Prevalence and correlation of chronic atrophic gastritis, intestinal metaplasia and other precancerous lesions of stomach in Iran: a historical cohort study
Introduction
Gastric cancer (GC) is the second main cause of cancer deaths worldwide (1,2); however its incidence and mortality has been declined in industrialized countries in the last few years (3). GC post-operative 5-year survival is highly dependent on the time of detection which indicated the higher survivability in early GC stages compared with advanced GC (4,5). In a randomized controlled trail Helicobacter pylori (H. pylori) eradication therapy accompanied with dietary supplements has been recommended as an effective strategy to prevent GC raised from regression of gastric precancerous lesions including chronic atrophic gastritis (CAG), intestinal metaplasia (IM) and epithelial dysplasia (ED) (6). CAG is a histopathologic entity characterized by chronic inflammatory processes of gastric mucosa that finally results in loss of gastric glandular cells and reduction of gastric secretory function. In addition, atrophy is known as a precursor factor for GC that develops as a result of autoimmune gastritis and it may be due to H. pylori (4,7). Several GC cases reported to begins with non-atrophic gastritis which results in CAG, IM, ED and finally adenocarcinoma (8,9). It usually takes decades for this process to be completed and the role of gastric Helicobacter pylori infection (HPI) is known as an initiation factor (10-12). H. pylori induce CAG, IM and ED with a low risk to develop stomach cancer. As the atrophy develops, the H. pylori density in stomach mucosa may adversely decrease and finally disappear in the late stages of atrophy (8). In a systematic review by Adamu and colleagues, reported the association between HPI and CAG incidence. They revealed that the occurrence of CAG is very low in lack of HPI (13). On the other hand, there are some regions with a high rate of HPI where the incidence of precancerous lesions and GC are low (14,15). In the current study, we evaluated the correlations between CAG and other precancerous lesions such as IM and ED and the HPI in Iranian population.
Materials and methods
In this historical cohort study a total number of 1,098 subjects who underwent diagnostic upper gastrointestinal (GI) endoscopy due to different medical complaints from 2009 to 2013 in Milad hospital were respectively enrolled in our study. The pathological report files of the subjects with gastritis diagnoses, who provided informed consent, were evaluated. Standard upper GI video endoscopy examinations were carried out and assessed by endoscopy experts under sedation with midazolam, under monitoring and local pharyngeal mucosa anaesthesia with 10% lidocaein spray. Then all suspicious findings in the esophagus, stomach and duodenum were captured. A specially designed coding system to record different lesions in the pathology reports was used. One or two biopsies were taken from antrum and then flattened and placed by the muscularis mucosa side on small pieces of filter paper and were immersed immediately in neutral buffer formalin containers and labeled. A coding system was designed to register biopsies information including their numbers, sites and tissue sufficiency. All the process of biopsy preparation, fixation, labeling and recording was supervised by the pathologist in charge. The patients were kept under close observation in the recovery room according to the standard post endoscopy cautions. The biopsies were embedded in paraffin wax blocks then stained by the haematoxylin and eosin method and sectioned, all based on standard pathologic protocols. The prepared histological sections were observed by three pathologists who own special interest and experience in GI pathology. They were not aware of endoscopic and clinical presentations of study patients but were informed of the sites of biopsies. Around 10% of the samples were randomly rechecked by all pathologist to evaluate and minimize inter observer variation bias. Gastric biopsies were fixed in 10 % formalin and embedded in paraffin wax by routine methods. An expert pathologist evaluated the 5-micron thick sections under haematoxylin and eosin and Giemsa staining for histopathological analysis and the intensity of the colonizing H. pylori, respectively. The diagnosis of HPI was done by direct light microscopy observation of the organism with Giemsa staining. We used the worldwide accepted grading scales for histopathological features, and for formulation of diagnosis as a comprehensive standardized one according to the updated Sydney System (16). All statistical analyses were performed using IBM SPSS statistics 19 for windows. Categorical variables were analyzed using Chi-square test or Fisher’s exact test and binary logistic regression. The P values of less than 0.05 were considered statistically significant.
Results
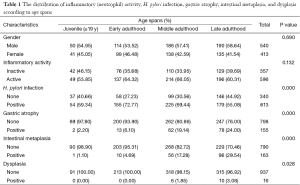
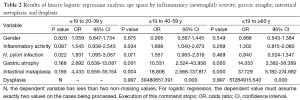
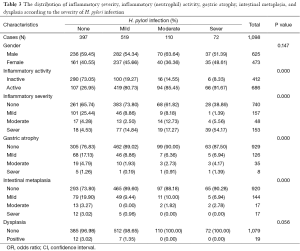
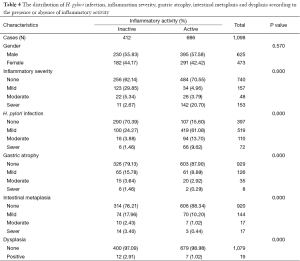
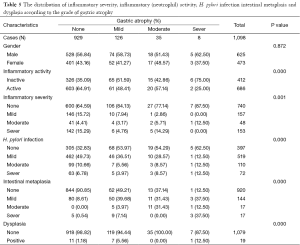
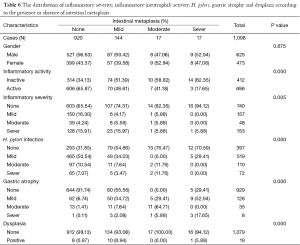
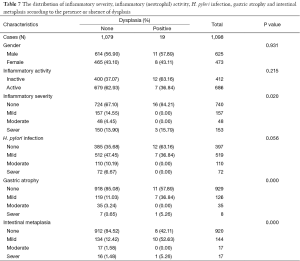
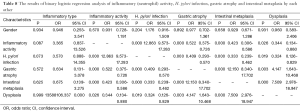
One thousand and ninety eight patients including: 625 (56.9%) men, 473 (43.0%) women were enrolled in this study. The youngest participant was 2 and the oldest 91 (mean age, 49.39; SD, 20.36) years old. The age of 953 cases were recorded and 145 ones’ were missing so we had to limit our population to 953 in order to find out how age might affect the other variables of our study. In the Table 1, the distribution of inflammatory activity (IA), HPI, CAG, IM and ED of stomach according to age is shown. In this table, the patients were classified into age groups that range 20 years; juvenile (≤19 y), early adulthood (20-39 y), middle adulthood (40-59 y) and late adulthood (≥60 y). The presence of all IA (53.58-64.32%), HPI (59.34-72.77%), CAG (2.20-6.10%) and IM (1.10-4.69%) increases between juvenile and early adulthood. Between adulthood age spans CAG (6.10 to 19.14 to 24.00), IM (4.69 to 17.28 to 29.54) and ED (0.00 to 1.85 to 3.08) increase steadily but no remarkable enhancement was seen in IA (64.32 to 66.05 to 60.31) HPI (72.77 to 69.44 to 55.08) between the mentioned age spans. By means of Chi-square analysis, prevalence of HPI, CAG, IM and ED revealed no significant (P=0.05) correlation by increase in age. Binary logistic regression analysis was employed to predict the probability of change in the rate of IA, HPI, CAG, IM and ED by means of an alteration in the age spans. Table 2 shows the results applying a 0.05 criterion of statistical significance. Alteration from juvenile to early adulthood just adds to the probability of enhancement in HPI by an odds ratio (OR) of 1.831 and confidence interval (CI) of 1.093 to 3.067. Similarly, alteration of age spans from early to middle adulthood significantly predicts an increase in the rates of IA (OR =1.668, 95% CI: 1.040-2.673), CAG (OR =10.531, 95% CI: 2.524-43.936) and IM (OR =18.806, 95% CI: 2.566-137.817). Finally, alteration from middle to late adulthood causes a probable increase in the rate of CAG (OR =14.053, 95% CI: 3.382-58.389) and IM (OR =37.729, 95% CI: 5.182-274.682). There were 701 subjects (63.84% of total cases) with HPI; among these patents, 579 subjects (82.60%) had simultaneous IA. In Table 3, the distribution of different grades of inflammatory severity (IS), IA, CAG, IM and ED of stomach according to severity of HPI is shown. According to Chi-square analysis results, there was a significant correlation between HPI severity and IS, IA, gastric atrophy (GA) and IM. For example IS was remarkably higher in the presence of sever HPI (54.17% of all 39 severely H. pylori infected cases had simultaneous sever inflammation comparing with 14.84%, 17.27% of all 77 mild and 19 moderate cases). The positivity of HPI was significantly higher in inflammatory active patients (84.40% of a total 686 subjects) compared with those without activity (29.61% of a total 412 subjects). The Table 4 shows the distribution of IS, HPI, GA, IM and ED according to the presence or absence of IA. There was a significant correlation between IA and all above variables (P<0.05). There were 169 subjects (15.39% of total cases) with CAG; 4.73% of them had severe CAG. The percentage of IA and HPI positivity in subjects with sever CAG (12.50% and 37.50%) was remarkably lower than that of the mild (48.41% and 46.03%) and moderate (57.14% and 45.71%). The presence of IM and ED was remarkably higher in CAG positive patients than CAG negatives (55.03% and 4.73% to 9.15% and 1.18%). In Table 5, the distribution of IS, IA, HPI, IM and ED of stomach according to the grade of CAG was shown. There was a significant correlation between CAG and all the other study variables (P<0.05). A notable increase in IM and ED was shown in association with more sever grade of CAG (Table 5). There were 178 patients (16.21% of total cases) with IM, 52.25% of them had simultaneous CAG. The Table 6 shows the distribution of IA, IS, HPI, CAG and ED according to the grading of IM. There’s a significant correlation between IM and IA, IS, HPI, CAG and ED (P<0.05). A total of 19 cases (1.73% of total cases) had ED. A total of 57.89% of them had simultaneous IM, 36.84% were HPI positive, and 42.11% had GAC. ED significantly correlates with IA (P=0.020), GAC (P=0.000) and IM (P=0.000) (Table 7). We conducted a binary logistic regression test on the IA, HPI, CAG, IM and ED everyone with each other. As presented in Table 8, IA significantly (P=0.05) adds to the odd of HPI (OR =12.863, 95% CI: 9.573-17.283) and inversely increase in HPI cause a probable enhancement of IA (OR =12.863, 95% CI: 9.573-17.283). Moreover, binary logistic regression analysis of coupled CAG-IM, CAG-ED and IM-ED showed that CAG adds to odds of IM (OR =12.150, 95% CI: 8.340-17.702) and ED (OR =4.147, 95% CI: 1.643-10.468) and similarly IM and ED add to the odd of CAG by an equivalent extent of OR. Finally, increase in IM leads to a probable enhancement of ED (OR =7.509, 95% CI: 2.976-18.947) and reversely ED adds to the odd of IM by the same extent of OR.
Full table
Full table
Full table
Full table
Full table
Full table
Full table
Full table
Discussion
More than half of the world’s population have H. pylori colonization in their stomachs that is known as a key factor in pathogenesis of some gastro duodenal diseases (17). In 1994, the International Agency for Research on Cancer classified H. pylori as a type I carcinogen agent that means a definite cause of cancer in human beings (18). In spite of recently emerging evidences that confirms decrease in the prevalence of H. pylori in all age groups, the image of its attributable diseases continues to change. GC development is considered as multistep progression which begins with a sequence of chronic gastritis development to GA, IM to dysplasia, and finally invasive cancer (19), this process could be triggered by chronic HPI. In our study analysis of HPI distribution by 20 years age groups Chi-square test revealed a significant correlation between increase in age and increase in HPI prevalence and intensity as demonstrated in Table 1, but the binary logistic regression test showed just alteration from juvenile to early adulthood may possibly add to rate of HPI (OR =1.831, CI: 1.093-3.067). Our results confirmed the results of other studies that indicated HPI increase with age in developing and developed countries (20,21). A recent study by Chen et al. which has similarity in method they used to ours, found that the incidence of HPI decreased with age (22). Eighty four point forty percent of our HPI positive patients had simultaneously IA and Chi-square test revealed a significant correlation between HPI and IA and the results of binary logistic regression test of HPI and IA confirmed this correlation (OR =12.863, 95% CI: 9.573-17.283). In line of our study, Parsonnet et al. reported that in a series of patients with gastroduodenal disorders, cases with H. pylori developed GC (23). Mizuno et al. revealed that the presence of HP infection and atrophic gastritis determines by serological evaluation has a predictive value in risk of individuals for GC (24). Our results are consistent with those of Chen et al. (22) and Tanko et al. (25) who demonstrated HPI may result in neutrophil activation and chronic gastritis. Our study showed a 45.56% prevalence of HPI in CAG positive patients which was not significantly low comparing with 21.01% that was found in the similar study by Chen et al. (22). In a study by Haziri et al., the very high percentage of HPI among patients with precancerous lesions (IM: 71.7% and ED: 71.4%) was determined (26). Since atrophy may reverse after effective eradication therapy, eradication therapy of H. pylori after detection of atrophy is recommended (5). Applying Chi-square test, CAG significantly correlated with HPI but by binary logistic regression test no remarkable OR (0.409) was found in comparison with Weck et al. study who reported OR of 2.9% (95% CI: 2.3-3.6) and with Fontham et al. (OR =6.4) (11,27). So in contrast with other studies, our results didn’t support the positive association between HPI and CAG, this maybe because of the hypothesis that attributes lower sensitivity of HPI detecting methods in presence of CAG to a low H. pylori density in the presence of atrophy (11,12) or as a result of HPI declining prevalence due to improvement in the standard of living in economically rapidly developing counties (28). In the current study, the presence of IM and ED was remarkably higher in CAG positive patients than CAG negatives (55.03% and 4.73% to 9.15% and 1.18%). According to the results of Chi-square test, prevalence and intensity of IM significantly correlates with IA, HPI, and CAG. Binary logistic regression test showed CAG significantly cause probable increase in prevalence of IM (OR =12.150, 95% CI: 8.340-17.702) and ED (OR =4.147, 95% CI: 1.643-10.468). These results match with those of demonstrated by Chen et al. (22) and Rugge et al. (29). Beside the very high Iranian’s rate of HPI comparing with other populations, age of infection seems to be very low (30). Few studies with different methods are fulfilled on CAG and other GC precancerous lesions in Iran, for instance CAG prevalence varies from 21.9% of cardiac and 39.3% of cardiac species from Ardebil (Northwest of Iran) by histologic method (31) to 53% in Babol (North of Iran) by the serologic pepsinogen I/II method (32). A cross sectional study by Malekzadeh et al. showed prevalence of 8.7% and 0.2% for IM and ED in Ardabil in Northwest of Iran (31). So as a conclusion of our carrier, HPI incidence in Iran that resembles a pattern of HPI distribution in the developing countries, increases in the first years of human life then its increase rate slows down in the early adulthood and turns to diminish in the rest of life. In contrast to HPI, CAG and IM steadily progress whole the life with an additive rate of increase in the adulthood. In this study unlike former studies which supported the evident role of HPI in the development of gastric precancerous lesions, our study showed HPI does not own a significant direct effect on the incidence of mentioned lesions but CAG alone, makes a considerable risk for occurrence of IM and ED.
Acknowledgements
This study was supported by AJA Cancer Research Center (ACRC), AJA University of Medical Sciences, Tehran, Iran.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ly QP, Sasson AR. Modern surgical considerations for gastric cancer. J Natl Compr Canc Netw 2008;6:885-94. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- DuVall MB, Caparosa RJ, Bailey HA Jr. Sensorineural hearing loss in the unoperated-on otosclerotic ear. Laryngoscope 1981;91:197-204. [PubMed]
- Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the "different disease" hypothesis. Cancer 2000;88:921-32.
- Park JM, Ryu WS, Kim JH, et al. Prognostic factors for advanced gastric cancer: stage-stratified analysis of patients who underwent curative resection. Cancer Res Treat 2006;38:13-8. [PubMed]
- Correa P, Fontham ET, Bravo JC, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst 2000;92:1881-8. [PubMed]
- Correa P. A human model of gastric carcinogenesis. Cancer Res 1988;48:3554-60. [PubMed]
- Beales IL, Crabtree JE, Scunes D, et al. Antibodies to CagA protein are associated with gastric atrophy in Helicobacter pylori infection. Eur J Gastroenterol Hepatol 1996;8:645-9. [PubMed]
- Asaka M, Sugiyama T, Nobuta A, et al. Atrophic gastritis and intestinal metaplasia in Japan: results of a large multicenter study. Helicobacter 2001;6:294-9. [PubMed]
- Chen XY, van Der Hulst RW, Shi Y, et al. Comparison of precancerous conditions: atrophy and intestinal metaplasia in Helicobacter pylori gastritis among Chinese and Dutch patients. J Clin Pathol 2001;54:367-70. [PubMed]
- Fontham ET, Ruiz B, Perez A, et al. Determinants of Helicobacter pylori infection and chronic gastritis. Am J Gastroenterol 1995;90:1094-101. [PubMed]
- Dursun M, Yilmaz S, Yükselen V, et al. Evaluation of optimal gastric mucosal biopsy site and number for identification of Helicobacter pylori, gastric atrophy and intestinal metaplasia. Hepatogastroenterology 2004;51:1732-5. [PubMed]
- Adamu MA, Weck MN, Gao L, et al. Incidence of chronic atrophic gastritis: systematic review and meta-analysis of follow-up studies. Eur J Epidemiol 2010;25:439-48. [PubMed]
- Kim N, Park YS, Cho SI, et al. Prevalence and risk factors of atrophic gastritis and intestinal metaplasia in a Korean population without significant gastroduodenal disease. Helicobacter 2008;13:245-55. [PubMed]
- Weck MN, Brenner H. Prevalence of chronic atrophic gastritis in different parts of the world. Cancer Epidemiol Biomarkers Prev 2006;15:1083-94. [PubMed]
- Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161-81. [PubMed]
- Kandulski A, Selgrad M, Malfertheiner P. Helicobacter pylori infection: a clinical overview. Dig Liver Dis 2008;40:619-26. [PubMed]
- Herrera V, Parsonnet J. Helicobacter pylori and gastric adenocarcinoma. Clin Microbiol Infect 2009;15:971-6. [PubMed]
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process--First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res 1992;52:6735-40. [PubMed]
- Bruden DL, Bruce MG, Miernyk KM, et al. Diagnostic accuracy of tests for Helicobacter pylori in an Alaska Native population. World J Gastroenterol 2011;17:4682-8. [PubMed]
- Veldhuyzen van Zanten SJ, Pollak PT, Best LM, et al. Increasing prevalence of Helicobacter pylori infection with age: continuous risk of infection in adults rather than cohort effect. J Infect Dis 1994;169:434-7. [PubMed]
- Chen S, Ying L, Kong M, et al. The Prevalence of Helicobacter pylori Infection Decreases with Older Age in Atrophic Gastritis. Gastroenterol Res Pract 2013;2013:494783.
- Parsonnet J, Samloff IM, Nelson LM, et al. Helicobacter pylori, pepsinogen, and risk for gastric adenocarcinoma. Cancer Epidemiol Biomarkers Prev 1993;2:461-6. [PubMed]
- Mizuno S, Miki I, Ishida T, et al. Prescreening of a high-risk group for gastric cancer by serologically determined Helicobacter pylori infection and atrophic gastritis. Dig Dis Sci 2010;55:3132-7. [PubMed]
- Tanko MN, Manasseh AN, Echejoh GO, et al. Relation between Helicobacter pylori, inflammatory (neutrophil) activity, chronic gastritis, gastric atrophy and intestinal metaplasia. Niger J Clin Pract 2008;11:270-4. [PubMed]
- Haziri A, Juniku-Shkololli A, Gashi Z, et al. Helicobacter pylori infection and precancerous lesions of the stomach. Med Arh 2010;64:248-9. [PubMed]
- Weck MN, Gao L, Brenner H. Helicobacter pylori infection and chronic atrophic gastritis: associations according to severity of disease. Epidemiology 2009;20:569-74. [PubMed]
- Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol 2009;24:1587-600. [PubMed]
- Rugge M, Farinati F, Baffa R, et al. Gastric epithelial dysplasia in the natural history of gastric cancer: a multicenter prospective follow-up study. Interdisciplinary Group on Gastric Epithelial Dysplasia. Gastroenterology 1994;107:1288-96. [PubMed]
- Malekzadeh R, Derakhshan MH, Malekzadeh Z. Gastric cancer in Iran: epidemiology and risk factors. Arch Iran Med 2009;12:576-83. [PubMed]
- Malekzadeh R, Sotoudeh M, Derakhshan MH, et al. Prevalence of gastric precancerous lesions in Ardabil, a high incidence province for gastric adenocarcinoma in the northwest of Iran. J Clin Pathol 2004;57:37-42. [PubMed]
- Ghadimi R, Taheri H, Suzuki S, et al. Host and environmental factors for gastric cancer in Babol, the Caspian Sea Coast, Iran. Eur J Cancer Prev 2007;16:192-5. [PubMed]


