Mast cells, disease and gastrointestinal cancer: A comprehensive review of recent findings
Abstract
Paul Ehrlich, a German scientist, discovered what is known as the mast cell in the late 1800’s, which has proven to be an important player in the immune system of vertebrates. Mast cells are ubiquitous throughout the tissues of the human body and play numerous roles, both beneficial and destructive. We know they are important in our army of immunity warrior cells, which defend us against viruses, bacteria and parasitic invaders. They are also very well known for the havoc they wreak, causing uncomfortable symptoms due to their release of histamine and other mediators which cause the all too familiar itching, sneezing, urticaria and rhinorrhea of allergic responses. Mast cell activities are diverse and include painful inflammatory reactions in autoimmune conditions such as rheumatoid arthritis. In the gastrointestinal system, mast cells are implicated in diverse actions such as increased gastric acid secretion, polyp formation and uncomfortable conditions such as Irritable Bowel Syndrome. The role of immunology and mast cells in these areas is intriguing but less well understood than their role in allergic responses. Because mast cells have been implicated in both physiologic as well as pathogenic processes, they have been the subjects of avid study. Review of the current literature on mast cell biology reveals that there are many studies of their presence within the tumor microenvironment and evidence, which supports mast cell influence on tumor angiogenesis, tumor invasion, and immune suppression. The studies reviewed in this article concentrate largely on mast cells in human GI malignancies. This review also provides background information regarding mast cells, such as their origination, their location within the body, how they are activated and how they function as mediators.
Key words
Mast cells; gastrointestinal cancer
Mast cell origination, location and activation
Location
Mast cells are important components of the immune system of vertebrates. Mast cells are found in varying quantities in virtually all tissues of the human body and are stationed much like sentinel cells of the immune system at bodily portals of entry. The numbers of mast cells are highest at locations where they can respond to foreign organisms and antigens, thereby concentrating heavily in the dermis, intestinal mucosa and submucosa, conjunctiva, pulmonary alveoli and airways. They are even present in the atrial appendage of the heart (1-4). Mast cells are found in the choroid plexus of the brain, the vascular bed of the meninges and in low numbers in the kidneys and bone marrow. Often located closely to blood vessels, nerves and lymphatics, their concentration averages an estimated density of 7,000 to 20,000 mast cells per cubic millimeter of tissue (5-11).
Origination
Mast cells originate in the bone marrow and spleen, arising from pluripotent CD34+ stem cells (12-14) and differentiate along the myeloid pathway and leave the hematopoietic tissues as committed progenitors (12,15). Their migration is directed out of the circulation by signals only partially characterized and home to specified tissues where they differentiate further during the maturation process (16,17).
Anatomy
Mast cells are round, mononuclear cells with variation in their cytoplasm and contain numerous metachromatic cytoplasmic granules ranging in size from 0.3 to 0.8 micrometers. From their vantage point surrounding blood vessels, their actions can influence the function of vascular structures, monitor blood for inflammatory and infectious changes and distribute mediators, which they release in response to a specific stimulus (18). Mast cell-derived mediators are of three basic types and include: (I) preformed mediators stored in secretory granules, which can be released into the extracellular environment within seconds after mast cell activation, (II) newly-synthesized lipid mediators, and (III) cytokines and chemokines (19,20). The preformed mediators are responsible for the acute symptoms of mast cell-mediated allergic reactions (20). These mediators are stored in cytoplasmic granules include histamine, neutral proteases, heparin proteoglycans, and cytokines such as TNF-α.
Histamine and histamine receptors
Histamine can produce powerful physiologic effects and its actions are mediated through specific receptors located on target cells labeled as H1, H2, H3 and H4 receptors (21-25). H1 actions includes: increased vascular permeability, bronchial and intestinal smooth muscle contraction, increased nasal mucus production, increased heart rate and cardiac output, flushing, T cell neutrophil and eosinophil chemotaxis (4,26). H2-mediated actions include increased gastric acid secretion, airway mucus production, but at the same time, inhibition of neutrophil and eosinophil influx into a tissue (26,27). H3 receptors have been found in the brain and H4 receptors can act as chemoattractants for bone marrow derived mast cells and modulation of calcium influx (28,29). Histamine and other mast cell-derived mediators have been studied intensely with regard to their physiologic and pathogenic actions.
Growth
The growth of mast cells is influenced mostly by stem cell factor (SCF), which is produced by stromal and endothelial cells and fibroblasts (30-32). The surface receptor for SCF is the receptor tyrosine kinase c-kit (CD117), which is expressed by stem cells and present during myeloid differentiation. CD117 is an early marker for mast cell precursors and expressed throughout their lifetime (2,12,15). SCF participates in each stage of growth and differentiation of mast cells including differentiation, proliferation, chemotaxis, adhesion and survival (33). It has been suggested that this global influence of SCF results in the ubiquitous presence of mast cells (13). There are numerous growth and differentiation factors other than SCF, which have been shown to affect mast cell functions including several of the type 2 helper T cell cytokines (2).
Physiology
In keeping with their role as important components of the immune system, mast cells increase in number at inflammatory sites in diseases, which may be atopic, inflammatory or malignant (20,34,35). Mast cells are present in urticaria, acute allergic reactions, rhinitis and asthma (20) and fight viral, bacterial and parasitic infections (20). Mast cells are present in inflammatory conditions such as rheumatoid arthritis, inflammatory bowel disease, psoriasis, pulmonary fibrosis, atherosclerotic cardiovascular diseases, cardiomyopathies and a number of malignancies (36-45). It is not clear if the role of mast cells in these diseases is regenerative, pathogenic or possibly both. Mast cell numbers increase significantly at multiple areas in the medical condition known as mastocytosis (46-48). Their actions in this disease process are responsible for the abnormal changes observed. Patients with mastocytosis suffer flushing, gastrointestinal cramps, hypotension, and a skin finding known are urticaria pigmentosa (49-52).
In the study of mast cells, it has been unclear as to why they appear to have both physiologic and pathogenic actions. It is known that they are important in the body’s defense against infecting organisms and are key effector cells in both innate and acquired immunity, capable of inducing and amplifying both these responses (1,3,4). Mast cells are capable of identifying microbial products through surface pattern recognition receptors and are involved in the recruitment of other leukocytes for containing infections or enabling tissue repair (26,33,53). Mast cells can trigger increased intestinal motility, bronchoconstriction and also epithelial sloughing, which have rid epithelial surfaces of infectious agents (4,54). Mast cells can stimulate fibroblast proliferation and collagen synthesis, thereby blocking and limiting the spread of infections (55-57). Mast cell numbers increase within uterine tissues during pregnancy and contribute to enhancing immunity through secretion of cytokines and proteases (58). Mast cells and T regulatory cells have been shown to mediate allograft tolerance in mice (59,60).
Mast cells appear to have some functions that can yield detrimental outcomes including urticaria, asthma, rhinitis, and atopic dermatitis (43,61-63). Mast cells also play a significant role in allergic responses including the extreme response known as anaphylaxis (64-66). The overgrowth of mast cells can lead to the varying forms of mastocytosis (19,67,68). Mast cells have been noted for many years to be present within human atherosclerotic lesions although their function within these lesions is unclear (69). Mouse models suggest the promotion of atherosclerotic changes occurs through the release of pro-inflammatory cytokines and possibly proteases (26,71). Mast cells are observed in abundance in numerous autoimmune illnesses including multiple sclerosis and rheumatoid arthritis. In both these disease processes, mast cell deficient mice showed a reduction in pathologic processes that was reversed upon restoration of mast cells in the mice (72,73). Figure 1 summarizes the role of mast cells in different processes including tumor biology, host defense, cardiovascular disease and tissue pathology [Reprinted with permission from Nature Immunology, New developments in mast cell biology 2008; 9: 1215-1223.].
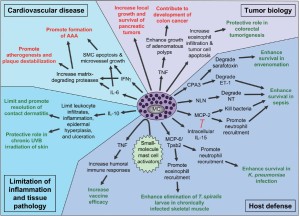
Mast cells and disease
The tumor microenvironment is composed of immune cells, stromal cells, endothelial cells and other components and provides a supportive niche to promote the invasion and growth of tumors (74-78). This environment also creates a suppressive barrier to effective immune responses against tumors and is consequently emerging as a target for cancer immunotherapies (79). Mast cell (MC) accumulation in the tumor microenvironment is associated with a poor prognosis in aggressive cancers, however, high densities of intratumor T effector cells are associated with a favorable prognosis (79). The relation of mast cells in cancers seems diversified and not yet well understood. Current evidence supports the notion that MCs influence tumor angiogenesis, tumor invasion, immune suppression and contribute to the immune suppressive tumor microenvironment (79). Important future investigations will be to identify molecules that provide signals with MCs during the initiation of tumor growth, identify the molecules produced, and understand how these molecules influence the outcome of antitumor immune responses (79).
Gastric cancer
It is known that gastric ulcers in humans cause an increased risk of gastric cancers. In a study designed to ascertain the relationship of mast cell density in gastric ulcers and cancer, Mukherjee et al. studied mass cell density (MCD) in patients with gastric ulcers, well-differentiated cancers and in poorly differentiated cancers (80). Biopsies were sectioned, stained and reviewed and MCD determined. It was concluded that the accumulation of mast cells in gastric ulcers is an inflammatory response. The MCD was increased in well-differentiated cancers and thought to be a mast cell mediated immune response and also possibly caused tumor angiogenesis and produced factors for tumor progression (80). In the poorly-differentiated cancer group, the mast cell mediated anti-tumor response was lacking, with no clear explanation (80).
Ribatti et al. studied the role of mast cells in gastric cancer angiogenesis in an attempt to clarify their role (81). Correlation between microvascular density and tryptaseand chymase-positive masts cells with histopathological type in gastric cancer was utilized as the study substrate. Specimens of gastric adenocarcinomas were obtained from 30 patients who had curative gastrectomies (81). These specimens were stained with anti-CD3l antibody to stain endothelial cells and anti-tryptase and anti-chymase antibodies to stain mast cells. Stage IV gastric carcinoma has a higher degree of vascularization than earlier stages and mast cells were increased accordingly and thought to be highly correlated with the extent of angiogenesis (81). Further understanding the mechanisms of angiogenesis in this malignancy may contribute to the development of an antiangiogenic therapy (81).
Sinnomon et al. demonstrated in their study the protective role of mast cells in intestinal tumorigenesis and noted that mast cells have been observed in numerous types of tumors, however, their role in carcinogenesis has been poorly understood (82). Strangely, much epidemiologic evidence has suggested a negative association between the presence of mast cells and tumor progression in lung, colon and breast cancers. Murine studies carried out by these authors, however, found that mast cell deficient mice developed more benign and malignant tumors (82). They were able to show that masts cells act as upstream regulators of numerous inflammatory cells, which provided a protective, antitumor role of mast cells in early stage intestinal tumorigenesis (82).
Esophageal cancer
Esophageal cancer in humans has historically been an aggressive disease with a poor prognosis. In a study aimed to identify new treatment modalities, Tinge et al. investigated the role of mast cells in esophageal cancer patients to determine whether a higher number of mast cells is associated with better survival and could possibly be a marker for further immunotherapeutic studies (83). At the conclusion of their study of the 61 patients in which tissue samples were stained and mast cell numbers quantified, it was found that there was no significant difference in survival found between patients with higher numbers of mast cells than those with the lower number (83).
Colorectal cancer
Colorectal cancer (CRC) is a common and potentially lethal malignancy (84-86). It has long been recognized that inflammatory bowel diseases cause an increased risk of colon cancer (87-89). Evidence from experimental animals has now implicated the innate immune system in the development of adenomatous polyps, which are precursors to cancer. In the review of Heijmans et al. the interaction between the immune system and the adenoma to carcinoma sequence is focused on with an emphasis on mast cells and the role they play in adenoma development (90). In indicating that the “jury is still out,” this article points to the need for more studies in the field of mast cell biology as they influence the growth of pre-malignant polyps as they advance into fully developed malignancies (90).
Tumor cells need an enriched blood supply to thrive and angiogenesis plays a critical role in several types of malignancies (91-93). In the study of colorectal cancer, Yodavudh et al. found that there is a significant correlation existing between increased microvessel density (MVD) and MCD. In relation to this, patients with tumors of low MVD and low MCD (counts) had significantly longer survival rates than those with high MVD and high MCD. The results of this study could possibly serve as a prognostic tool for patients with this malignancy (94). In addition to this, a multivariate Cox hazard revealed that MVD and distant metastasis were independent poor prognostic factors to survival in patients with colorectal cancer. Their studies have also shown that the patients with high MVD (hypervascular) tumors and distant metastasis had 1.9 and 2.5 times higher death rates than the corresponding hypovascular and non-metastatic groups. This being said, the authors believe that thorough ascertainment of microvessel density in the invasive front of primary colorectal carcinoma could possibly serve as a prognostic tool for patients with this malignancy (94).
Mast cells have been studied as possibly affecting the development of colonic polyps (95). Colon polyps classified as adenomatous are felt to be pre-malignant, ultimately undergoing neoplastic transformation if not discovered and removed (95). Recent data has suggested that the tumor stromal environment is implicated in the development of these polyps. Polyps are infiltrated with proinflammatory mast cells and their precursors (95). If these are depleted, a profound remission of existing polyps is observed. Based on this, the data suggests that mast cells are an essential hematopoietic component for preneoplastic polyp development and a possible target for therapeutic intervention (95).
Other studies of interest in the field of colon cancer and their relationship to mast cells include those in which patients with advanced stage cancers are the subjects. In the study by Xia et al., it was seen that mast cell count in the mucosa adjacent to the colon cancer was higher than in the stroma of the cancer (96). Also, there was no difference in mast cell counts observed between the stroma in metastatic lymph nodes and the lymph tissue adjacent to the metastatic nodes. Further, the mast cell count in the regionaldraining nodes not containing cancer cells was significantly higher than that of the stroma of lymph node metastasis and surrounding lymph tissue (96). It was not possible to conclude, however, that any of the mast cells were related to five-year survival. From this, the authors concluded that mast cells do not contribute to the progression of advanced colon cancer (96). In the study by Xianrui Wu, et al., the clinic and prognostic significance of tumor-infiltrating mast cells (TIM) in patients with CRC was investigated (97). TIM infiltration in 325 CRC specimens was quantified by immunohistochemistry. Multivariate Cox regression analysis showed that a high amount of TIM was a risk factor for overall survival as well as for disease-free survival (97). The authors postulated that high numbers of tumor-infiltrating mast cells can be a useful biomarker for predicting poor survival of patients with CRC (97).
Biliary and hepatocellular carcinomas
Cholangiocarcinoma
Cholangiocarcinomas manifest from the epithelia of the bile ducts of the liver and also originate around the bile ducts (23,98-101). Although cholangiocarcinoma is an uncommon diagnosis in the United States, it tends to be a deadly cancer due to the advanced stage at its first presentation (23,98- 101). The term cholangiocarcinoma has been used to refer to bile duct cancers arising in the intrahepatic, perihilar, or extrahepatic biliary tree, exclusive of gallbladder or ampulla of Vater (102-104). Intrahepatic cholangiocarcinomas can manifest from either small intrahepatic ductules or large intrahepatic ducts proximal to the bifurcation of the right and left hepatic ducts (105-107). Tumors occurring in the proper hepatic duct bifurcation are referred to as Klatskin tumors (108,109). Cholangiocarcinomas are normally asymptomatic until they reach an advanced stage, at which point cholangiocarcinomas present symptoms such as painless jaundice when the tumor obstructs the biliary drainage system, pruritus, right upper quadrant abdominal pain and weight loss (102,110,111). The etiology of cholangiocarcinoma remains poorly understood (104,112).
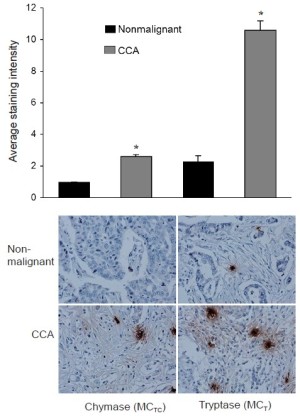
In a study pertaining to the progression of growth of cholangiocarcinoma tumors, Francis et al. studied the endogenous activity of histidine decarboxylase (HDC) which is an enzyme stimulating histamine synthesis (100). It was found that cholangiocarcinoma cells displayed elevated HDC and decreased monoamine oxidase B expression resulting in increased histamine secretion and increased expression of Hl-H4 histamine receptors. Inhibiting HDC and antagonizing HlHR decreased histamine synthesis. In vivo methods using a xenograft tumor model were utilized to measure tumor volume after treatment with histamine or inhibition of histamine synthesis by manipulation of HDC. Treatment with histamine increased proliferation and VEGF expression in cholangiocarcinoma tumors that were blocked by HDC inhibition and the HlHR antagonist (100. In nude mice, histamine increased tumor growth and VEGF expression whereas reducing HDC and inhibiting histamine synthesis ablated the autocrine stimulation of histamine on tumor growth (100). Therefore, a novel concept that an autocrine loop consisting of enhanced histamine synthesis by HDC, which sustains cholangiocarcinoma growth is proposed. While this study has important clinical implications, it did not address the potential role of mast cells in regulating cholangiocarcinoma growth. In unpublished work from Alpini & Francis et al., they demonstrated that cholangiocarcinoma tumors express increased levels of both chymase and tryptase when compared to non-malignant tissue (Figure 2). This suggests that mast cells potentially play a key role as part of the tumor microenvironment of cholangiocarcinoma and ongoing studies in these laboratories is underway to investigate this hypothesis.
Hepatocellular carcinoma (HCC)
HCC is a primary tumor of the liver, generally developing in the setting of chronic liver disease such as chronic Hepatitis B and C (113-116). The tumor grows silently until advanced (113-116). It has become common practice to monitor patients with chronic hepatitis in clinics with the use of imaging studies and lab tests which as a-fetoprotein, which begins to rise as a tumor develops and progresses (113,117-119). As with other GI malignancies mentioned above, there is active research in progress regarding the possible role of mast cells in hepatocellular carcinoma (120-123).
By studying mast cell actions in non-diseased liver tissue compared to that of hepatocellular carcinoma (HCC) neoplasms and intrahepatic cholangiocarcinoma (ICC) neoplasms, Terada and Matsunaga observed MC roles in carcinogenesis of these two diseases (124). Immunohistochemical studies demonstrated that densities of mast cells in HCC and ICC were significantly higher than those observed in non-diseased livers (124). Also, the density of mast cells in ICC was significantly higher than those found in HCC. In another study, the density of sinusoidal mast cells was much higher in HCC than in non-diseased livers (124). With these results, Terada and Matsunaga postulate that mast cell number increases during intrahepatic cholangiocarcinoma and hepatocellular carcinoma and mast cells may also play a role in fibrosis or tumor immunology in HCC and ICC (124).
In the study from Ju et al., the relevance of peritumoral mast cells (MCs) and T regulatory cells to HCC outcomes was studied (122). Tryptase positive MCs and T-regulatory cells (Tregs) were studied using immunohistochemistry enumeration in tissues from 207 HCC patients. The higher peritumoral MCs were associated with poorer clinical outcomes and also related to increased 5-year recurrence incidence of malignancy (122). High density MCs were especially related to increased incidence of early reoccurrence, within 2 years. Also, Tregs were correlated with MCs in density and reversely related to HCC outcomes. Of interest, mast cells in combination with Tregs pointed to better prognostic outcomes than MCs alone (122). MCs were positively correlated to serum alanine aminotransferase, a liver enzyme that is an inflammatory marker for hepatic parenchymal damage. Peritumoral MCs could be used as prognostic parameters for HCC through inflammation response-related mechanisms and MCs and Tregs might cooperate with each other and result in a poorer prognosis (122).
In a study by Grizzi et al., the density of MCs in HCC was studied to determine whether the MC density had any correlation with histologic grading, staging or basic characteristics (121). A tissue staining method was used to quantify the MCs within and around the neoplasm and data statistically analyzed. In this study, it was suggested that MC density did not correlate with the age, gender, or serum alanine aminotransferase or aspartate aminotransferase levels or the stage of grade of the HCC (121). No differences were seen between the MC density of lesions with and without hepatitis C infection but were significantly higher in specimens with marked sinusoidal capillarisation. The conclusion was that based on the above observations, there is no causal relationship between MC recruitment and HCC (121). However, as capillarisation proceeds along with development of the arterial blood supply during hepatocarcinogenesis, MCs may be considered to have an important role in the transition from sinusoidal to capillarytype endothelial cells and growth of the HCC lesions (121).
An interesting study from Lampiasi, et al., examined the role of spontaneous histamine release from mast cell granules on the growth of human HCC cells (123). Two malignant HCC cell lines, HA22T/VGH and HuH-6 with differing characteristics, biological behavior and genetic defects were utilized in the study (123). Total mast cell releasate, exocytosed granules and histamine reduced cell viability in the HuH-6 cells. In the HA22T/VGH cells, granule remnants and histamine induced a weak but significant cell growth increase (123). Both the cells lines expressed H1 and H2 histamine receptors. The selective H1 antagonist terfenadine reverted the histamine-induced inhibition of HuH-6 cell growth whereas the selective H2 antagonist ranitidine inhibited the histamine-induced cell growth of HA22T/VGH cells (123). Histamine downregulated the expression of beta-catenin, COX-2 and survivin in HuH-6 cells. This was associated with caspase-3 activation and PARP cleavage. In contrast, in the HA22T/ VGH cells, expression of survivin and b-catenin increased after being treated with the granule remnants and histamine. The overall results suggested that mediators stored in mast cell granules and histamine may affect the growth of HCC cells. Mast cells and histamine appear to play different roles depending on the tumor cell features (123). It was suggested that histamine and histamine receptor agonists/antagonists might be considered as possible therapeutic drugs to inhibit liver tumor growth (123).
Terada et al., performed a study of human mast cells divided into the tryptase only [MC(T)] and those with both tryptase and chymase inclusions [(MC(TC) ] (124). The authors studied MCs in non-diseased livers, HCC and ICC by double immunostaining and quantitative morphometry and found that in the non-diseased livers, MCs were located to a greater extent in portal tracts and found to a lesser extent in sinusoids (124). In HCC, mast cells were noted in the tumoral sinusoids and in the fibrous septa. In ICC, there were many mast cells present in the tumoral stroma. Morphometry demonstrated that the density of MCs was significantly higher in the cancerous lesions than in the non-diseased livers. Accordingly, it was concluded that MCs increase during carcinogenesis in HCC and ICC and may play a role in fibrosis or tumor immunology in these diseases (124).
Pancreatic tumors
At the present time, pancreatic cancer is the fourth leading cause of cancer death in the United States with an overall five-year survival rate of less than 5% (125-127). It is known that chronic inflammation and a history of pancreatitis are significant risk factors for the development of this disease, increasing the risk 12-18 fold compared to the general population (128-130). Mast cells have been and are being extensively studied for their conducting role of allergic reactions and auto-immunity. In addition, mast cells have been increasingly recognized as crucial components of the tumor stromal microenvironment in a number of human malignancies (131-133). In pancreatic cancer, there has only been one study, which examines the association between mast cells and angiogenesis (133). The authors of this study reported that mast cell infiltration in pancreatic cancer is associated with an angiogenic phenotype but they did not find a correlation with survival and did not evaluate the correlation between mast cell infiltration and other pathological variables such as tumor stage and grade (133). Patients with pancreatic cancer had elevated serum tryptase activity and in vitro, cultured pancreatic cells induced mast cell migration (133). Mast cell conditioned media induced pancreatic cancer cell migration, proliferation and invasion but had no effect on normal ductal cells. In vitro, the interaction between mast cells and pancreatic cancer cells promote tumor growth and invasion (133). The most important finds of this study established that (1) high numbers of tumor infiltrating mast cells are associated with higher grade tumors and decreased survival and (2) that mast cell infiltration was significantly higher in pancreatic cancer compared to normal pancreatic tissue (133). Figure 3 demonstrates the infiltration of mast cells in human pancreatic adenocarcinoma tumors [Reprinted with permission from Clinical Cancer Research, Crosstalk between mast cells and pancreatic cells contributes to pancreatic cancer progression 2010;16:2257-65.].
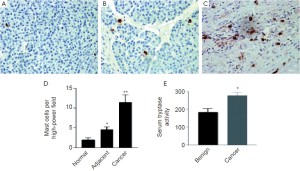
Mast cells in the tumor microenvironment might also be essential for pancreatic ductal adenocarcinoma (PDAC) tumorigenesis (134). Using a spontaneous mouse model of PDAC, the presence of inflammatory cells at various stages of PDAC development was determined. The study shows that in the spontaneous mouse model of PDAC, there was an early influx of mast cells to the tumor microenvironment (134). Although PDAC tumor growth was inhibited in mast cell-deficient mice, aggressive PDAC growth was restored when PDAC cells were injected into mast cell-deficient mice reconstituted with wild-type bone marrow-derived mast cells. The authors predicted that mast cells infiltrating into the tumor microenvironment may be a contributing factor towards PDAC’s poor prognosis, thus making mast cells a potential novel therapeutic target (134).
Concluding remarks
In closing, review of the scientific literature available on MCs presents a puzzling collection of information. While MCs are important components of the immune system, which is essential for the life of humans and other vertebrate organisms, they display characteristics and actions, which are both beneficial as well as harmful. They are essential to fight viruses, bacteria, parasites and other invaders at portals of entry of our bodies. They can be likened to an invading army in allergic reactions and can produce havoc with the release of their granules which cause problems ranging from swelling, hives, itching, running eyes and runny noses all the way to lethal anaphylactic shock. They have been observed to be present in auto-immune inflammatory diseases where they seem to have a detrimental role in increasing swelling, pain and dysfunction. They have further been observed in numerous malignancies and, as we have seen, play roles alone and in cooperation with other cells of the immune system. Their capabilities in angiogenesis, immune suppression, tumor growth, support of tumor metastases, and actions in the tumor microenvironment have been the subject of numerous sophisticated scientific studies. What can be observed from the studies being done in this field is that there are some consistent findings as well as some fragmented findings. Putting all the pieces together will be an exciting challenge to come. It does seem likely, however, that the knowledge existing at present as well as the scientific developments to come on the role of mast cells will contribute significantly to the understanding of the pathogenesis and treatment of human malignancies.
Acknowledgements
Portions of this work was partly supported by the NIH grants DK58411 and DK76898 and by the Scott & White Nicholas C. Hightower Endowed Chair of Gastroenterology (G. Alpini).
Disclosure: The authors declare no conflict of interest.
References
- Silphaduang U, Noga EJ. Peptide antibiotics in mast cells of fish. Nature 2001;414:268-9.
- Boyce JA. The biology of the mast cell. Allergy Asthma Proc 2004;25:27-30.
- Echtenacher B, Mannel DN, Hultner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature 1996;381:75-7.
- Marshall JS, Jawdat DM. Mast cells in innate immunity. J Allergy Clin Immunol 2004;114:21-7.
- Baddeley SM, Bacon AS, McGill JI, et al. Mast cell distribution and neutral protease expression in acute and chronic allergic conjunctivitis. Clin Exp Allergy 1995;25:41-50.
- Cowen T, Trigg P, Eady RA. Distribution of mast cells in human dermis: development of a mapping technique. Br J Dermatol 1979;100:635-40.
- Eady RA, Cowen T, Marshall TF, et al. Mast cell population density, blood vessel density and histamine content in normal human skin. Br J Dermatol 1979;100:623-33.
- Irani AM, Butrus SI, Tabbara KF, et al. Human conjunctival mast cells: distribution of MCT and MCTC in vernal conjunctivitis and giant papillary conjunctivitis. J Allergy Clin Immunol 1990;86:34-40.
- Schulman ES, MacGlashan DW Jr, Peters SP, et al. Human lung mast cells: purification and characterization. J Immunol 1982;129:2662-7.
- Sperr WR, Bankl HC, Mundigler G, et al. The human cardiac mast cell: localization, isolation, phenotype, and functional characterization. Blood 1994;84:3876-84.
- Strobel S, Miller HR, Ferguson A. Human intestinal mucosal mast cells: evaluation of fixation and staining techniques. J Clin Pathol 1981;34:851-8.
- Arinobu Y, Iwasaki H, Gurish MF, et al. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci U S A 2005;102:18105-10.
- Gurish MF, Boyce JA. Mast cells: ontogeny, homing, and recruitment of a unique innate effector cell. J Allergy Clin Immunol 2006;117:1285-91.
- Kirshenbaum AS, Kessler SW, Goff JP, et al. Demonstration of the origin of human mast cells from CD34+ bone marrow progenitor cells. J Immunol 1991;146:1410-5.
- Kirshenbaum AS, Goff JP, Semere T, et al. Demonstration that human mast cells arise from a progenitor cell population that is CD34(+), c-kit(+), and expresses aminopeptidase N (CD13). Blood 1999;94:2333-42.
- Kitamura Y, Oboki K, Ito A. Molecular mechanisms of mast cell development. Immunol Allergy Clin North Am 2006;26:387-405; v.
- Toru H, Ra C, Nonoyama S, et al. Induction of the highaffinity IgE receptor (Fc epsilon RI) on human mast cells by IL-4. Int Immunol 1996;8:1367-73.
- Kunder CA, St John AL, Abraham SN. Mast cell modulation of the vascular and lymphatic endothelium. Blood 2011;118:5383-93.
- Castells M. Mast cell mediators in allergic inflammation and mastocytosis. Immunol Allergy Clin North Am 2006;26:465-85.
- Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol 2010;125:S73-80.
- Francis H, Franchitto A, Ueno Y, et al. H3 histamine receptor agonist inhibits biliary growth of BDL rats by downregulation of the cAMP-dependent PKA/ERK1/2/ ELK-1 pathway. Lab Invest 2007;87:473-87.
- Francis H, Glaser S, Demorrow S, et al. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/ CaMK I/CREB pathway. Am J Physiol Cell Physiol 2008;295:C499-513.
- Onori P, Gaudio E, Franchitto A, et al. Histamine regulation of hyperplastic and neoplastic cell growth in cholangiocytes. World J Gastrointest Pathophysiol 2010;1:38-49.
- Repka-Ramirez MS. New concepts of histamine receptors and actions. Curr Allergy Asthma Rep 2003;3:227-31.
- Vesuna F, Raman V. Histamine: a potential therapeutic agent for breast cancer treatment? Cancer Biol Ther 2006;5:1472-3.
- Bryce PJ, Mathias CB, Harrison KL, et al. The H1 histamine receptor regulates allergic lung responses. J Clin Invest 2006;116:1624-32.
- Leino L, Lilius EM. Histamine receptors on leukocytes are expressed differently in vitro and ex vivo. Int Arch Allergy Appl Immunol 1990;91:30-5.
- Arrang JM, Devaux B, Chodkiewicz JP, et al. H3-receptors control histamine release in human brain. J Neurochem 1988;51:105-8.
- Hofstra CL, Desai PJ, Thurmond RL, et al. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J Pharmacol Exp Ther 2003;305:1212-21.
- Anderson DM, Lyman SD, Baird A, et al. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell 1990;63:235-43.
- Huang E, Nocka K, Beier DR, et al. The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell 1990;63:225-33.
- Lammie A, Drobnjak M, Gerald W, et al. Expression of c-kit and kit ligand proteins in normal human tissues. J Histochem Cytochem 1994;42:1417-25.
- Boyce JA. Mast cells: beyond IgE. J Allergy Clin Immunol 2003;111:24-32; quiz 33.
- Amin K, Lúdvíksdóttir D, Janson C, et al. Inflammation and structural changes in the airways of patients with atopic and nonatopic asthma. BHR Group. Am J Respir Crit Care Med 2000;162:2295-301.
- Fischer M, Harvima IT, Carvalho RF, et al. Mast cell CD30 ligand is upregulated in cutaneous inflammation and mediates degranulation-independent chemokine secretion. J Clin Invest 2006;116:2748-56.
- Church MK, Levi-Schaffer F. The human mast cell. J Allergy Clin Immunol 1997;99:155-60.
- de Paulis A, Marinò I, Ciccarelli A, et al. Human synovial mast cells. I. Ultrastructural in situ and in vitro immunologic characterization. Arthritis Rheum 1996;39:1222-33.
- Fox CC, Lazenby AJ, Moore WC, et al. Enhancement of human intestinal mast cell mediator release in active ulcerative colitis. Gastroenterology 1990;99:119-24.
- Goodfield M, Hull SM, Holland D, et al. Investigations of the ‘active’ edge of plaque psoriasis: vascular proliferation precedes changes in epidermal keratin. Br J Dermatol 1994;131:808-13.
- Gotis-Graham I, McNeil HP. Mast cell responses in rheumatoid synovium. Association of the MCTC subset with matrix turnover and clinical progression. Arthritis Rheum 1997;40:479-89.
- Harvima IT, Naukkarinen A, Paukkonen K, et al. Mast cell tryptase and chymase in developing and mature psoriatic lesions. Arch Dermatol Res 1993;285:184-92.
- Levick SP, Loch DC, Taylor SM, et al. Arachidonic acid metabolism as a potential mediator of cardiac fibrosis associated with inflammation. J Immunol 2007;178:641-6.
- Maurer M, Wedemeyer J, Metz M, et al. Mast cells promote homeostasis by limiting endothelin-1-induced toxicity. Nature 2004;432:512-16.
- Naukkarinen A, Harvima IT, Aalto ML, et al. Mast cell tryptase and chymase are potential regulators of neurogenic inflammation in psoriatic skin. Int J Dermatol 1994;33:361-6.
- Nolte H, Spjeldnaes N, Kruse A, et al. Histamine release from gut mast cells from patients with inflammatory bowel diseases. Gut 1990;31:791-4.
- Hartmann K, Hermes B, Rappersberger K, et al. Evidence for altered mast cell proliferation and apoptosis in cutaneous mastocytosis. Br J Dermatol 2003;149:554-9.
- Janssens AS, Heide R, den Hollander JC, et al. Mast cell distribution in normal adult skin. J Clin Pathol 2005;58:285-9.
- Metcalfe DD. Regulation of normal and neoplastic human mast cell development in mastocytosis. Trans Am Clin Climatol Assoc 2005;116:185-203; discussion 203-4.
- Escribano L, Akin C, Castells M, et al. Current options in the treatment of mast cell mediator-related symptoms in mastocytosis. Inflamm Allergy Drug Targets 2006;5:61-77.
- Hermine O, Lortholary O, Leventhal PS, et al. Casecontrol cohort study of patients’ perceptions of disability in mastocytosis. PLoS One 2008;3:e2266.
- Valent P, Akin C, Sperr WR, et al. Mastocytosis: pathology, genetics, and current options for therapy. Leuk Lymphoma 2005;46:35-48.
- Valent P, Horny HP, Escribano L, et al. Diagnostic criteria and classification of mastocytosis: a consensus proposal. Leuk Res 2001;25:603-25.
- Malaviya R, Ikeda T, Ross E, et al. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature 1996;381:77-80.
- Lawrence CE, Paterson YY, Wright SH, et al. Mouse mast cell protease-1 is required for the enteropathy induced by gastrointestinal helminth infection in the mouse. Gastroenterology 2004;127:155-65.
- Garbuzenko E, Nagler A, Pickholtz D, et al. Human mast cells stimulate fibroblast proliferation, collagen synthesis and lattice contraction: a direct role for mast cells in skin fibrosis. Clin Exp Allergy 2002;32:237-46.
- Hermes B, Feldmann-Böddeker I, Welker P, et al. Altered expression of mast cell chymase and tryptase and of c-Kit in human cutaneous scar tissue. J Invest Dermatol 2000;114:51-5.
- Noli C, Miolo A. The mast cell in wound healing. Vet Dermatol 2001;12:303-13.
- Garfield RE, Irani AM, Schwartz LB, et al. Structural and functional comparison of mast cells in the pregnant versus nonpregnant human uterus. Am J Obstet Gynecol 2006;194:261-7.
- Hunt JE, Friend DS, Gurish MF, et al. Mouse mast cell protease 9, a novel member of the chromosome 14 family of serine proteases that is selectively expressed in uterine mast cells. J Biol Chem 1997;272:29158-66.
- Lu LF, Lind EF, Gondek DC, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature 2006;442:997-1002.
- Crimi E, Chiaramondia M, Milanese M, et al. Increased numbers of mast cells in bronchial mucosa after the latephase asthmatic response to allergen. Am Rev Respir Dis 1991;144:1282-6.
- Mihm MC Jr, Soter NA, Dvorak HF, et al. The structure of normal skin and the morphology of atopic eczema. J Invest Dermatol 1976;67:305-12.
- Williams CM, Galli SJ. The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. J Allergy Clin Immunol 2000;105:847-59.
- Brockow K, Jofer C, Behrendt H, et al. Anaphylaxis in patients with mastocytosis: a study on history, clinical features and risk factors in 120 patients. Allergy 2008;63:226-32.
- Karasuyama H, Tsujimura Y, Obata K, et al. Role for basophils in systemic anaphylaxis. Chem Immunol Allergy 2010;95:85-97.
- Nauta A, Knippels L, Garssen J, et al. Animal models of anaphylaxis. Curr Opin Allergy Clin Immunol 2007;7:355-9.
- Akin C, Metcalfe DD. Systemic mastocytosis. Annu Rev Med 2004;55:419-32.
- Ma Z, Jiao Z. Mast cells as targets of pimecrolimus. Curr Pharm Des 2011;17:3823-9.
- Jeziorska M, McCollum C, Woolley DE. Mast cell distribution, activation, and phenotype in atherosclerotic lesions of human carotid arteries. J Pathol 1997;182:115-22.
- Lee-Rueckert M, Kovanen PT. Mast cell proteases: physiological tools to study functional significance of high density lipoproteins in the initiation of reverse cholesterol transport. Atherosclerosis 2006;189:8-18.
- Sun J, Sukhova GK, Wolters PJ, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med 2007;13:719-24.
- Lee DM, Friend DS, Gurish MF, et al. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science 2002;297:1689-92.
- Secor VH, Secor WE, Gutekunst CA, et al. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med 2000;191:813-22.
- Lawler J. Introduction to the tumour microenvironment review series. J Cell Mol Med 2009;13:1403-4.
- Leonardi GC, Candido S, Cervello M, et al. The tumor microenvironment in hepatocellular carcinoma (review). Int J Oncol 2012;40:1733-47.
- Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem 2007;101:805-15.
- Mbeunkui F, Johann DJ Jr. Cancer and the tumor microenvironment: a review of an essential relationship. Cancer Chemother Pharmacol 2009;63:571-82.
- Witz IP. The tumor microenvironment: the making of a paradigm. Cancer Microenviron 2009;2:9-17.
- Dalton DK, Noelle RJ. The roles of mast cells in anticancer immunity. Cancer Immunol Immunother 2012. [Epub ahead of print].
- Mukherjee S, Bandyopadhyay G, Dutta C, et al. Evaluation of endoscopic biopsy in gastric lesions with a special reference to the significance of mast cell density. Indian J Pathol Microbiol 2009;52:20-4.
- Ribatti D, Guidolin D, Marzullo A, et al. Mast cells and angiogenesis in gastric carcinoma. Int J Exp Pathol 2010;91:350-6.
- Sinnamon MJ, Carter KJ, Sims LP, et al. A protective role of mast cells in intestinal tumorigenesis. Carcinogenesis 2008;29:880-6.
- Tinge B, Molin D, Bergqvist M, et al. Mast cells in squamous cell esophageal carcinoma and clinical parameters. Cancer Genomics Proteomics 2010;7:25-9.
- Horton KM, Abrams RA, Fishman EK. Spiral CT of colon cancer: imaging features and role in management. Radiographics 2000;20:419-30.
- Miura K, Karasawa H, Sasaki I. cIAP2 as a therapeutic target in colorectal cancer and other malignancies. Expert Opin Ther Targets 2009;13:1333-45.
- Zitt M, Zitt M, Müller HM. DNA methylation in colorectal cancer--impact on screening and therapy monitoring modalities? Dis Markers 2007;23:51-71.
- Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol 2004;287:G7-17.
- Terzić J, Grivennikov S, Karin E, et al. Inflammation and colon cancer. Gastroenterology 2010;138:2101-14.e5.
- Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res 2009;29:2727-37.
- Heijmans J, Büller NV, Muncan V, et al. Role of mast cells in colorectal cancer development, the jury is still out. Biochim Biophys Acta 2012;1822:9-13.
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol 2002;29:15-8.
- Jain RK. Tumor angiogenesis and accessibility: role of vascular endothelial growth factor. Semin Oncol 2002;29:3-9.
- Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med 1998;49:407-24.
- Yodavudh S, Tangjitgamol S, Puangsa-art S. Prognostic significance of microvessel density and mast cell density for the survival of Thai patients with primary colorectal cancer. J Med Assoc Thai 2008;91:723-32.
- Gounaris E, Erdman SE, Restaino C, et al. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci U S A 2007;104:19977-82.
- Xia Q, Wu XJ, Zhou Q, et al. No relationship between the distribution of mast cells and the survival of stage IIIB colon cancer patients. J Transl Med 2011;9:88.
- Wu X, Zou Y, He X, et al. Tumor-Infiltrating Mast Cells in Colorectal Cancer as a Poor Prognostic Factor. Int J Surg Pathol 2012. [Epub ahead of print].
- Blechacz BR, Gores GJ. Cholangiocarcinoma. Clin Liver Dis 2008;12:131-50, ix.
- DeMorrow S, Onori P, Venter J, et al. Neuropeptide Y inhibits cholangiocarcinoma cell growth and invasion. Am J Physiol Cell Physiol 2011;300:C1078-89.
- Francis H, DeMorrow S, Venter J, et al. Inhibition of histidine decarboxylase ablates the autocrine tumorigenic effects of histamine in human cholangiocarcinoma. Gut 2012;61:753-64.
- Meng F, Han Y, Staloch D, et al. The H4 histamine receptor agonist, clobenpropit, suppresses human cholangiocarcinoma progression by disruption of epithelial mesenchymal transition and tumor metastasis. Hepatology 2011;54:1718-28.
- Blechacz B, Komuta M, Roskams T, et al. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2011;8:512-22.
- Deoliveira ML, Schulick RD, Nimura Y, et al. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology 2011;53:1363-71.
- Jarnagin W, Winston C. Hilar cholangiocarcinoma: diagnosis and staging. HPB (Oxford) 2005;7:244-51.
- Callea F, Sergi C, Fabbretti G, et al. Precancerous lesions of the biliary tree. J Surg Oncol Suppl 1993;3:131-3.
- Choi BI, Han JK, Hong ST, et al. Clonorchiasis and cholangiocarcinoma: etiologic relationship and imaging diagnosis. Clin Microbiol Rev 2004;17:540-52, table of contents.
- Soyer P, Bluemke DA, Reichle R, et al. Imaging of intrahepatic cholangiocarcinoma: 1. Peripheral cholangiocarcinoma. AJR Am J Roentgenol 1995;165:1427-31.
- Childs T, Hart M. Aggressive surgical therapy for Klatskin tumors. Am J Surg 1993;165:554-7.
- van Gulik TM, Gouma DJ. Changing perspectives in the assessment of resectability of hilar cholangiocarcinoma. Ann Surg Oncol 2007;14:1969-71.
- Kosar I, Ataseven H, Yönem O, et al. A new variant of bile duct duplication with coexistence of distal cholangiocarcinoma. Nat Rev Gastroenterol Hepatol 2010;7:527-30.
- Yusoff AR, Siti ZM, Muzammil AR, et al. Cholangiocarcinoma: a 10-year experience of a single tertiary centre in the multi ethnicity-Malaysia. Med J Malaysia 2012;67:45-51.
- Sirica AE. Cholangiocarcinoma: molecular targeting strategies for chemoprevention and therapy. Hepatology 2005;41:5-15.
- Chiappini F. Circulating tumor cells measurements in hepatocellular carcinoma. Int J Hepatol 2012;2012:684802.
- Hussain K, El-Serag HB. Epidemiology, screening, diagnosis and treatment of hepatocellular carcinoma. Minerva Gastroenterol Dietol 2009;55:123-38.
- Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet 2003;362:1907-17.
- Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int 2009;29:502-10.
- Kienle P, Weitz J, Klaes R, et al. Detection of isolated disseminated tumor cells in bone marrow and blood samples of patients with hepatocellular carcinoma. Arch Surg 2000;135:213-8.
- Komeda T, Fukuda Y, Sando T, et al. Sensitive detection of circulating hepatocellular carcinoma cells in peripheral venous blood. Cancer 1995;75:2214-19.
- Lemoine A, Le Bricon T, Salvucci M, et al. Prospective evaluation of circulating hepatocytes by alpha-fetoprotein mRNA in humans during liver surgery. Ann Surg 1997;226:43-50.
- Cervello M, Foderàa D, Florena AM, et al. Correlation between expression of cyclooxygenase-2 and the presence of inflammatory cells in human primary hepatocellular carcinoma: possible role in tumor promotion and angiogenesis. World J Gastroenterol 2005;11:4638-43.
- Grizzi F, Franceschini B, Chiriva-Internati M, et al. Mast cells and human hepatocellular carcinoma. World J Gastroenterol 2003;9:1469-73.
- Ju MJ, Qiu SJ, Gao Q, et al. Combination of peritumoral mast cells and T-regulatory cells predicts prognosis of hepatocellular carcinoma. Cancer science 2009;100:1267-74.
- Lampiasi N, Azzolina A, Montalto G, et al. Histamine and spontaneously released mast cell granules affect the cell growth of human hepatocellular carcinoma cells. Exp Mol Med 2007;39:284-94.
- Terada T, Matsunaga Y. Increased mast cells in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Hepatol 2000;33:961-6.
- Kelley RK, Ko AH. Erlotinib in the treatment of advanced pancreatic cancer. Biologics 2008;2:83-95.
- Riall TS, Lillemoe KD. Underutilization of surgical resection in patients with localized pancreatic cancer. Ann Surg 2007;246:181-2.
- Zhang L, Jamaluddin MS, Weakley SM, et al. Roles and mechanisms of microRNAs in pancreatic cancer. World J Surg 2011;35:1725-31.
- Algül H, Treiber M, Lesina M, et al. Mechanisms of disease: chronic inflammation and cancer in the pancreas- -a potential role for pancreatic stellate cells? Nat Clin Pract Gastroenterol Hepatol 2007;4:454-62.
- Farrow B, Evers BM. Inflammation and the development of pancreatic cancer. Surg Oncol 2002;10:153-69.
- Farrow B, Sugiyama Y, Chen A, et al. Inflammatory mechanisms contributing to pancreatic cancer development. Ann Surg 2004;239:763-9; discussion 769-71.
- Lohela M, Werb Z. Intravital imaging of stromal cell dynamics in tumors. Curr Opin Genet Dev 2010;20:72-8.
- Srikrishna G, Freeze HH. Endogenous damageassociated molecular pattern molecules at the crossroads of inflammation and cancer. Neoplasia 2009;11:615-28.
- Strouch MJ, Cheon EC, Salabat MR, et al. Crosstalk between mast cells and pancreatic cancer cells contributes to pancreatic tumor progression. Clin Cancer Res 2010;16:2257-65.
- Chang DZ, Ma Y, Ji B, et al. Mast cells in tumor microenvironment promotes the in vivo growth of pancreatic ductal adenocarcinoma. Clin Cancer Res 2011;17:7015-23.


