Robotic gastrectomy for gastric cancer—an American perspective
Introduction
Robotic surgery is the most innovative surgical approach for the treatment of gastric cancer. Surgeon pioneers have judiciously embraced the robotic surgical technology, currently only available as the Da Vinci Surgical Systems (Intuitive Surgical, Sunnyvale, CA, USA), by critically evaluating its safe application, its’ potential advantages, and defining how robotic gastrectomy will fit into a comprehensive surgical strategy for the treatment of our gastric cancer patients (1). In the United States, the adoption of robotic surgery for gastric cancer, like many other complex abdominal operations, is in its nascent stages (2-7). The optimum role of robotic gastrectomy for gastric cancer is being critically defined based on the substantial body of literature mostly from South Korea and growing number of individual surgeon’s training and experiences.
The goals of robotic radical gastrectomy for our gastric cancer patients are the same as for open or laparoscopic approaches: to achieve long-term survival and to preserve quality of life after surgery. In the United States indications for the robotic approach for gastric cancer operations have included those patients who met a spectrum of the clinical criteria for curative resection (Figure 1) (2,3). The extent of surgical resections and the lymph node dissections has been consistent between the robotic approaches to the curative resection and the open operations, adhering to the oncologic principles of gastric cancer surgery (8-10). In South Korea and Japan, robotic gastrectomies were initially performed only on early stage lesions and expanded to advanced disease with growing robotic surgery expertise (11,12). However, robotic surgery experience for gastric cancer in the United States as well as Italy and China, has included mostly patients with locally advanced disease (13).
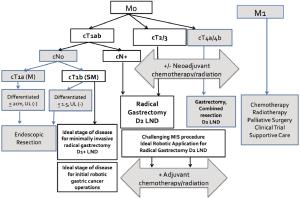
For the American gastric cancer patient population, the ability to expand minimally invasive radical gastrectomy with D2 lymphadenectomy to an increasing number of patients with surgical resectable advanced gastric cancer is one of the most appealing perceived advantages of the robotic surgical approach. The decision to integrate robotic surgery for gastric cancer patients as a part of the surgical armamentarium is built on the growing body of evidence, which supports its oncologic safety and the outcomes studies which demonstrate equivalence to laparoscopy in its patient benefits over open operations (14-16). Robotic radical gastrectomy with D2 lymphadenectomy is a minimally invasive option especially well suited for the surgical treatment of gastric cancer in the United State, where there are low hospital volumes, advanced stage of disease at the time of diagnosis, and a relatively limited surgeon experience with gastric cancer surgery (17). As surgeons better define the role of robotic surgery for gastric cancer, guidelines to direct the optimum application of robotic surgery in the overall strategy for gastric cancer treatment should be developed. Concurrently, proper robotic gastrectomy training, prerequisite to the safe and advantageous integration of robotic surgery into practice, will need to be considered.
Within the global context
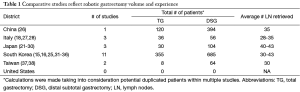
Within the last decade, robotic radical gastrectomy has emerged as an oncologically sound surgical approach for the treatment of early gastric cancer (15,18). In 2002 and 2003 pioneering surgeons from Japan (Hashizume et al.) (19) and the United States (Giulianotti et al.) (3) independently reported the initial safety and feasibility of robotic gastrectomy for cancer. Subsequently, South Korea, Japan, and Italy experienced early adoption of robotic gastrectomy (20-22). The single institutional safety and feasibility studies were quickly followed by comparative evaluations, which demonstrated the robotic advantages of minimally invasive surgery for gastric cancer patients (23-25). Most of the robotic gastrectomy experience comes from South Korea who have the highest number of published studies, whereas the largest number of patients evaluated in one study was from the first reported Chinese experience (Table 1). Moreover, most robotic gastrectomies in South Korea, Japan, and Taiwan have been performed in early gastric cancer patients while those of the United States, Italy, and China were performed for advanced gastric cancers. This trend reflects the relatively rapid dissemination of minimally invasive surgery for the treatment of early gastric cancer in the former countries and limited number of early stage cases in China and the West.
Full table
The largest experience of robotic radical gastrectomy comes from Yonsei University Severance Hospital in South Korea where 948 robotic gastric cancer operations were achieved between 2005-2014 (1). Moreover, the largest study overall and the only multiinstitutional prospective comparative study, which included surgeons from Yonsei University, was also conducted in South Korea (31). Of the total of 434 patients enrolled, 185 patients in the robotic treatment group were compared to the 185 patients in the laparoscopic group. While the results of this prospective trial concluded that the surgeon perceived advantages of superior operative environment of robotic surgery did not translate into clinical outcomes, it did support the previous findings of the smaller retrospective studies. It demonstrated robotic gastrectomy for gastric cancer to provide the same minimally invasive benefits to the patient as laparoscopic surgery over the open approach.
The minimally invasive benefits of robotic radical gastrectomy are clearly defined and include less intraoperative blood loss, shorter hospital stay, decreased use of pain medicine, and earlier return of gastrointestinal function when compared to open operations (18,23,27,32). There are no significant differences in the average number of lymph nodes retrieved, percentage of positive surgical margins, or long-term survivals representing adherence to oncologic principals during surgery without compromise in oncologic outcome (14). While longer operative times and higher operative costs have been the consistently reported disadvantages of the robotic approach for gastric cancer, the durations of the robotic gastrectomy operations appear to improve with the surgeons’ increasing experience (37,38). A cost analysis found the robotic approach not to have a higher financial impact on the cost of gastric cancer treatment in the United States (4).
The adoption of robotic radical gastrectomies by American general surgeons and surgical oncologists remain in its nascent stages of development. The majority of the gastric cancer operations, like most complex abdominal cancer surgery, are performed using the traditional open approach. In major academic centers, approximately 10% of radical gastrectomies are performed minimally invasively with an estimated 2.3% done using robot-assistance (4). These statistics are not surprising, considering that a combination of 1,490 different community, teaching and comprehensive cancer hospitals treated the relatively limited number of 37,124 gastric cancer patients over a 5 years period between 2001-2006. In this study 7,470 patients were treated at the highest volume hospitals which performed >13 cases/year. Another 7,229 patients received their surgery at hospitals with <3 cases/year volume. Moreover, only a few hospitals in the United States have a gastric cancer surgical volume exceeding 100 cases per year, with a limited number of surgeons having sufficient number of gastric cancer operations to readily overcome the learning curve of laparoscopic radical gastrectomy with D2 lymphadenectomy (39).
This snapshot of the United States gastric cancer surgical practices belies the lack of large volume studies in minimally invasive surgery for gastric cancer. It also speaks to the difficulty of obtaining sufficient training and experience to provide our gastric cancer patients with the most oncologically appropriate minimally invasive operations. The robotic surgical option adds another layer of complexity and decision making in the treatment of gastric cancer patients that requires both expertise in gastric cancer surgery and robotic operations; and preferably, robotic gastrectomy with D2 lymphadenectomy. The comparative outcome studies in the United States should provide a better understanding of the challenges of a radical gastrectomy with D2 lymphadenectomy particularly using the laparoscopic approach and the potential advantages of the robotic features specific to certain more complex portions of procedure. This should provide insight into how American surgeons can capitalize on the superiority of the robotic surgical systems.
The challenges of laparoscopic surgeons & advantages of the specific robotic features
The minimally invasive techniques of both the subtotal distal gastrectomies and the total gastrectomies with D2 lymph node dissections have been well described in detail in previously published studies and in textbooks (25,40). First, the standard laparoscopic radical gastrectomy and D2 lymphadenectomy is performed using five access ports and requires two assistant surgeons, one to control the camera and another to help provide the proper exposure during the procedure (41). Second, at least one of the assistants is preferably an experienced advanced laparoscopist and must provide prolonged and steady retraction of various organs including the stomach, pancreas, and major vasculature for proper and timely exposure. Thirdly, the D2 lymphadenectomy requires steady and precise dissection along the head of the pancreas (#6 lymph node station), the vessels in the porta hepatis (#12 lymph node station), the common hepatic artery (#8 lymph node station), the celiac axis (#9 lymph node station), the left gastric artery (#7 lymph node station) the splenic artery (#11 lymph node station), and at times in the splenic hilum (#10 lymph node station). Fourthly, the camera operator must provide a clear and steady view of the operative field with directed movements while using challenging angles in the suprapancreatic area, posterior to the stomach, and within the esophageal hiatus for total gastrectomies. Most importantly, the laparoscopic radical gastrectomy with D2 lymphadenectomy has a steep learning curve, which is difficult to achieve without a high volume practice (41-44).
Control of four instruments
The Da Vinci Surgical System (Si and Xi) possesses key robotic features to address these challenges during a robotic radical gastrectomy. The robotic arms are docked on the four of the five access ports used during the procedure. This gives the surgeon control of four robotic arms leaving one port for the bedside assistant. Only one assist surgeon is needed to help change the robotic instruments, suction, or staple, and to provide the safety of a surgeon who is scrubbed at the patient side. At the operative console, the primary surgeon controls the camera and three additional instruments. This provides almost complete control of the operative environment allowing the surgeon to provide timely and appropriate repositioning of the camera and third arm retraction throughout the operation without delay and irrespective of the assistant’s skill level. This feature maybe especially useful in situations where two assistants are difficult to obtain for a single operation and especially in teaching hospitals or academic centers where the assistant is a trainee.
Steady and magnified 3-dimensional view of the operative field
Another distinct advantage for the surgeon is the 3D view of the operative field. The 3D magnified robotic visualization provides the improved depth perception superior to that of the laparoscopic flat 2-dimensional images, with the option of 30° angulation offering a high definition view of the operative field not readily observed during an open operation. The camera remains steady without unwanted movements since the robotic camera arm does not fatigue as a human assistant would and moves with the same steadiness and accuracy. This permits a well-coordinated steady 3D magnified operative view, which is especially useful during the D2 lymphadenectomy when numerous angles around the vessels can be very helpful during the dissection.
Tremor filtered precise operative movements
Besides the camera, the surgeon controls three additional robotic arms, which can be fitted with several different robotic instruments as per surgeon preference, all with more precise and tremor filtered capabilities. A feature to shift control between two arms allows the surgeon to alternate the use of two different instruments on one side of the dissection. For example, the surgeon can position one of the left sided arms for retraction and help improve exposure prior to dissection of a certain area using the two other instruments. During the suprapancreatic lymphadenectomy, the third robotic arm holding a Cardiere forceps can be used to gently and precisely retract the pancreas for improved exposure of the celiac axis and splenic artery. The exposure can be maintained without movement, allowing a tremor filtered precise dissection, identifying the major vessels and retrieving the soft tissues along these vessels without unwanted injury. The Cardiere grasper is often used for the third arm retraction while the harmonic (or the hook electrocautery) in the left arm and a Maryland bipolar forceps on the right arm. Although no direct correlation can be made, the precision of these dissections may correspond to the statistically less blood loss during robotic operations compared to both laparoscopic and open operations.
Endowrist instruments
Another useful advantage of the robotic gastrectomy is derived from the robotic Endowrist capability, which allow for angled dissection and ease of suturing. The Large Needle Driver and the Mega ™ Suture Needle Driver are Endowristed with 7-degrees of articulation facilitating ease of intracorporal suturing of the gastrojejunostomy during a distal gastrectomy, the jejunojejunostomy during a total gastrectomy, the oversewing of staple lines, or gaining quicker and more precise control of bleeding vessels. The robotic skills required to perform these complex minimally invasive maneuvers and perform the robotic gastrectomy safely and without compromise to oncologic techniques, require specific training in robotic surgery and gastric cancer operations along with the necessary operative experience.
Robotic surgery certification and specific hospital privileges for robotic surgery are uniformly required prior to performing an operation using the robot. Moreover a formal robotic surgical training curriculum, which includes gastric cancer procedures, is necessary and actively being developed in the United States. The robotic surgery curriculum will incorporate the currently available simulation and dry lab experience along with the hands on porcine and cadaveric training provided by Intuitive Surgical, along with structured practical experience to take into account the learning curve patterns of the robotic gastrectomy.
The learning curve in robotic gastrectomy for cancer
The technical difficulty of performing an extended lymphadenectomy recommended for all surgically resectable patients with Stage II or greater gastric cancer is well recognized even in open operations (45,46). The learning curve for laparoscopic radical gastrectomy with extended node dissection for even experienced gastric cancer surgeons are steep (40-100 cases), with differences in operative time and postoperative patient outcomes between surgeons who have reached their plateau and those who have not (47,48). In contrast, the learning curve of robotic radical gastrectomy demonstrate a quicker adaptation with most studies reporting 11 to 25 cases to be sufficient for experienced gastric cancer surgeons (Table 2) (24,33,49-51). This very important advantage can provide an increasing number of surgeons the opportunity to achieve proficiency in minimally invasive gastric cancer operations and offer the benefits of the robotic approach to the gastric cancer patients in the United States.

Full table
Conclusions
Robotic surgery is a promising surgical approach for the treatment of gastric cancer patients in the United States. Surgeons with a thorough understanding of the principles of gastric cancer treatment, including strict adherence to the proper oncologic technique and proper training in robotic gastric cancer surgery can provide an oncologically sound minimally invasively operation for our gastric cancer patients. Minimizing surgical trauma enhances postoperative recovery, essential to the long-term survival and quality of life of gastric cancer patients, and should be weighed heavily in the surgeon’s selection of the operative approach. As expertise in robotic gastric cancer treatment grows, the optimal role of the ever-evolving robotic surgical technology for the treatment of gastric cancer will be better defined.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Son T, Hyung WJ. Robotic gastrectomy for gastric cancer. J Surg Oncol 2015;112:271-8. [PubMed]
- Anderson C, Ellenhorn J, Hellan M, et al. Pilot series of robot-assisted laparoscopic subtotal gastrectomy with extended lymphadenectomy for gastric cancer. Surg Endosc 2007;21:1662-6. [PubMed]
- Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg 2003;138:777-84. [PubMed]
- Glenn JA, Turaga KK, Gamblin TC, et al. Minimally invasive gastrectomy for cancer: current utilization in US academic medical centers. Surg Endosc 2015. [Epub ahead of print]. [PubMed]
- deSouza AL, Prasad LM, Ricci J, et al. A comparison of open and robotic total mesorectal excision for rectal adenocarcinoma. Dis Colon Rectum 2011;54:275-82. [PubMed]
- Shakir M, Boone BA, Polanco PM, et al. The learning curve for robotic distal pancreatectomy: an analysis of outcomes of the first 100 consecutive cases at a high-volume pancreatic centre. HPB (Oxford) 2015;17:580-6. [PubMed]
- Leung U, Fong Y. Robotic liver surgery. Hepatobiliary Surg Nutr 2014;3:288-94. [PubMed]
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [PubMed]
- Degiuli M, Sasako M, Ponti A, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg 2014;101:23-31. [PubMed]
- Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer 2011;14:97-100. [PubMed]
- Song J, Kang WH, Oh SJ, et al. Role of robotic gastrectomy using da Vinci system compared with laparoscopic gastrectomy: initial experience of 20 consecutive cases. Surg Endosc 2009;23:1204-11. [PubMed]
- Isogaki J, Haruta S, Man-I M, et al. Robot-assisted surgery for gastric cancer: experience at our institute. Pathobiology 2011;78:328-33. [PubMed]
- Obama K, Sakai Y. Current status of robotic gastrectomy for gastric cancer. Surg Today 2015. [Epub ahead of print]. [PubMed]
- Coratti A, Fernandes E, Lombardi A, et al. Robot-assisted surgery for gastric carcinoma: Five years follow-up and beyond: A single western center experience and long-term oncological outcomes. Eur J Surg Oncol 2015;41:1106-13. [PubMed]
- Woo Y, Hyung WJ, Pak KH, et al. Robotic gastrectomy as an oncologically sound alternative to laparoscopic resections for the treatment of early-stage gastric cancers. Arch Surg 2011;146:1086-92. [PubMed]
- Kim KM, An JY, Kim HI, et al. Major early complications following open, laparoscopic and robotic gastrectomy. Br J Surg 2012;99:1681-7. [PubMed]
- Dudeja V, Gay G, Habermann EB, et al. Do hospital attributes predict guideline-recommended gastric cancer care in the United States? Ann Surg Oncol 2012;19:365-72. [PubMed]
- Pernazza G, Gentile E, Felicioni L, et al. Improved Early Survival After Robotic Gastrectomy in Advanced Gastric Cancer. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 2006;16:286.
- Hashizume M, Shimada M, Tomikawa M, et al. Early experiences of endoscopic procedures in general surgery assisted by a computer-enhanced surgical system. Surg Endosc 2002;16:1187-91. [PubMed]
- Song J, Oh SJ, Kang WH, et al. Robot-assisted gastrectomy with lymph node dissection for gastric cancer: lessons learned from an initial 100 consecutive procedures. Ann Surg 2009;249:927-32. [PubMed]
- Uyama I, Kanaya S, Ishida Y, et al. Novel integrated robotic approach for suprapancreatic D2 nodal dissection for treating gastric cancer: technique and initial experience. World J Surg 2012;36:331-7. [PubMed]
- Patriti A, Ceccarelli G, Bellochi R, et al. Robot-assisted laparoscopic total and partial gastric resection with D2 lymph node dissection for adenocarcinoma. Surg Endosc 2008;22:2753-60. [PubMed]
- Kim MC, Heo GU, Jung GJ. Robotic gastrectomy for gastric cancer: surgical techniques and clinical merits. Surg Endosc 2010;24:610-5. [PubMed]
- D'Annibale A, Pende V, Pernazza G, et al. Full robotic gastrectomy with extended (D2) lymphadenectomy for gastric cancer: surgical technique and preliminary results. J Surg Res 2011;166:e113-20. [PubMed]
- Son T, Lee JH, Kim YM, et al. Robotic spleen-preserving total gastrectomy for gastric cancer: comparison with conventional laparoscopic procedure. Surg Endosc 2014;28:2606-15. [PubMed]
- Junfeng Z, Yan S, Bo T, et al. Robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: comparison of surgical performance and short-term outcomes. Surg Endosc 2014;28:1779-87. [PubMed]
- Caruso S, Patriti A, Marrelli D, et al. Open vs robot-assisted laparoscopic gastric resection with D2 lymph node dissection for adenocarcinoma: a case-control study. Int J Med Robot 2011;7:452-8. [PubMed]
- Pugliese R, Maggioni D, Sansonna F, et al. Subtotal gastrectomy with D2 dissection by minimally invasive surgery for distal adenocarcinoma of the stomach: results and 5-year survival. Surg Endosc 2010;24:2594-602. [PubMed]
- Suda K, Man-I M, Ishida Y, et al. Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc 2015;29:673-85. [PubMed]
- Noshiro H, Ikeda O, Urata M. Robotically-enhanced surgical anatomy enables surgeons to perform distal gastrectomy for gastric cancer using electric cautery devices alone. Surg Endosc 2014;28:1180-7. [PubMed]
- Kim HI, Han SU, Yang HK, et al. Multicenter Prospective Comparative Study of Robotic Versus Laparoscopic Gastrectomy for Gastric Adenocarcinoma. Ann Surg 2015. [Epub ahead of print]. [PubMed]
- Hyun MH, Lee CH, Kwon YJ, et al. Robot versus laparoscopic gastrectomy for cancer by an experienced surgeon: comparisons of surgery, complications, and surgical stress. Ann Surg Oncol 2013;20:1258-65. [PubMed]
- Kang BH, Xuan Y, Hur H, et al. Comparison of Surgical Outcomes between Robotic and Laparoscopic Gastrectomy for Gastric Cancer: The Learning Curve of Robotic Surgery. J Gastric Cancer 2012;12:156-63. [PubMed]
- Son SY, Lee CM, Ahn SH, et al. Clinical Outcome of Robotic Gastrectomy in Gastric Cancer in Comparison with Laparoscopic Gastrectomy: A Case-Control Study. Journal of Minimally Invasive Surgery 2012;15:27.
- Yoon HM, Kim YW, Lee JH, et al. Robot-assisted total gastrectomy is comparable with laparoscopically assisted total gastrectomy for early gastric cancer. Surg Endosc 2012;26:1377-81. [PubMed]
- Park JY, Jo MJ, Nam BH, et al. Surgical stress after robot-assisted distal gastrectomy and its economic implications. Br J Surg 2012;99:1554-61. [PubMed]
- Huang KH, Lan YT, Fang WL, et al. Comparison of the operative outcomes and learning curves between laparoscopic and robotic gastrectomy for gastric cancer. PLoS One 2014;9:e111499. [PubMed]
- Huang KH, Lan YT, Fang WL, et al. Initial experience of robotic gastrectomy and comparison with open and laparoscopic gastrectomy for gastric cancer. J Gastrointest Surg 2012;16:1303-10. [PubMed]
- Enzinger PC, Benedetti JK, Meyerhardt JA, et al. Impact of hospital volume on recurrence and survival after surgery for gastric cancer. Ann Surg 2007;245:426-34. [PubMed]
- Kim HI, Hur H, Kim YN, et al. Standardization of D2 lymphadenectomy and surgical quality control (KLASS-02-QC): a prospective, observational, multicenter study BMC Cancer 2014;14:209. [NCT01283893]. [PubMed]
- Kim MC, Jung GJ, Kim HH. Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol 2005;11:7508-11. [PubMed]
- Kang SY, Lee SY, Kim CY, et al. Comparison of Learning Curves and Clinical Outcomes between Laparoscopy-assisted Distal Gastrectomy and Open Distal Gastrectomy. J Gastric Cancer 2010;10:247-53. [PubMed]
- Kim JH, Jung YS, Kim BS, et al. Learning curve of a laparoscopy assisted distal gastrectomy for a surgeon expert in performing a conventional open gastrectomy. J Korean Gastric Cancer Assoc 2006;6:167-72.
- Zhou D, Quan Z, Wang J, et al. Laparoscopic-assisted versus open distal gastrectomy with D2 lymph node resection for advanced gastric cancer: effect of learning curve on short-term outcomes. a meta-analysis. J Laparoendosc Adv Surg Tech A 2014;24:139-50. [PubMed]
- Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med 1999;340:908-14. [PubMed]
- Cuschieri A, Fayers P, Fielding J, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet 1996;347:995-9. [PubMed]
- Jung DH, Son SY, Park YS, et al. The learning curve associated with laparoscopic total gastrectomy. Gastric Cancer 2014. [Epub ahead of print]. [PubMed]
- Moon JS, Park MS, Kim JH, et al. Lessons learned from a comparative analysis of surgical outcomes of and learning curves for laparoscopy-assisted distal gastrectomy. J Gastric Cancer 2015;15:29-38. [PubMed]
- Zhou J, Shi Y, Qian F, et al. Cumulative summation analysis of learning curve for robot-assisted gastrectomy in gastric cancer. J Surg Oncol 2015;111:760-7. [PubMed]
- Park SS, Kim MC, Park MS, et al. Rapid adaptation of robotic gastrectomy for gastric cancer by experienced laparoscopic surgeons. Surg Endosc 2012;26:60-7. [PubMed]
- Marano A, Choi YY, Hyung WJ, et al. Robotic versus Laparoscopic versus Open Gastrectomy: A Meta-Analysis. J Gastric Cancer 2013;13:136-48. [PubMed]


