if available. In confirmed LS patients, prophylactic surgery by age 40 is likely the best option today to prevent future gynecologic cancers.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Disclaimer: The views expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the United States Government.
References
- Lu KH, Dinh M, Kohlmann W, et al. Gynecologic cancer as a "sentinel cancer" for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet Gynecol 2005;105:569-74. [PubMed]
- Lynch HT, Krush AJ. Cancer family "G" revisited: 1895-1970. Cancer 1971;27:1505-11. [PubMed]
- Douglas JA, Gruber SB, Meister KA, et al. History and molecular genetics of Lynch syndrome in family G: a century later. JAMA 2005;294:2195-202. [PubMed]
- Warthin AS. Heredity with reference to carcinoma as shown by the study of the cases examined in the pathological laboratory of the University of Michigan, 1895-1913. Arch Intern Med 1913;12:546-55.
- Committee on Practice Bulletins-Gynecology, Society of Gynecologic Oncology. ACOG Practice Bulletin No. 147: Lynch syndrome. Obstet Gynecol 2014;124:1042-54. [PubMed]
- de la Chapelle A. The incidence of Lynch syndrome. Fam Cancer 2005;4:233-7. [PubMed]
- Lancaster JM, Powell CB, Chen LM, et al. Society of Gynecologic Oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecol Oncol 2015;136:3-7. [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [PubMed]
- Practice Bulletin No. 149: Endometrial cancer. Obstet Gynecol 2015;125:1006-26. [PubMed]
- Schmandt RE, Iglesias DA, Co NN, et al. Understanding obesity and endometrial cancer risk: opportunities for prevention. Am J Obstet Gynecol 2011;205:518-25. [PubMed]
- Chui MH, Ryan P, Radigan J, et al. The histomorphology of Lynch syndrome-associated ovarian carcinomas: toward a subtype-specific screening strategy. Am J Surg Pathol 2014;38:1173-81. [PubMed]
- Crijnen TE, Janssen-Heijnen ML, Gelderblom H, et al. Survival of patients with ovarian cancer due to a mismatch repair defect. Fam Cancer 2005;4:301-5. [PubMed]
- Soliman PT, Broaddus RR, Schmeler KM, et al. Women with synchronous primary cancers of the endometrium and ovary: do they have Lynch syndrome? J Clin Oncol 2005;23:9344-50. [PubMed]
- Provenzale D, Jasperson K, Ahnen DJ, et al. NCCN Clinical Practice Guidelines in Oncology, Genetic/Familial High-Risk Assessment: Colorectal, Version I.2015. Cited 4 May, 2015. Available online: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site
- Ovarian cancer symptoms consensus statement internet. Accessed May 3, 2015. Cited May 3, 2015. Available online: http://www.foundationforwomenscancer.org/about-the-foundation/allied-support-group/ovarian-cancer-symptoms-consensus-statement/
- Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of Lynch syndrome: a consensus statement by the US Multi-Society Task Force on colorectal cancer. Gastroenterology 2014;147:502-26. [PubMed]
- Frolova AI, Babb SA, Zantow E, et al. Impact of an immunohistochemistry-based universal screening protocol for Lynch syndrome in endometrial cancer on genetic counseling and testing. Gynecol Oncol 2015;137:7-13. [PubMed]
- Chui MH, Gilks CB, Cooper K, et al. Identifying Lynch syndrome in patients with ovarian carcinoma: the significance of tumor subtype. Adv Anat Pathol 2013;20:378-86. [PubMed]
- Renkonen-Sinisalo L, Bützow R, Leminen A, et al. Surveillance for endometrial cancer in hereditary nonpolyposis colorectal cancer syndrome. Int J Cancer 2007;120:821-4. [PubMed]
- Gerritzen LH, Hoogerbrugge N, Oei AL, et al. Improvement of endometrial biopsy over transvaginal ultrasound alone for endometrial surveillance in women with Lynch syndrome. Fam Cancer 2009;8:391-7. [PubMed]
- Dove-Edwin I, Boks D, Goff S, et al. The outcome of endometrial carcinoma surveillance by ultrasound scan in women at risk of hereditary nonpolyposis colorectal carcinoma and familial colorectal carcinoma. Cancer 2002;94:1708-12. [PubMed]
- Ketabi Z, Bartuma K, Bernstein I, et al. Ovarian cancer linked to Lynch syndrome typically presents as early-onset, non-serous epithelial tumors. Gynecol Oncol 2011;121:462-5. [PubMed]
- Weiss NS, Sayvetz TA. Incidence of endometrial cancer in relation to the use of oral contraceptives. N Engl J Med 1980;302:551-4. [PubMed]
- Schmeler KM, Lynch HT, Chen LM, et al. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N Engl J Med 2006;354:261-9. [PubMed]
- Kwon JS, Sun CC, Peterson SK, et al. Cost-effectiveness analysis of prevention strategies for gynecologic cancers in Lynch syndrome. Cancer 2008;113:326-35. [PubMed]