A rare cause of recurrent cholangitis: neuroendocrine tumour of the main bile duct: case report and review of literature
Introduction
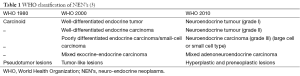
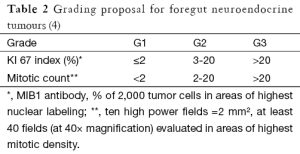
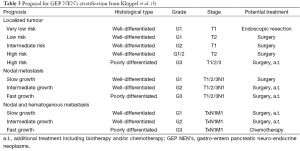
After an initial report in 1888 by Lubarsch, the German pathologist Oberndorfer invented the term “karzinoide” or “carcinoma-like” to describe a well-differentiated neuroendocrine tumour in 1907 (1,2). As more and more cases were reported, the limits of his definition became apparent. Carcinoids appeared to be a heterogeneous group of tumours varying in location, size and clinical symptoms. Given the difference in functional and biological behaviour, the World Health Organization (WHO) abandoned the term carcinoid in 2000 and established criteria to classify neuro-endocrine neoplasms (NEN’s) irrespective of their site of origin, which could predict the biological behaviour with high probability (3) (Table 1). In 2006 the European neuro-endocrine tumour society (ENETS) proposed a TNM staging system for foregut NEN’s, based on size and invasion grade (4). Its usefulness was confirmed in 2008 by Pape et al. after reviewing 202 cases (48 gastric, 23 duodenal and 131 pancreatic) (5). In 2010 the WHO came with a new set of criteria to classify NEN’s (3). The former well-differentiated tumour and carcinoma are now called simply neuro-endocrine tumour, and the poorly differentiated carcinomas are called neuro-endocrine carcinoma. Further differentiation is made after microscopic evaluation of structure, mitotic count and Ki 67-index (Table 2). Based on growth features, TNM stage and grade treatment stratification for gastro-entero pancreatic (GEP) NEN’s was suggested by Klöppel et al. (3) (Table 3).
NEN’s originate from enterochromaffin cells, named after their staining properties. These cells are part of the diffuse endocrine system and produce specific peptides and amines (6). Modlin et al. reviewed 13,715 cases from a US database between 1950 and 1999 (7). Main sites of location were the gastrointestinal (GI) tract (67.5%) and the bronchopulmonary system (25.3%). Within the gastro-intestinal tract, most carcinoid tumours occurred in the small intestine (41.8%), rectum (27.4%), and stomach (8.7%). NEN’s of the gallbladder (GB) and extra-hepatic biliary tract represent respectively 0.2% and 0.01% of NEN’s of all sites (7). This low frequency is due to the paucity of enterochromaffin cells in the biliary tract. It has been hypothesized that the origin of NEN’s of the biliary tract could be intestinal metaplasia induced by chronic inflammation of the bile duct (8). Another hypothesis was suggested by Roskams et al. (9). It appears that bile ductular cells and hepatocytes can express a neuro-endocrine phenotype in normal conditions. This characteristic is up regulated during ductular reaction, as observed in cholestatic liver disease. The subsequent increase in number of endocrine cells acts as a predisposing factor for the development of NEN’s.
Case report
We present the case of a 75-year-old female with repeating episodes of cholangitis, itching and limited weight loss (4 kg in 6 months). She was suffering from arterial hypertension and hypercholesterolemia for which she received medical treatment and her surgical history merely consisted of resection of a benign nodule in the right axilla. Further investigations were conducted. Lab results showed a positive serology for previous hepatitis A infection and an increased liver set (total bilirubin 10.2 mg/dL; direct bilirubin 8.7 mg/dL; c-GTP 350 IU/L; AST/ALT 65/105 IU/L). Tumour markers (carcinoembryonic antigen and carbohydrate antigen 19-9) were normal. Abdominal ultrasound showed dilated intra- and extra-hepatic bile ducts and cholecystolithiasis. To visualize the expected common bile duct (CBD) stones an endoscopic ultrasound was performed, which revealed an oval opaque nodular mass (16×10 mm2) in the supra-pancreatic part of the CBD. Cytology after ultrasound-guided puncture with 22 G needle could not reveal any signs of malignancy. Computed tomography and magnetic resonance imaging confirmed the mass and showed irregular thickening of the CBD wall extending from its mid- to its supra-pancreatic portion. There were no signs of spreading to regional lymph nodes or distant metastasis. A resection of the CBD, including the GB, was decided upon. Peroperative frozen sections of the distal and proximal margins were negative. Lymphnode dissection up to the level of the coeliac trunk was added and a Roux-en-Y hepatico-jejunostomy (HJ) was used as reconstruction. The postoperative course was complicated by an intra-abdominal abscess on day 6, located in the GB bed without arguments for leakage and treated by percutaneous drainage and antibiotics. On day 17 she suffered from catheter sepsis for which she received a second course of antibiotics and on day 18 a pleural puncture had to be performed for pleural effusion on the right. Eventually she was discharged on the 30th day postoperative.
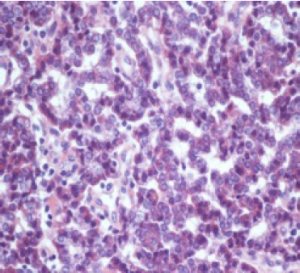
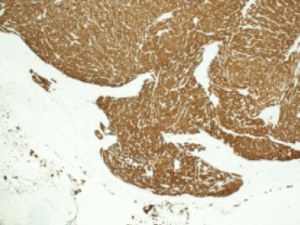
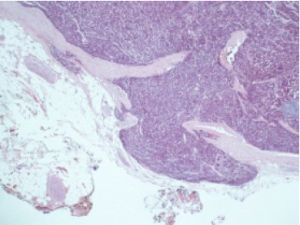
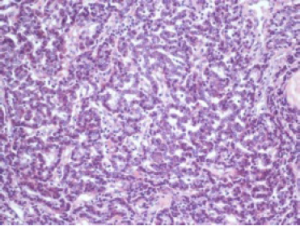
Histological evaluation was performed on a formalin-fixed, paraffin-embedded specimen. Macroscopy of the resected specimen showed a 12 mm grayish infiltrative intraluminally bulging tumour arising from the CHD. Microscopic examination showed a submucosal polypoidal tumour bulging into the CBD lumen (Figure 1) with extension into the adjacent vascular and periductal soft tissue (Figure 2). Metaplasia was not seen. The tumour was composed of medium-sized polygonal to roundish cells arranged in microtubules and embedded in sclerotic stroma (Figure 3). The cells had oval nuclei with small nucleoli and finely dotted chromatin (salt en pepper chromatin) (Figure 4). Immunohistochemical evaluation was positive for chromogranin, neuron-specific-enolase and cytokeratin, but negative for CK7, CK20 and p53 (Figure 5). The MIB-1 proliferation index was 8%. Specific test for functional NEN were negative. There were no arguments for lymph node or skip metastasis, corresponding with a grade II neuroendocrine tumour (NET) according to the 2010 WHO classification.
Discussion
The differential diagnosis of a patient with jaundice is quite extensive. Pre-, intra- and post-hepatic diseases might be the cause. To come to a specific diagnosis further investigations are essential. Determining the type of hyperbilirubinemia [(un)conjugated] and an abdominal ultrasound are the next diagnostic steps. If conjugated hyperbilirubinemia is present, combined with dilated extra and intrahepatic bile ducts, the differential diagnostic list is narrowed down to extra-hepatic obstructive causes. This is most frequently caused by choledocholithiasis, strictures after invasive procedures, pancreatitis or intrinsic/extrinsic tumours. In case of intrinsic tumours, cholangiocarcinoma accounts for 80-90% of cases (3). In the remaining 10-20% neuro-endocrine carcinoma, malignant melanoma, lymphoma, botryoid rhabdomyosarcoma, benign epithelial tumours [(cyst)adenoma and papilloma], paraganglioma and traumatic neuroma have been reported in literature (10). If a tumour is not suspected on echography, endoscopy is most frequently the next diagnostic step, allowing further differentiation. If a tumour is present brush cytology can be attempted, however one must bear in mind the high rate of false negative results. Sensitivities as low as 30% have been reported (11). This combined with the submucosal localisation of NEN’s, implicates that preoperative differentiation between cholangiocarcinoma and non-functional NEN’s remains difficult. Further investigations include magnetic resonance imaging (MRI) and or computed tomography (CT) to exclude distant metastasis.
Even though the first description of a carcinoid tumour dates back to 1907, it was only in 1959 that Davies et al. described the first carcinoid tumour of the distal CBD and pancreatic duct (2,12). After a thorough literature search we found 142 cases of extrahepatic bile duct (EHBD) NEN’s. The largest series being 31 cases is the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute in the US (13). A summary of the results is provided in Table 4. Because only summarized data is available from the SEER program, these results are discussed separately when indicated.
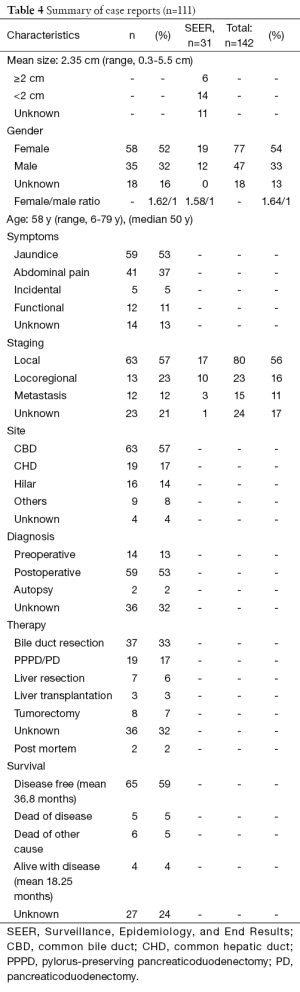
Full table
NEN’s appear to be most common in middle aged women (median 50 years; mean 58 years; 1.63/1 ratio) most frequently presenting with jaundice (53%) and/or abdominal pain (37%). This correlates with the SEER data, where they found a mean age of 58.2 years and a 1.58/1 female to male ratio. Although they can be hormonally active, clinical symptoms due to hormone production (gastrine, somatostatin, and serotonin) are rare. We found seven patients (6.3%) who presented with Zollinger-Ellison syndrome (ZES) (14-17) and five patients (4.5%) with an increased urinary 5-hydroxy-indolic-acetic acid (5-HIAA) (18-22). None of the ZES patients presented with jaundice as opposed to all of the patients with increased urinary 5-HIAA. In five cases (4.5%) the tumour was an incidental finding (23-27).
As stated, preoperative diagnosis remains difficult and was achieved in only 13% (14/111) of cases. Eleven of these cases were functional NEN’s (seven ZES, four increased urinary 5-HIAA). In two cases the diagnosis was preoperatively confirmed after endoscopic biopsy (28,29) and in the other case clinical suspicion arose after angiography (30).
Within the biliary tract the most common site is the bile duct (57%, 63/111), followed by the common hepatic duct (CHD) (17%, 19/111) and the hilar region (14%, 16/111). We found seven cases of NEN’s in the cystic duct, one at the level of an anterior sectional branch and one in a choledocal cyst.
In 56% (80/141) of reported cases the disease was local, in 16% (23/141) locoregional (positive lymph nodes or evidence of invasion into adjacent structures) and in 11% (16/141) metastasized at the moment of diagnosis (liver, pancreas, lung or peritoneum). Suggesting that this specific location enables earlier diagnosis compared to their other foregut relatives. In 17% (24/141) no data was provided. In the SEER program 14 patients had a tumour of 2 cm or more, six had a tumour of less than 2 cm. Data were lacking for 11 patients. In 54 of the remaining 111 cases tumour diameters were mentioned, with a mean size of 2.35 cm and a range of 0.3-5.5 cm. When we look at the correlation between tumour size and staging, we see respective mean sizes of 2.3 cm (range, 0.3-5.5 cm), 2.1 cm (range, 1.4-4.5 cm) and 2.95 cm (range, 0.7-5 cm) for local, locoregional and metastatic disease, suggesting that size is not the best predictor of biologic behavior, as opposed to what was stated in 2000 by Hamilton et al. (31). This finding is also reflected in the current staging system, where grade is more important than size to guide treatment and predict prognosis (4).
As stated by Chamberlain (32) and supported by others (10,33), surgical resection with negative histologic margins remains the cornerstone of treatment and affords the best chance of long-term, disease-free survival, even in the setting of metastatic disease. In most cases, this implicates EHBD excision with portal lymphadenectomy and Roux-en-Y biliary reconstruction with or without partial hepatectomy. In the cases we reviewed, 33% (37/111) of patients underwent a bile duct resection with reconstruction, 17% (19/111) a (pylorus preserving) pancreaticoduodenectomy, 6% (7/111) a liver resection, 7% (8/111) an unspecified tumorectomy and 3% (3/111) a liver transplantation. In 32% (36/111) the type of surgery could not be retrieved and in two cases the diagnosis was made at autopsy. The role of adjuvant therapy is controversial, with most studies failing to demonstrate a survival advantage (34).
As opposed to other GEP NEN’s or cholangiocarcinoma, grade I and II EHBD NEN’s tend to have a more indolent biologic behaviour, even when metastatic (35). In the SEER data they compared grade I and II EHBD NEN’s with GB carcinoids and small cell carcinomas (the current grade III NEN’s) and found a clear difference in survival. They reported 5- and 10-year survival rates of 84% and 80% for grade I and II EHBD NEN’s, compared to 44%-36%, 8%-0% and 0%-0% for grade I and II GB NEN’s, grade III GB NEN’s and grade III EHBD NEN’s respectively. Ten-year survival for patients with localized EHBD NEN’s was 85% and dropped to 74% for locoregional ones. They also found that in 10% of cases grade I and II NEN’s of the GB and EHBD were associated with a second primary neoplasm. These were most frequently located in the GI system. Therefore close surveillance of colon, lung and other organs appears mandatory.
In 2008 Modlin et al. reported on the prognosis of other GEP NEN’s, illustrating their variable clinical behavior and emphasizing the necessity of abandoning the idea of GEP NEN’s being a slow growing and fairly benign pathology. In case of an insulinoma 85-95% of patients are cured after enucleation, but in case of other pancreatic NEN’s or NEN’s of the small bowel, most patients present with metastatic disease worsening their surgical outcome and overall prognosis (33).
When we look at the survival rates in the remaining case reports, 59% (65/111) of patients remained disease free after a mean of 36.8 months. Eleven patients were reported dead, 5% (5/111) due to the disease, 5% (6/111) of unrelated causes. Four percent (4/111) of patients were alive with the disease after a mean of 18.25 months.
Conclusions
NEN’s of the EHBD are rare tumours which appear to have a more indolent biological behaviour compared to their foregut relatives. Based on the current knowledge (142 case reports) grade appears to be more important than size for prognosis and R0 resection remains the cornerstone for long-time survival, even in a metastasized setting.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gosset A, Masson P. Tumeurs endocrines de l'appendice. Presse Med 1914;25:237-9.
- Oberndorfer S. Karzinoide Tumoren des Dünndarms. Frankf Z Pathol 1907;1:426-32.
- Klöppel G. Classification and pathology of gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer 2011;18 Suppl 1:S1-16. [PubMed]
- Rindi G, Klöppel G, Alhman H, et al. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch 2006;449:395-401. [PubMed]
- Pape UF, Jann H, Müller-Nordhorn J, et al. Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer 2008;113:256-65. [PubMed]
- Montuenga LM, Guembe L, Burrell MA, et al. The diffuse endocrine system: from embryogenesis to carcinogenesis. Prog Histochem Cytochem 2003;38:155-272. [PubMed]
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934-59. [PubMed]
- Barrón-Rodríguez LP, Manivel JC, Méndez-Sánchez N, et al. Carcinoid tumor of the common bile duct: evidence for its origin in metaplastic endocrine cells. Am J Gastroenterol 1991;86:1073-6. [PubMed]
- Roskams T, van den Oord JJ, De Vos R, et al. Neuroendocrine features of reactive bile ductules in cholestatic liver disease. Am J Pathol 1990;137:1019-25. [PubMed]
- Noronha YS, Raza AS. Well-differentiated neuroendocrine (carcinoid) tumors of the extrahepatic biliary ducts. Arch Pathol Lab Med 2010;134:1075-9. [PubMed]
- Vasilieva LE, Papadhimitriou SI, Dourakis SP. Modern diagnostic approaches to cholangiocarcinoma. Hepatobiliary Pancreat Dis Int 2012;11:349-59. [PubMed]
- Davies AJ. Carcinoid Tumours (Argentaffinomata): Hunterian Lecture delivered at the Royal College of Surgeons of England on 3rd March 1959. Ann R Coll Surg Engl 1959;25:277-97. [PubMed]
- Albores-Saavedra J, Batich K, Hossain S, et al. Carcinoid tumors and small-cell carcinomas of the gallbladder and extrahepatic bile ducts: a comparative study based on 221 cases from the Surveillance, Epidemiology, and End Results Program. Ann Diagn Pathol 2009;13:378-83. [PubMed]
- Tonelli F, Giudici F, Nesi G, et al. Biliary tree gastrinomas in multiple endocrine neoplasia type 1 syndrome. World J Gastroenterol 2013;19:8312-20. [PubMed]
- Price TN, Thompson GB, Lewis JT, et al. Zollinger-Ellison syndrome due to primary gastrinoma of the extrahepatic biliary tree: three case reports and review of literature. Endocr Pract 2009;15:737-49. [PubMed]
- Martignoni ME, Friess H, Lübke D, et al. Study of a primary gastrinoma in the common hepatic duct - a case report. Digestion 1999;60:187-90. [PubMed]
- Wu PC, Alexander HR, Bartlett DL, et al. A prospective analysis of the frequency, location, and curability of ectopic (nonpancreaticoduodenal, nonnodal) gastrinoma. Surgery 1997;122:1176-82. [PubMed]
- Nesi G, Lombardi A, Batignani G, et al. Well-differentiated endocrine tumor of the distal common bile duct: a case study and literature review. Virchows Arch 2006;449:104-11. [PubMed]
- Juturi JV, Maghfoor I, Doll DC, et al. A case of biliary carcinoid presenting with pancreatitis and obstructive jaundice. Am J Gastroenterol 2000;95:2973-4. [PubMed]
- Chan C, Medina-Franco H, Bell W, et al. Carcinoid tumor of the hepatic duct presenting as a Klatskin tumor in an adolescent and review of world literature. Hepatogastroenterology 2000;47:519-21. [PubMed]
- Vitaux J, Salmon RJ, Languille O, et al. Carcinoid tumor of the common bile duct. Am J Gastroenterol 1981;76:360-2. [PubMed]
- Little JM, Gibson AA, Kay AW. Primary common bile-duct carcinoid. Br J Surg 1968;55:147-9. [PubMed]
- Maitra A, Krueger JE, Tascilar M, et al. Carcinoid tumors of the extrahepatic bile ducts: a study of seven cases. Am J Surg Pathol 2000;24:1501-10. [PubMed]
- Ligato S, Furmaga W, Cartun RW, et al. Primary carcinoid tumor of the common hepatic duct: A rare case with immunohistochemical and molecular findings. Oncol Rep 2005;13:543-6. [PubMed]
- Ferrone CR, Tang LH, D'Angelica M, et al. Extrahepatic bile duct carcinoid tumors: malignant biliary obstruction with a good prognosis. J Am Coll Surg 2007;205:357-61. [PubMed]
- Tsalis K, Vrakas G, Geroukis T, et al. Primary neuroendocrine tumor of the extrahepatic biliary tree mimicking Klatskin tumor. J Gastrointestin Liver Dis 2010;19:341-2. [PubMed]
- Bergdahl L. Carcinoid tumours of the biliary tract. Aust N Z J Surg 1976;46:136-8. [PubMed]
- Hubert C, Sempoux C, Berquin A, et al. Bile duct carcinoid tumors: an uncommon disease but with a good prognosis? Hepatogastroenterology 2005;52:1042-7. [PubMed]
- Johnson G, Hatfield A, Webster G, et al. A diagnosis of an intraluminal carcinoid tumor of the bile duct by using cholangioscopy. Gastrointest Endosc 2010;71:622-3; discussion 623. [PubMed]
- Gusani NJ, Marsh JW, Nalesnik MA, et al. Carcinoid of the extra-hepatic bile duct: a case report with long-term follow-up and review of literature. Am Surg 2008;74:87-90. [PubMed]
- Hamilton SR, Aaltonen LA. Pathology and Genetics of Tumours of the Digestive System. Lyon, France: IARC Press, 2000:214-217 .
- Chamberlain RS, Blumgart LH. Carcinoid tumors of the extrahepatic bile duct. A rare cause of malignant biliary obstruction. Cancer 1999;86:1959-65. [PubMed]
- Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;9:61-72. [PubMed]
- El Rassi ZS, Mohsine RM, Berger F, et al. Endocrine tumors of the extrahepatic bile ducts. Pathological and clinical aspects, surgical management and outcome. Hepatogastroenterology 2004;51:1295-300. [PubMed]
- Squillaci S, Marchione R, Piccolomini M, et al. Well-differentiated neuroendocrine carcinoma (malignant carcinoid) of the extrahepatic biliary tract: report of two cases and literature review. APMIS 2010;118:543-56. [PubMed]