Revising robotic surgery for stomach, potential benefits revised II: prevention of pancreatic fistula
Introduction
Gastric cancer is the fourth most common malignant tumor and the second leading cause of cancer-related death worldwide (1). Surgical resection remains the only curative treatment option, and regional lymphadenectomy is recommended as part of radical gastrectomy (2). According to the Japanese Classification of Gastric Cancer, D2 gastrectomy is recommended for advanced gastric cancer (AGC) (3); however, D2 lymphadenectomy, especially when combined with splenectomy or pancreaticosplenectomy, has been reported to increase morbidity and mortality (4-7). In particular, postoperative pancreatic fistula (POPF) has been one of the major complications following radical gastrectomy. It sometimes induces lethal complications such as abdominal abscesses, secondary anastomotic leakage, and intra-abdominal hemorrhage (8,9).
Laparoscopic gastrectomy has been increasingly performed mainly for the early gastric cancer (EGC) as a minimally invasive surgical approach that provides significant advantages for short-term outcomes as opposed to open surgical procedures (10-13). We previously reported that laparoscopic approach improved short-term postoperative courses in comparison with open approach even in radical gastrectomy for AGC; however, there still was no significant reduction in postoperative complications, suggesting that reduction in complications by any means, might further improve postoperative courses following minimally invasive gastrectomy (14).
The da Vinci Surgical System (Intuitive, Sunnyvale, California, USA) has been developed to overcome some of the disadvantages of standard minimally invasive surgery (15,16). This robotic system facilitates precise dissection in a confined surgical field with impressive dexterity (15,16). Thus, it may be postulated that use of the robot in minimally invasive radical gastrectomy attenuate postoperative complications especially related to surgical manipulation, e.g., POPF (17).
This article provides the updates on POPF following radical gastrectomy for gastric cancer and discusses effectiveness of the use of the robotic system in reducing POPF based on our experience and review of the literature.
Definition, diagnosis, and incidence of POPF following radical gastrectomy
A general definition of pancreatic fistula is an abnormal communication between the pancreatic ductal epithelium and another epithelial surface containing pancreas-derived, enzyme-rich fluid (18). Although the diagnosis of POPF is suspected when the drain amylase level is at least three times as high as the upper normal limit of the serum amylase level on the postoperative day 3, it was comprehensively diagnosed according to not only drain amylase levels, but also changes in the properties of the drain and the clinical, laboratory, and imaging findings including computed tomographic scans (8,9,17,18).
There had been no universally recognized definition of POPF following gastrectomy for gastric cancer until recently (9). Accordingly, different definitions of POPF had been applied in each clinical study, resulting in highly variable rates of POPF ranging from 5.8% to 49.7% (9). To evaluate the incidence and severity of POPF more accurately, the International Study Group on Pancreatic Fistula (ISGPF) definition and Clavien-Dindo (CD) classification have increasingly been used of late (8,9,18-21).
The ISGPF definition, graded primarily on clinical impact, was developed by an international panel of pancreatic surgeons to formulate an acceptable and objective definition of POPF that decreases interobserver variability (18). This definition has been utilized to determine the incidence, severity and treatment outcomes of POPF following gastrectomy since Obama, et al. reported the feasibility of laparoscopic gastrectomy with radical lymphadenectomy for gastric cancer (9,22). POPF is graded according to the ISGPF criteria as follows: grade A, no clinical impact requiring little change in management or deviation from the normal clinical pathway; grade B, requiring a change in management or adjustment in the clinical pathway; grade C, requiring a major change in clinical management or deviation from the normal clinical pathway (18,22).
Clavien-Dindo classification was developed by Clavien and Dindo in 2004 with the aim of presenting an objective, simple, reliable, and reproducible way of reporting negative events after surgery (19,20,23). According to this classification, surgical complications are classified as follows based on the intensity of therapeutic interventions required to treat the complication: grade I, any deviation from the normal postoperative course without the need for pharmacological treatment or surgical, endoscopic, and radiological interventions; grade II, requiring pharmacological treatment; grade III, requiring surgical, endoscopic or radiological intervention; grade IV, life-threatening complication requiring IC/ICU management; grade V, death of a patient (19,20). The CD classification may be more advantageous than the ISGPF in terms of the fact that, using CD classification, not only POPF but also any other kind of postoperative complications could be quantitatively determined on the same scale, although the principle of these grading systems are quite similar.
Incidences of POPF following radical gastrectomy determined based on these grading systems are summarized in Table 1.
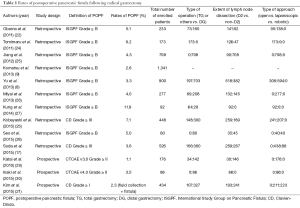
Full table
Therapeutic strategy for POPF following radical gastrectomy in correspondence with its severity
Patients with high drain amylase level and no abnormal physical and laboratory findings are observed without any treatment (ISGPF Grade A, CD Grade I) (17). The abdominal drainage tube is removed basically after the drain amylase level was sufficiently recovered. Patients with high drain amylase level accompanied by abnormal findings such as fever, abdominal pain and high inflammatory markers, are intensively treated with antibiotics, octreotide acetate and parenteral nutrition while the drainage tube position is urgently confirmed using computed tomographic scans and radiographic contrast study (ISGPF Grade B, CD Grade II) (17). When the drainage tube position is not appropriate, an additional or alternative drainage tube is placed into the fluid cavity using percutaneous computed tomography or ultrasonography-guided technique (ISGPF Grade C, CD Grade IIIa), and irrigation and drainage with saline is performed (17). Parenteral nutrition is gradually switched to enteral nutrition without delay, once pancreatic fistula is confined to a certain space and inflammatory response is settled. To be noted, patients requiring only repositioning but not replacement of their drainage tubes are classified as ISGPF Grade B or CD Grade II (9,31). If these series of conservative treatments were not effective, open drainage and debridement for POPF abscess by laparotomy would be performed and the irrigation type drainage tube and an enteral feeding tube would be placed (ISGPF Grade C, CD Grade ≥ IIIb) (9).
Etiology and prevention of POPF following radical gastrectomy: use of the robot?
Causes and risk factors
The incidence of POPF has reportedly been associated with greater extent of resection and lymph node dissection, i.e. total gastrectomy, splenectomy, pancreaticosplenectomy, and D2 lymphadenectomy, suggesting that surgical manipulation of the suprapancreatic area and splenic hilum with excessive retraction of pancreatic body may cause pancreatic injury leading to POPF (8,9,17,27). Open gastrectomy, age, male gender, and obesity were also reported as significant risk factors in relation to POPF, however, the cause-effect relationship between these factors and POPF has been unclear (8,25,32).
Prevention
It is needless to say that excessive resection and lymph node dissection should be avoided to prevent POPF. Particularly, the practical importance of station ten lymph node dissection and splenectomy in D2 total gastrectomy has been controversial (5-7,10). At present, according to the latest Japanese gastric cancer treatment guidelines 2014 (ver. 4), complete clearance of station 10 nodes by splenectomy should still be considered for potentially curable T2-T4 tumors invading the greater curvature of the upper stomach (3). However, in patients with T2-4/N0-2/M0 gastric cancer not invading the greater curvature, the Japan Clinical Oncology Group (JCOG) 0110 trial demonstrated that prophylactic splenectomy should be avoided to improve operative safety and survival (2,33). In addition to this, no one would argue against the possibility that combination of pancreas-protective operative maneuver and the use of surgical devices which may attenuate tissue damage and make the surgical procedures easier might lower the risk of POPF. The following strategies have been tested so far.
Evolution of surgical energy devices
In open and conventional laparoscopic gastrectomy, ultrasonically activated scalpel and/or vessel sealing system have been used over a decade. The tip temperature and the degree of lateral thermal spread of these devices were lower than those of monopolar diathermy (34); however, the incidence of POPF has still been reported as 1.7-22.1% (laparoscopic gastrectomy for EGC, 1.7-7%; open total gastrectomy, 13.0-22.1%) (8). So far there has been no report that clearly determined the effectiveness of these devices in reducing POPF.
The outermost layer-oriented medial approach
To improve the safety, efficacy, and reproducibility of suprapancreatic nodal dissection, we developed our original methodology called outermost layer-oriented medial approach (35,36). In this approach, the layer between the autonomic nerve sheaths of the major arteries and the adipose tissue bearing lymphatic tissue is dissected (35,36). Although the chance of intraoperative pancreatic injury could undoubtedly decrease just by keeping the appropriate layer while performing suprapancreatic dissection, POPF occurred in as high as 4.3% of the patients who underwent conventional laparoscopic radical gastrectomy at our institute (17). This might be at least partly because retraction of pancreas, which could potentially traumatize pancreas, was required to create sufficient operative field for conventional laparoscopic approach (8,9,17,22).
Use of the robot
According to our previous retrospective cohort study, the use of the robot reduced surgery-related complications including POPF, leading to further improvement in short-term postoperative courses following minimally invasive radical gastrectomy (17). Moreover, the greater the extent of gastric resection and lymphadenectomy, the more effective the use of the robot to reduce postoperative complications and to improve short-term outcomes, suggesting that the best indication for the use of the robot should be radical gastrectomy for AGC accompanied by D2 dissection (17). Strikingly, no POPF took place in the robotic group (17). This might be brought about not only because of the integrity of the robot-specific functions including articulating forceps, natural three-dimensional magnified view with high definition, tremor filtering, and motion scaling, which enables us to conduct suprapancreatic lymph node dissection with little touch on the pancreas, but also because of the outermost layer-oriented medial approach to the suprapancreatic area, and our original setup using da Vinci’s plane and the monitor-quadrisection theories (4,17,35,36). In addition, the “double bipolar” method characterized by simultaneous use of Maryland bipolar forceps (bipolar forced coagulation, 420172, Intuitive) with the right hand and Fenestrated bipolar forceps (bipolar soft coagulation, 420205, Intuitive) with the left hand might also facilitate pancreas-protective dissection in robotic gastrectomy (15,17,35). Actually, heat production in bipolar devices was demonstrated to be lower than ultrasonic cutting devices (37).
Current status and future perspectives on the role of the robot in radical gastrectomy for gastric cancer
According to the latest meta-analysis and multi-institutional RCT on the short-term outcomes of robotic vs. conventional laparoscopic gastrectomy, use of the robot significantly increased operative time and cost, whereas there were no significant differences in other short-term outcomes including POPF (21,38). Contribution of robotic gastrectomy to long-term outcomes has yet to be demonstrated (17,21). These results suggested that use of the robot might even deteriorate the cost-effectiveness (38). In other words, the greatest issue around robotic surgery is that clear benefits of the robotic system which justify the longer operative time and higher cost have never been clarified (21). However, apart from our aforementioned previous study in which 43% of the robotic group had pStage II or III diseases (17), most of the patients enrolled in these previous studies had pStage I diseases (21,38). Moreover, Harmonic Scalpel (420275, Intuitive) or monopolar cautery but not the Fenestrated bipolar forceps was used as the principal energy device in these studies. Thus, multi-institutional prospective studies in which considerable number of patients with AGC are enrolled should be required to determine whether use of the robotic system for AGC, notably combined with the double bipolar method, truly attenuates POPF, possibly leading to improvement in long-term outcomes.
In reality, since the beginning of October, 2014, we have been conducting a multi-institutional single-arm prospective study, which Japanese Ministery of Health, Labor, and Welfare has recently approved for Advanced Medical Technology (“senshiniryo”) (Figure 1). This study was designed to determine the impact of the use of the robot, for minimally invasive radical gastrectomy to treat resectable gastric cancer, on short-term outcomes, mainly focusing on postoperative complications, as well as long-term outcomes and cost. The specific hypothesis of the present study was that the use of the robot in patients with cStage I or II diseases reduces the morbidity (CD ≥ III) of 6.4% in conventional laparoscopic gastrectomy down to 3.2%. All the patients will be registered in 2 years and followed up for 3 years, thus the expected study period should be 5 years in total. Interim analyses will be done once the initial 220 cases are registered.
Are there any solutions for longer operative time and higher cost in robotic gastrectomy? To shorten the operative time, not only reduction in time for docking, undocking, and exchanging forceps but also prevention of conflict of the robotic arms and forceps are supposed to be essential (17). Use of da Vinci Xi Surgical System may be of some help in this regard. To reduce the cost, competition between rival robots such as Telelap Alf-X (39) as well as efforts of the Intuitive Surgical Inc. to lower the price are desirable.
Conclusions
The use of the robot is assumed to provide a technically superior operative environment for minimally invasive surgery (21). The greatest advantage of the robotic procedure may be the potential that the use of the robot helps lots of surgeons perform technically demanding operations more easily in a less invasive manner (17). As long as POPF has still been an important issue on radical gastrectomy for gastric cancer, further investigation would be warranted to clarify the association between the use of the robot and reduction in POPF.
Acknowledgements
The authors thank Dr. Seiichiro Kanaya for his wonderful contribution to establishing our original method of robotic gastrectomy.
Footnote
Conflicts of Interest: K. Suda and I. Uyama are funded by Intuitive Surgical, Inc. in relation to the aforementioned “senshiniryo” study. M. Nakauchi, K. Inaba and Y. Ishida have no commercial association with or financial involvement that might pose a conflict of interest in connection with the submitted article.
References
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006;12:354-62. [PubMed]
- Sano T, Sasako M, Yamamoto S, et al. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy--Japan Clinical Oncology Group study 9501. J Clin Oncol 2004;22:2767-73. [PubMed]
- Japanese Gastric Cancer Association (Aug 25, 2014). JGCA Gastric Cancer Treatment Guidelines 2014 (ver. 4). Kanehara, Tokyo.
- Uyama I, Suda K, Satoh S. Laparoscopic surgery for advanced gastric cancer: current status and future perspectives. J Gastric Cancer 2013;13:19-25. [PubMed]
- Hartgrink HH, van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 2004;22:2069-77. [PubMed]
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [PubMed]
- Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 1999;79:1522-30. [PubMed]
- Yu HW. Risk factors of postoperative pancreatic fistula in curative gastric cancer surgery. J Gastric Cancer 2013;13:179-84. [PubMed]
- Komatsu S, Ichikawa D, Kashimoto K, et al. Risk factors to predict severe postoperative pancreatic fistula following gastrectomy for gastric cancer. World J Gastroenterol 2013;19:8696-702. [PubMed]
- NCCN Guidelines Version 2.2011 Gastric Cancer. Available online: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
- Bamboat ZM, Strong VE. Minimally invasive surgery for gastric cancer. J Surg Oncol 2013;107:271-6. [PubMed]
- Wei HB, Wei B, Qi CL, et al. Laparoscopic versus open gastrectomy with D2 lymph node dissection for gastric cancer: a meta-analysis. Surg Laparosc Endosc Percutan Tech 2011;21:383-90. [PubMed]
- Angst E, Hiatt JR, Gloor B, et al. Laparoscopic surgery for cancer: a systematic review and a way forward. J Am Coll Surg 2010;211:412-23. [PubMed]
- Shinohara T, Satoh S, Kanaya S, et al. Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc 2013;27:286-94. [PubMed]
- Suda K, Ishida Y, Kawamura Y, et al. Robot-assisted thoracoscopic lymphadenectomy along the left recurrent laryngeal nerve for esophageal squamous cell carcinoma in the prone position: technical report and short-term outcomes. World J Surg 2012;36:1608-16. [PubMed]
- Boone J, Schipper ME, Moojen WA, et al. Robot-assisted thoracoscopic oesophagectomy for cancer. Br J Surg 2009;96:878-86. [PubMed]
- Suda K, Man-I M, Ishida Y, et al. Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc 2015;29:673-85. [PubMed]
- Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005;138:8-13. [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [PubMed]
- Kim HI, Han SU, Yang HK, et al. Multicenter Prospective Comparative Study of Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma. Ann Surg 2015. [Epub ahead of print]. [PubMed]
- Obama K, Okabe H, Hosogi H, et al. Feasibility of laparoscopic gastrectomy with radical lymph node dissection for gastric cancer: from a viewpoint of pancreas-related complications. Surgery 2011;149:15-21. [PubMed]
- Kobayashi D, Iwata N, Tanaka C, et al. Factors related to occurrence and aggravation of pancreatic fistula after radical gastrectomy for gastric cancer. J Surg Oncol 2015;112:381-6. [PubMed]
- Tomimaru Y, Miyashiro I, Kishi K, et al. Is routine measurement of amylase concentration in drainage fluid necessary after total gastrectomy for gastric cancer? J Surg Oncol 2011;104:274-7. [PubMed]
- Jiang X, Hiki N, Nunobe S, et al. Postoperative pancreatic fistula and the risk factors of laparoscopy-assisted distal gastrectomy for early gastric cancer. Ann Surg Oncol 2012;19:115-21. [PubMed]
- Miyai H, Hara M, Hayakawa T, et al. Establishment of a simple predictive scoring system for pancreatic fistula after laparoscopy-assisted gastrectomy. Dig Endosc 2013;25:585-92. [PubMed]
- Kung CH, Lindblad M, Nilsson M, et al. Postoperative pancreatic fistula formation according to ISGPF criteria after D2 gastrectomy in Western patients. Gastric Cancer 2014;17:571-7. [PubMed]
- Seo HS, Shim JH, Jeon HM, et al. Postoperative pancreatic fistula after robot distal gastrectomy. J Surg Res 2015;194:361-6. [PubMed]
- Katai H, Sasako M, Fukuda H, et al. Safety and feasibility of laparoscopy-assisted distal gastrectomy with suprapancreatic nodal dissection for clinical stage I gastric cancer: a multicenter phase II trial (JCOG 0703). Gastric Cancer 2010;13:238-44. [PubMed]
- Inaki N, Etoh T, Ohyama T, et al. A Multi-institutional, Prospective, Phase II Feasibility Study of Laparoscopy-Assisted Distal Gastrectomy with D2 Lymph Node Dissection for Locally Advanced Gastric Cancer (JLSSG0901). World J Surg 2015;39:2734-41. [PubMed]
- Japan Clinical Oncology Group. Postoperative Complication Criteria according to Clavien-Dindo Classification ver. 2.0. Available online: http://www.jcog.jp/doctor/tool/Clavien_Dindo.html
- Tanaka K, Miyashiro I, Yano M, et al. Accumulation of excess visceral fat is a risk factor for pancreatic fistula formation after total gastrectomy. Ann Surg Oncol 2009;16:1520-5. [PubMed]
- Sano T, Sasako M, Mizusawa J, et al. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma (JCOG0110): Final survival analysis. J Clin Oncol 2015;33:abstr 103.
- Sutton PA, Awad S, Perkins AC, et al. Comparison of lateral thermal spread using monopolar and bipolar diathermy, the Harmonic Scalpel and the Ligasure. Br J Surg 2010;97:428-33. [PubMed]
- Uyama I, Kanaya S, Ishida Y, et al. Novel integrated robotic approach for suprapancreatic D2 nodal dissection for treating gastric cancer: technique and initial experience. World J Surg 2012;36:331-7. [PubMed]
- Kanaya S, Haruta S, Kawamura Y, et al. Video: laparoscopy distinctive technique for suprapancreatic lymph node dissection: medial approach for laparoscopic gastric cancer surgery. Surg Endosc 2011;25:3928-9. [PubMed]
- Seehofer D, Mogl M, Boas-Knoop S, et al. Safety and efficacy of new integrated bipolar and ultrasonic scissors compared to conventional laparoscopic 5-mm sealing and cutting instruments. Surg Endosc 2012;26:2541-9. [PubMed]
- Hyun MH, Lee CH, Kim HJ, et al. Systematic review and meta-analysis of robotic surgery compared with conventional laparoscopic and open resections for gastric carcinoma. Br J Surg 2013;100:1566-78. [PubMed]
- Gidaro S, Buscarini M, Ruiz E, et al. Telelap Alf-X: a novel telesurgical system for the 21st century. Surg Technol Int 2012;22:20-5. [PubMed]



