Pancreatic neoplasm: a unique size and presentation
Introduction
VIPomas are rare pancreatic endocrine tumors (PETs), detected in 1 in 10 million people per year and usually present as a constellation of well-defined clinical features characterized by watery diarrhea, hypokalemia, and achlorhydria (WDHA) (1,2). Theses tumors secrete an excess of vasoactive intestinal peptide (VIP) and are typically diagnosed only after they have metastasized liver, lymph nodes and lungs (60% to 80%) (3-6). The diagnosis is confirmed by identifying hyper secretion of VIP in a setting of the localized pancreatic tumor (7). Symptomatic pancreatic VIPomas are usually solitary, more than 3 cm in diameter, and occur in the tail of pancreas in 75 percent of patients (4). We demonstrate a rare glimpse at an unusual small size of VIPoma at its earliest clinical presentation.
Case presentation
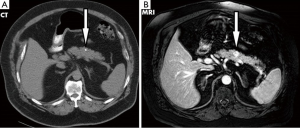
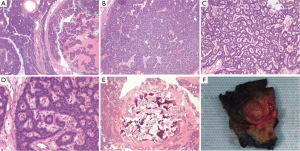
A 71-year-old male veteran with a past medical history of insulin-dependent diabetes mellitus, obesity and hypertension who was referred to our GI clinic for a three month history of worsening diarrhea. Prior to onset of symptoms, the patient had occasional diarrhea with a stool output ranging from 1 to 3 L daily and two formed stools per day. Within a month period, he progressed to 5–6 bowel movements daily. One month prior to the onset of worsening diarrhea, he presented to the ED with complains of upper quadrant abdominal pain as a new 0.4 cm calcified lesion within the pancreas was identified on an abdominal CT (Figure 1). This new finding was not evident on a previous CT abdomen 1 year ago. On examination he was dehydrated with elevated creatinine/BUN level (2.1/28) from baseline (1.4/21) and leukocytosis (18.1) while denying abdominal pain, fever/chills, bleeding per rectum, nausea/vomiting, dysuria hematuria or any recent travel. Abdominal examination revealed non-specific tenderness without any rigidity or guarding, with increased bowel sounds. Initial blood investigations in the ED demonstrated normal potassium 4.7 mmol/L (range, 3.5–5.1 mmol/L), normal sodium 142 mmol/L (range, 135–145 mmol/L) and normal chloride 102 mmol/L (range, 98–106 mmol/L). Serum albumin was of 4.2 g/L with a corrected calcium level of 9.1 mmol/L. Full blood count showed normal hemoglobin of 16.3 g/dL and a platelet count of 392×103/mcL while liver enzymes were within normal limits (AST U/L 24; ALT U/L 43; total bilirubin 0.7 mg/dL). In fact our patient never demonstrated any evidence of electrolyte abnormalities dating as far back as 1999. In the clinic a VIP level, MRI of the abdomen was ordered as he was placed on metamucil. Outpatient MRI exhibited a well circumscribed 13 mm solitary lesion within the ventral pancreatic body consistent with pancreatic neuroendocrine tumor (Figure 1). Serum VIP level was in excess of 261 pg/mL (normal <60 pg/mL) with a repeat of 245.7 pg/mL. Gastrin level was normal at 108 pg/mL and C-peptide also within normal limits at 3.4 ng/mL. A total body indium-111 octreotide scan failed to demonstrate any evidence of metastasis or somatostatin receptor avid tumors. Following distal pancreatectomy with splenectomy the pathological results described gland like structures nested in a trabecular growth pattern and well differentiated cells with a moderate amount of amphophilic cytoplasm and nuclei showing some prominent nucleoli and granular chromatin (Figure 2). The findings were consistent with VIPoma as margins were negative for neoplasm. Patient had an uneventful post operative hospital course. Nearly 1 year following his diagnosis he has demonstrated a resolution of his symptomatology with normalization of VIP levels.
Discussion
First described in 1958 by Verner and Morrison, fewer than 300 cases of VIP-secreting islet cell tumors have been reported in the medical literature with an estimated incidence rate between 0.2 and 2 cases per million people/year (3,8-10). Perfuse secretory diarrhea is the hallmark of the disease process with 1 L to more than 3 L of stool output daily (11,12). In a review of 241 patients by Soga and colleagues, diarrhea was noted in all patients, hypokalemia in 89%, achlorhydria in 43%, and weight loss in 36% (13). Associated symptoms include abdominal pain (50%), hypercalcemia (50%), hyperglycemia (40%) and flushing (20%) (14). Such clinical constellation of symptoms is a result of excessive and unregulated secretion of VIP by the tumor. This 28-amino acid polypeptide is secreted from non-beta islet cells of the pancreas which causes activation of cellular adenylate cycles and c-AMP at the intestinal epithelial cells. Such activation leads to, excessive secretion of electrolytes from the small intestines leading to hypokalemia and severe dehydration. It also causes smooth muscles relaxation of the gastrointestinal tract, bicarbonate secretion from the pancreas and inhibits gastric acid production (12).
The majority of tumors are malignant in nature with most patients presenting with metastatic disease and local lymph node involvement (78%) (13). Primary tumors are largely solitary ranging from 2–6 cm in size with a preponderance of the tumors in the body and tail (90%) (15). Giving the relatively large size and somatostatin dense receptor of the tumors, localization with CT and staging with somatostatin receptor scintigraphy (SRS) using radiolabeled octreotide is the preferred technique for tumor detection (16,17). Octreotide scan has a unique advantage in which it allows instantaneous whole body scanning and detection of metastases outside of the abdominal region. Since most tumors are >3 cm, CT of the abdomen is usually sufficient with a sensitivity of >80%, but helical, contrast-enhanced triple phase CT or MRI is the preferred choice, as such modalities will also help identify metastatic lesion to the liver. If the presence of a pancreatic tumor is still indeterminate, an endoscopic ultrasound (EUS) would be able to identify pancreatic tumors as small as 2 to 3 mm. It would also provide accurate information regarding the local extent of disease and permit for transmucosal needle biopsy of pancreatic lesions.
Pancreatic neuroendocrine tumors are staged by TNM classification. The American Joint Committee on Cancer (AJCC) and the European Neuroendocrine Tumor Society (ENETS) provide the two staging systems in which provides information regarding prognosis and overall survival (18,19).
Patient with VIPoma are initially managed conservatively with adequate fluid resuscitation and electrolyte replacement. Surgical resection or debulking has become the mainstay of therapy, as it offers immediate symptomatic cure with increase survival rates. In a study of 20 patients with functioning of PET, 90% had complete resolution of their symptoms and normalization of their VIP levels status post-surgical resection with a two year survival of 60% (20). However, due to the slow growing nature of theses tumors, medical therapy with somatostatin is a viable option for non-surgical candidates or for patients with recurrent disease. The effectiveness of medical therapy in regards to symptomatic therapy has been as high as 78% with limited side effects (10).
Our patient’s presentation was inconsistent with the WDHA syndrome as he failed to demonstrate hypovolemia,
Conclusions
VIPoma is a very rare clinical entity but nevertheless an important condition to consider when dealing with patients suffering from chronic diarrhea. Early diagnosis is a key prognostic factor as surgical resection or debulking has become the mainstay of therapy, as it offers immediate symptomatic cure with increase survival rates.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Friesen SR. Update on the diagnosis and treatment of rare neuroendocrine tumors. Surg Clin North Am 1987;67:379-93. [PubMed]
- Van Heerden JA, Thompson GB. Islet cell tumours of the pancreas. In: Trede M, Carter DC, editors. Surgery of the pancreas. Edinburgh: Churchill Livingstone, 1993;545-61.
- Perry RR, Vinik AI. Clinical review 72: diagnosis and management of functioning islet cell tumors. J Clin Endocrinol Metab 1995;80:2273-8. [PubMed]
- Smith SL, Branton SA, Avino AJ, et al. Vasoactive intestinal polypeptide secreting islet cell tumors: a 15-year experience and review of the literature. Surgery 1998;124:1050-5. [PubMed]
- Mozell E, Stenzel P, Woltering EA, et al. Functional endocrine tumors of the pancreas: clinical presentation, diagnosis, and treatment. Curr Probl Surg 1990;27:301-86. [PubMed]
- Ayub A, Zafar M, Abdulkareem A, et al. Primary hepatic vipoma. Am J Gastroenterol 1993;88:958-61. [PubMed]
- Vinik AI, Raymond E. Pancreatic neuroendocrine tumors: approach to treatment with focus on sunitinib. Therap Adv Gastroenterol 2013;6:396-411. [PubMed]
- Verner JV, Morrison AB. Islet cell tumor and a syndrome of refractory watery diarrhea and hypokalemia. Am J Med 1958;25:374-80. [PubMed]
- Soga J, Yakuwa Y. Vipoma/diarrheogenic syndrome: a statistical evaluation of 241 reported cases. J Exp Clin Cancer Res 1998;17:389-400. [PubMed]
- Peng SY, Li JT, Liu YB, et al. Diagnosis and treatment of VIPoma in China: (case report and 31 cases review) diagnosis and treatment of VIPoma. Pancreas 2004;28:93-7. [PubMed]
- Matsumoto KK, Peter JB, Schultze RG, et al. Watery diarrhea and hypokalemia associated with pancreatic islet cell adenoma. Gastroenterology 1966;50:231-42.
- Long RG, Bryant MG, Mitchell SJ, et al. Clinicopathological study of pancreatic and ganglioneuroblastoma tumours secreting vasoactive intestinal polypeptide (vipomas). Br Med J (Clin Res Ed) 1981;282:1767-71. [PubMed]
- Eriksson B, Oberg K. An update of the medical treatment of malignant endocrine pancreatic tumors. Acta Oncol 1993;32:203-8. [PubMed]
- Ito T, Igarashi H, Jensen RT. Pancreatic neuroendocrine tumors: clinical features, diagnosis and medical treatment: advances. Best Pract Res Clin Gastroenterol 2012;26:737-53. [PubMed]
- Von Hoff D, Evans D, Hruban R, editors. Pancreatic cancer. Sudbury, MA: Jones and Bartlett Publishers, 2005.
- Kirkwood KS, Debas HT. Neuroendocrine tumors: common presentations of uncommon diseases. Compr Ther 1995;21:719-25. [PubMed]
- Nikou GC, Toubanakis C, Nikolaou P, et al. VIPomas: an update in diagnosis and management in a series of 11 patients. Hepatogastroenterology 2005;52:1259-65. [PubMed]
- Strosberg JR, Cheema A, Weber JM, et al. Relapse-free survival in patients with nonmetastatic, surgically resected pancreatic neuroendocrine tumors: an analysis of the AJCC and ENETS staging classifications. Ann Surg 2012;256:321-5. [PubMed]
- Strosberg JR, Cheema A, Weber J, et al. Prognostic validity of a novel American Joint Committee on Cancer Staging Classification for pancreatic neuroendocrine tumors. J Clin Oncol 2011;29:3044-9. [PubMed]
- Matthews BD, Smith TI, Kercher KW, et al. Surgical experience with functioning pancreatic neuroendocrine tumors. Am Surg 2002;68:660-5. [PubMed]


