Updating advances and controversies on the multidisciplinary therapy of gastric cancer
Abstract
Gastric cancer is one of the most common malignancies. In recent years, the overall treatment modality of gastric cancer has been an integrated mode that combines standardized surgery and perioperative adjuvant therapies based on anatomy, tumor biology and immunology. Reasonable staging of gastric cancer is of great significance in guiding the choice of integrated treatment programs, determination of the efficacy and prognosis. Despite more detailed and accurate staging for gastric cancer and prognosis identification in the updated staging system, more are left to be solved. At the same time, the role of free intraperitoneal cytology and laparoscopy in staging should also be given adequate attention. In terms of operations, after years of extensive debate and exploration, D2 lymph node dissection involving nodes around named branches of the celiac trunk has been considered as a standard treatment. While the development of perioperative adjuvant treatment has substantially improved the outcomes of advanced gastric cancer, altered treatment strategies have also brought new challenges. In recent years, the rapidly developed new treatment technologies and targeted therapy combined with traditional chemotherapy provide a new opportunity for breaking through the existing bottleneck in this field.
Key words
Gastric cancer; multidisciplinary therapy; advances; controversies
Introduction
Gastric cancer is one of the most common malignant tumors. Data show a global annual increase of about 934,000 gastric cancer patients and about 734,000 deaths worldwide, with 56% from China and Japan (1). Although surgery is still the primary treatment option for gastric cancer, the treatment model has undergone significant changes: the previously used simple gastrectomy has been replaced by radical approaches aiming at lymph node dissection; and anatomy-based operations are giving their place to an integrated mode that combines standardized surgery and perioperative adjuvant therapies based on anatomy, tumor biology and immunology. This article summarizes the latest research advances and clinical significance of the multidisciplinary management of gastric cancer in recent years as follows.
Staging of gastric cancer
Reasonable staging is the first step in the multidisciplinary management of gastric cancer, a significant link for the choice of treatment programs and determination of the efficacy and prognosis. Since its first edition in 1977, the TNM staging system has been used as a basis for the clinical staging of gastric cancer and a standard staging method in each update of the clinical diagnosis and treatment guidelines of gastric cancer. On January 1, 2010, the American Joint Committee on Cancer (AJCC) and the International Union for Cancer Control (UICC) promulgated the 7th edition of TNM staging (2), including a new set of TNM staging criteria for gastric cancer. Compared with the 6th edition of TNM staging in 2003, the new system includes major adjustments regarding the identification of tumor invasion, lymph node metastasis and other aspects of gastric cancer.
These include:
(I) T stage: (i) The original T1 is divided into T1a (tumor invasion confined to the mucosa) and T1b (invasion of the submucosa); (ii) the original T2 is divided into T2 (tumor invasion of the muscle) and T3 (invasion of serosal connective tissues); and (iii) the original T3 and T4 are respectively changed to T4a [tumor invasion through the serosa (visceral peritoneum) but no invasion of adjacent structures], andT4b (tumor invasion of adjacent structures).
(II) N stage: Using a cutoff of three metastatic nodes, the original N1 was divided into N1 (metastasis of 1-2 regional lymph nodes) and N2 (3-6 regional lymph node); and The original N2 and N3 are combined as N3 (metastasis of 7 or more regional lymph nodes).
(III) M stage: Mx (distant metastasis unassessable) is removed.
The new staging system was subject to academic verification from different angles after its release. Qiu et al. (3) conducted a retrospective analysis of 1,000 patients with gastric cancer, and found that the 7th version was not as efficient as the 6th version in predicting the 5-year survival. Ahn et al. (4) compared the two staging criteria in 9,998 cases of gastric cancer, however, suggested that the new one better reflected the difference in survival between patient groups. Nevertheless, these changes signify more active and meticulous treatment strategies for gastric cancer patients with regional lymph node metastasis as developed by the international academic community, which is consistent with China’s past experience in this regard. In this revision, however, the original IV stage regarding non-distant metastases has been moved forward. Whether this adjustment is reasonable remains subject to further discussion, and the relevant verification and analysis is underway. Moreover, in light of the lacking of sufficient data on individualized treatment, the modification of treatment strategies in line with the updated staging also needs to be further studied.
In Japan, anatomic classification of lymph nodes based on the location of primary lesions has been used to determine the degree of metastasis (N1-N3, M1) and staging and define the corresponding dissection scope (D1-D3) until the provision of the 13th edition. However, in view of the complexity and lacking of objective identification of the location of primary lesions and metastatic lymph nodes, these staging criteria have not been accepted by nononcologists as well as investigators in other countries. Meanwhile, a growing number of studies have shown that classification based on the number of metastases is a better indicator of prognosis than the anatomic one. Therefore, the anatomic N stage staging has been abolished and replaced by the lymph node-based methodology in the new Japanese guidelines and management protocols. The current revision fully reflects the general applicability and objectivity of tumor staging valued by both Eastern and Western scholars.
At present, the primary means for diagnosing gastric cancer include endoscopy, endoscopic ultrasound, CT, PET-CT and MRI, where pathological diagnosis is still the gold standard. Difficulty in determining the depth of invasion and lacking the ability to identify metastases to lymph nodes and distant tissues make traditional endoscopy only a qualitative diagnostic tool, which can not be used for staging. Endoscopic ultrasound has an accuracy up to 80.3% in preoperative staging of gastric cancer, and has particularly great clinical significance in determining levels of tumor invasion. CT and MRI have a higher sensitivity for lymph node metastasis and distant metastasis. In addition, preoperative diagnostic laparoscopy enables accurate observation of the location and extent of the primary tumor, lymph nodes, peritoneal metastasis and invasion of adjacent tissues, and is thereby gaining more and more attention in recent years. Muntean et al. (5) conducted staging laparoscopy (SL) for 45 patients with gastric cancer and found that the tool had an overall sensitivity of 89%, specificity of 100% and diagnostic accuracy of 95.5%. It also showed 54.5% sensitivity, 100% specificity and 64.3% accuracy for lymph node metastases. On the other hand, PET/CT has been more and more valued in the assessment of resectable gastric cancer. Hur et al. (6) suggested in a study that a higher 18FDG uptake of the primary tumor and regional lymph nodes may indicate a higher degree of local progression and lower chance for radical treatment, hence reducing the possibility of a simple laparotomy.
Treatment options for early gastric cancer
The Japan Gastrointestinal Endoscopy Society first introduced the concept of early gastric cancer (EGC) in 1962 (7). EGC is confined to the intramucosal leision, regardless of its size or lymph node metastasis. It is generally believed that lymph node metastasis may occur even in early gastric cancer, and thus D2 resection has been regarded as the standard surgery for early gastric cancer. With deepened studies on the molecular biology and clinical pathology of EGC and gradual understanding of the pattern and biological behavior of lymph node metastasis, the treatment model has undergone great changes. Surgeries with narrowed scope of gastrectomy and lymph node dissection are introduced, including endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), laparoscopic wedge resection (LWR), intragastric mucosal resection, (IGMR), laparoscopicassisted radical gastrectomy and other surgical procedures. Many long-term follow-up results show that with appropriate surgical indications, minimally invasive surgery has benefits of less postoperative pain, faster recovery of gastrointestinal function and less blood loss without increasing postoperative recurrence of cancer.
Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD)
The currently accepted indications for EMR treatment of EGC include visible mucosal carcinoma (cT1a) with a size <2 cm, differentiated histological type and no formed ulcers. Studies have confirmed that lymph node metastasis is rare among cases with those indications. If pathological results confirm invasion of the superficial submucosa without involvement of vessels, gastrectomy or close follow-up may be applied. If SM1 is invaded with vascular and lymphatic involvement or infiltration of deep submucosal SM2, D2 gastric resection should be added. Introduced since 2000, ESD has the following advantages compared with EMR: (I) Resection with controllable scope and size, enabling complete removal of even large tumors; and (II) Ulcer lesions are no longer a contraindication for ESD. Therefore, ESD can achieve complete removal of larger even ulcer lesions. EMR or ESD currently facing the biggest problem is how to improve the accuracy of preoperative staging.
Laparoscopic assisted gastrectomy
In recent years, Japanese scholars put forward that laparoscopic gastrectomy is suitable for about 20% of candidates of gastric cancer surgery (8,9). So far, however, randomized controlled trials with a large sample comparing laparoscopic (assisted) surgery and open surgery are still lacking, and there are only a few small-scale controlled trials available (10,11). No high-level evidence was derived from these results to demonstrate the superiority of laparoscopic surgery, as a minimally invasive treatment, in the intraoperative bleeding volume, respiratory dysfunction, narcotic dosage, and length of hospital stay or other indicators (12). Hence, laparoscopic surgery is still considered only for IA, IB patients and as an experimental option. The recommendation grade of laparoscopic surgery for gastric cancer is merely “C” in the Japan Society of Laparoscopic Surgery Clinical Guidelines. Therefore, although it is technically feasible to perform laparoscopic surgery for a strict selection of gastric patients to achieve as effective D2 resection as open surgery, this modality needs to be further explored due to the lacking of clinical trial results with a large sample and evidence-based design.
Function-preserving minimally invasive surgery
This mainly includes the following types: (I) Laparoscopic assisted vagus-preserving radical surgery; (II) Pyloruspreserving gastrectomy (PPG); (III) Laparoscopic assisted vagus sparing segemental gastrectomy (LAVSSG). These approaches improve the quality of life by preserving the hepatic and celiac branches of the pyloric vagus and thus effectively improving postoperative digestive function and reducing the incidence of gallstones (13) and diarrhea. However, due to overlapping indications with endoscopic surgery, it is not commonly used in conventional therapy. Careful consideration should be given to older patients and those with poor body conditions. However, since functionpreserving local excision provides better quality of life (14) after operation, renewed assessment may be possible as diagnostic techniques (such as sentinel lymph node detection technology) advance and standard options change.
Multidisciplinary management of advanced gastric cancer
Surgical treatment
The long-term survival in patients with advanced gastric cancer is less than 30%. Surgery has dominated in the combined treatment for long. Two preliminary consensuses are present in gastric cancer surgery: Surgery alone can not provide biologically radical treatment even with extended resection and lymph node dissection; and palliative resection enables better outcomes in patients without distant metastasis than those untreated. For advanced gastric cancer, a commonly accepted practice is standard surgery for the purpose of radical resection, which requires removal of 2/3 or more of the stomach and D2 lymph node dissection to ensure R0 resection of the primary tumor (distance between gross margin and the original lesion >5 cm and microscopic negative margins). Correspondingly, non-standard operations may also be available with varying resection and dissection extents based on disease progression.
Scope of lymph node dissection
The scope of lymph node dissection has been a highly controversial topic in studies of gastric cancer. Most investigators from Japan, China, Korea and some from Europe and the US suggest extended lymph node dissection (ELND), which is advised against by most European and American investigators. In recent years, however, they have accepted most of the Asian opinions with the release of a series of large-scale randomized controlled trial results. A retrospective analysis of the data of 1,377 patients undergoing gastric cancer resection from the US-SEER database showed that the longest survival period of advanced patients was among those with 15 or more N2 lymph nodes or 20 or more N3 lymph nodes (15). A 15-year follow-up of the Dutch study also revealed increased survival after D2 dissection. A further analysis of the cause of death pointed out that the mortality related to gastric cancer after D2 operation was obviously lower than those undergoing D1 dissection (37% versus 48%, P=0.01), whereas higher perioperative mortality as a result of the combined splenectomy or pancreatectomy was a major cause of bias in the study (16). The Italian gastric cancer study group reported the results of pancreas-preserving D2 dissection, which confirmed that the perioperative morbidity and mortality of D2 was comparable to D1 surgery (17). Australia and Spainish studies also demonstrated that D2 surgery improved patients’ quality of life without increasing their risk of perioperative mortality (18,19). Enzinger et al. conducted a subgroup analysis of the highly controversial INT0116, showing that D1 or D2 surgery tended to improve survival in centers with a relatively large number of gastric cancer patients (20).
Therefore, starting from the 2010 version, NCCN guidelines for surgical treatment of gastric cancer have particularly provided that “modified” D2 surgery (not combined with pancreatectomy or splenectomy) performed by experienced surgeons in larger-scale cancer centers could actually provide lower mortality and better survival benefits. Hence, “radical surgery for gastric cancer should be completed by experienced surgeons in a large cancer center, which should include dissection of regional lymph nodes-perigastric lymph nodes (D1) and lymph nodes along the named vessels accompanying the celiac trunk for the purpose of examining at least 15 or more lymph nodes”. D2 lymph node dissection involving nodes around named branches of the celiac trunk has been considered as a standard treatment.
Extensive surgery
Extended radical resection is performed for primary gastric cancer or metastases that directly invade perigastric organs (T4) or those with lymph node metastasis of N2 where radical resection is still avaliable (Table 1). This includes: Extensive resection combined with removal of other organs; and D2 or above level lymph node dissection, such as surgeries targeted at IIIa, IIIb and some IV lesions involving the number 16 lymph nodes.
| Table 1 Randomised trials comparing the extent of lymphadenectomy | |||||||||||||||
| Arm | N | Morbidit |
Mortalit |
5-year Surviva l(%) | 10-year Survival (%) | 15-year Survival (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cuschieri et al. (1999) (21) | D1 | 200 | 28 | 6.5 | 35 | - | - | ||||||||
| D2 | 200 | 46 | 13 | 33 | - | - | |||||||||
| Bonenkamp et al. (1995) (22); Hartgrink et al. (2004) (23); Songun et al. (2010) (16) | D1 | 380 | 25 | 4 | 45 | 30 | 21 | ||||||||
| D2 | 331 | 43 | 10 | 47 | 35 | 29 | |||||||||
| Degiuli et al. (2004) (24) | D1 | 76 | 10.5 | 1.3 | - | - | - | ||||||||
| D2 | 86 | 16.3 | 0 | - | - | - | |||||||||
| Wu et al. (2006) (25) | D1 | 110 | 7.3 | 0 | 53.6 | - | - | ||||||||
| D3 | 111 | 17.1 | 0 | 59.5 | - | - | |||||||||
| Sasako et al. (2008) (26) | D2 | 263 | 20.9 | 0.8 | 69.2 | - | - | ||||||||
| D2+PAND | 260 | 28.1 | 0.8 | 70.3 | - | - | |||||||||
(I) Extended resection combined with removal of the pancreas and the spleen
Since dissection of numbers 10 and 11 lymph nodes is required for D2 dissection in upper gastric cancer, some investigators suggested combined resection of the left pancreas, the splenic artery and vein and the spleen. However, this brought to a high incidence of severe postoperative complications such as pancreatic fistula, intra-abdominal infections and diabetes. Wang et al. (27) randomly assigned 84 patients with advanced gastric cancer to receive pancreas-preserving radical resection (38 cases) and combined pancreatic resection (46 cases). As a result, postoperative complication rates were 23.7% and 52.2%; respectively, while the postoperative 5-year survival rates were the opposite -- 42.4% and 35.6%, suggesting that routine combined resection of the head and tail of the pancreas should be avoid in upper and medium advanced gastric cancer. Therefore, combined pancreatectomy is often not recommended when the lesion is not invading this organ and only metastasis of the lymph nodes around the splenic artery or splenic hilum is suspected. Left pancreatic resection combined with splenectomy is only indicated for patients whose gastric cancer has directly invaded the pancreas.
For advanced gastric cancer of the upper stomach, it has been controversial as to whether splenectomy should be combined for complete dissection of numbers 10 and 11d lymph nodes. In particular, European and American investigators have regarded this combination as a high-risk modality. Recent studies have found that the occurrence of splenic lymph node metastasis is mostly associated with gastric cancer at the cardia area, with an incidence of 9.8-14%, and is mainly observed in advanced tumors that have invaded into or beyond the serosa (T4). Since direct violation of the spleen is clinically rare, prophylactic splenectomy does not provide better outcome for the treatment of gastric cancer than spleenpreserving approaches and it is therefore not routinely advised. A number of clinical trials, including the (28) Japanese JCOG0110, are underway to explore this practice. Nonetheless, the preliminary consensus for now is that splenectomy should be performed as long as the spleen is directly invaded by IIIb and IV gastric cancer at the cardia or greater curvature, or circulation metastasis and splenic lymph node metastasis is present.
In short, for gastric cancer of the upper and medium part of stomach that invades the tail and head of pancreas, total gastrectomy should be combined with spleen and pancreatic resection; in the case of metastasis of the numbers 10 and 11 lymph nodes, combined splenectomy should be considered. Prophylactic splenectomy should not be performed when there is no metastasis to numbers 10 and 11 lymph nodes.
(II) Lymph node dissection at the level of D2 or above
The significance of extended dissection is unclear. The significance of prophylactic number 16 lymph node dissection has been denied by a Japanese randomized controlled trial (JCOG9501) (26). For metastasis to the number 16 lymph nodes without other non-radical curable factors, although R0 could be achieved by D2+No. 16 dissection, the outcomes remain poor. Whether D2 or D2+No.16 should be the choice following downstaging by preoperative chemotherapy is still under study.
Perioperative treatment
Perioperative chemotherapy
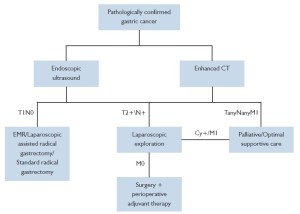
Changes in the trend of managing solid tumors such as breast cancer and lung cancer have in large part triggered a revolution in the field of tumor treatment. It is recognized that tumor is a systemic disease even in the early stages, which entails systemic management such as chemotherapy. Tumor recurrence and metastasis are associated with not only the completion of surgical resection and lymph node dissection, but also the presence of micrometastases and its further growth and proliferation, which play a more important role. For a long time, attempts have been made with adjuvant chemotherapy to control relapse and metastasis, though no satisfying, definite results have been produced. Adjuvant chemotherapy after the resection of primary lesions does not achieve individualized effects even applied according to the specific staging. Therefore, the concept of preoperative adjuvant therapy (also known as neoadjuvant therapy) has been introduced based on the experience of adjuvant therapy, which includes neoadjuvant chemotherapy, neoadjuvant radiotherapy and neoadjuvant chemotherapy. The introduction and application of preoperative neoadjuvant treatment has been a challenge of the new century to both cancer surgeons and physicians (Figure 1).
The most representative clinical trial regarding perioperative chemotherapy is the UK MAGIC study (29). In the study, three cycles of epirubicin combined with cisplatin and 5-FU (ECF regimen) chemotherapy were given respectively before and after surgery, and were well tolerated in the 86% patients who completed the preoperative chemotherapy. In the combination therapy group, 229 patients (92%) received surgical exploration, of which 69% received radical surgery, while only 66% patients received radical treatment in the surgery alone group. There was no significant difference in the postoperative mortality and surgically related mortality between the two groups. Pathological tumor size was used to evaluate the efficacy of treatment, and the results showed a significantly lower value in the combination therapy group than in the surgery alone group (P<0.001). The disease-free survival and 5-year survival rate in the combined treatment group were significantly prolonged, with a 25% decrease in the risk of recurrence and metastasis (HR=0.75, P=0.009). The results suggested that perioperative chemotherapy might improve long-term survival in patients with advanced gastric cancer, where neoadjuvant chemotherapy could downstage the T, N staging of locally advanced gastric cancer and improve the surgical cure rate.
Currently accepted principles of neoadjuvant chemotherapy necessitate control of micrometastasis in high-risk groups with locally advanced yet radically resectable cancer. The specific indications include clinical stage II-IIIb (cT3-4, cN1-2) with the use of following regimens: EEP (30), ECF (29), OLF (Oxaliplatin, leucovorin, 5FU) and so on. For cases whose lesions are not radically resectable, the objective will be downstaging III and IV advanced tumors with a larger size and extensive lymph node metastasis. The specific indications include cT3-4, cN2-, M1 (LYM) with the use of following programs: P-ELF (CDDP, etopiside, leucovorin, 5-FU), EAP (etopiside, ADR, CDDP), CPT-11 + CDDP (31), PLF (32), S -1 + CDDP, OLF (Oxaliplatin, leucovorin, 5-FU), DCF (Docetaxel, CDDP, 5-FU) and so on. Phase III clinical trial results have confirmed that radiotherapy is effective against tumors of the gastroesophageal junction (33). In addition, although there are reports that potent chemotherapy may achieve a higher negative conversion rate for patients with positive peritoneal free cells, a high level of clinical evidence is still lacking.
Regarding adjuvant chemotherapy after surgery, the INT0116 (34) study and MAGIC study (29) from the US have respectively proved the effectiveness of postoperative 5-FU/LV combined with radiotherapy and ECF (Epirubicin + CDDP + 5-FU) for preoperative/ postoperative chemotherapy, though neither of them is not as effective as the overall result in the Japanese trial. The latest ACTS-GC trial has (35) confirmed that one-year TS-1 adjuvant chemotherapy after D2 radical treatment for stage II and III gastric cancer is associated with increased survival (71.7% vs. 61.1%, HR=0.669, 95% CI 0.540- 0.828) and decreased risk of recurrence and metastasis by 34.7% (HR=0.653, 95% CI 0.537-0.793). The SPIRITS (36) study compared TS-1 combined with cisplatin and TS-1 single-drug treatment in 305 patients with gastric cancer from 38 centers in Japan. The results showed that the combined treatment group had significantly better overall and progression-free survival than the singleagent S-1 group. Therefore, for the initial treatment of gastric cancer patients with standard chemotherapy, Japanese investigators recommend TS-1 + CDDP36 (36), while the ECF program is still the traditional treatment recommended by western countries. Since 2009, NCCN guidelines have included paclitaxel -based chemotherapy (2B ev idence level ) in sys temic gast r ic cancer chemotherapy and valued sorafenib and other targeted agents in combination with conventional chemotherapy. With the announcement of the ToGA study results (37), the therapeutic value of chemotherapy combined with trastuzumab for HER2-positive advanced gastric cancer patients has been confirmed by investigators from various countries, and this therapy has been included in the standard program for metastatic or locally advanced gastric cancer treatment (2A evidence level). Throughout the recent years, targeted drugs may have been playing a increasingly important role in the non-surgical treatment of gastric cancer, a trend shown in relevant clinical trials.
Perioperative radiotherapy
Preoperative induction chemotherapy followed by chemoradiotherapy can produce significant pathological remission and prolong the survival of gastric cancer patients (Table 2). MacDonald et al. (34) conducted a randomized controlled study (INT0116) on 556 patients undergoing surgery alone or combination of postoperative radiotherapy and chemotherapy (5-FU/LV +45 Gy radiotherapy), which showed that postoperative radiotherapy and chemotherapy was associated with prolonged survival. Since then, the program became the standard treatment in the United States. At present the CALGB80101 study is comparing it with the ECF program. However, in view of the 10-year follow-up results from the INT0116 study, the efficacy was limited in all subgroups except for poorly differentiated adenocarcinoma. The Korean randomized controlled study using capecitabine/cisplatin (XP) as a control group is also in progress.
| Table 2 Randomized trials of surgery only versus surgery combined with chemotherapy or chemoradiotherapy | |||||||||||||||
| Arm | N | RFS | OS | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MacDonald et al. (2001) (34) | Surgery only | 275 | 31% (3-year) | 41% (3-year) | |||||||||||
| CRT | 281 | 48% (3-year) | 50% (3-year) | ||||||||||||
| Cunningham et al.(2006) (29) | Surgery only | 253 | - | 23% (5-year) | |||||||||||
| ECF | 250 | - | 36% (5-year) | ||||||||||||
| Sasako et al.(2011) (35) | Surgery only | 530 | 53.1% (5-year) | 61,1% (5-year) | |||||||||||
| S-1 | 529 | 65.4% (5-year) | 71.7% (5-year) | ||||||||||||
| Boige et al. (2007) (38) | Surgery only | 111 | 21% (5-year) | 24% (5-year) | |||||||||||
| FP | 113 | 34% (5-year) | 38% (5-year) | ||||||||||||
| CRT=postoperative chemoradiotherapy (fluorouracil plus leucovorin followed by 45 Gy radiotherapy); ECF=Three preoperative and three postoperative cycles of epirubicin, cisplatin, and fluorouracil; S-1=cycles of S-1 (orally active combination of tegafur, gimeracil, and oteracil) for 1 year postoperatively; FP=2–3 cycles of preoperative fluorouracil and cisplatin; postoperative FP was recommended for patients with a response or stable disease with pN+ | |||||||||||||||
Intraperitoneal hyperthermic chemotherapy
The postoperative recurrence rate gastric cancer is high and peritoneal recurrence is the most common form with an overall incidence up to 50% for patients with advanced gastric cancer postoperatively. Developed in recent years, the intraperitoneal chemo-hyperthermia (IPCH) is one of the highly valued therapeutic tools, which combines the anti-cancer effects of synergies from regional chemotherapy and hyperthermia. This easy-to-operate technology, showing significant effects both in the prevention and treatment of peritoneal metastasis or postoperative recurrence of advanced gastrointestinal cancer with small toxicity, has become an ideal surgical adjuvant therapy.
Gastric cancer patients with no distant metastasis that involves the liver, lung, brain or bone and no serious organic complication of the heart, lung , liver, kidney and other vital organs, who have had the primary foci cured or palliatively resected and have one of the following conditions, are eligible for IPCH treatment: (I) Positive for intraperitoneal free cancer cells (FCC); (II) Tumor invasion into or beyond the serosa, or peritoneal metastasis; and (III) Postoperative scattered peritoneal recurrence or small or moderate malignant ascites, for whom radical cytoreductive surgery is possible, i.e. surgical removal of as much visible metastases as possible, particularly nodules on the peritoneal surface. Relevant articles have noted that hyperthermic perfusion chemotherapy is only effective on nodules of 3-5 mm. Therefore, to achieve satisfying outcomes, it is recommended to perform IPCH therapy following minimization of the intra-abdominal tumor burden. In summary, the new mode of ‘surgery + perioperative therapy’ has come on the stage of gastric cancer treatment (Figure 2). With the development of medical technology and wide application of more and more novel technologies, evidence-based approaches in combination with the strengths of various treatments will be the key to multidisciplinary management of gastric cancer for ultimately improving the outcomes and quality of life of these patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol 2006;20:633-49.
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Qiu MZ, Wang ZQ, Zhang DS, et al. Comparison of 6th and 7th AJCC TNM staging classification for carcinoma of the stomach in China. Ann Surg Oncol 2011;18:1869-76.
- Ahn HS, Lee HJ, Hahn S, et al. Evaluation of the seventh American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer 2010;116:5592-8.
- Muntean V, Mihailov A, Iancu C, et al. Staging laparoscopy in gastric cancer. Accuracy and impact on therapy. J Gastrointestin Liver Dis 2009;18:189-95.
- Hur H, Kim SH, Kim W, et al. The efficacy of preoperative PET/CT for prediction of curability in surgery for locally advanced gastric carcinoma. World J Surg Oncol 2010;8:86.
- Urabe M, Mizugami T, Yamamoto K, et al. Histogenesis of the so-called early-stage gastric cancer. Rinsho Geka 1962;17:947-63.
- Abe N, Takeuchi H, Ohki A, et al. Long-term outcomes of combination of endoscopic submucosal dissection and laparoscopic lymph node dissection without gastrectomy for early gastric cancer patients who have a potential risk of lymph node metastasis. Gastrointest Endosc 2011;74:792-7.
- Takeuchi H, Oyama T, Kamiya S, et al. Laparoscopyassisted proximal gastrectomy with sentinel node mapping for early gastric cancer. World J Surg 2011;35:2463-71.
- Huscher CG, Mingoli A, Sgarzini G, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg 2005;241:232-7.
- Kitano S, Shiraishi N, Fujii K, et al. A randomized controlled trial comparing open vs laparoscopy-assisted distal gastrectomy for the treatment of early gastric cancer: an interim report. Surgery 2002;131:S306-11.
- Memon MA, Khan S, Yunus RM, et al. Meta-analysis of laparoscopic and open distal gastrectomy for gastric carcinoma. Surg Endosc 2008;22:1781-9.
- Furukawa H, Hiratsuka M, Imaoka S, et al. Phase II study of limited surgery for early gastric cancer: segmental gastric resection. Ann Surg Oncol 1999;6:166-70.
- Seto Y, Yamaguchi H, Shimoyama S, et al. Results of local resection with regional lymphadenectomy for early gastric cancer. Am J Surg 2001;182:498-501.
- Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable gastric cancer of advanced stage. Ann Surg Oncol 2007;14:317-28.
- Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49.
- Degiuli M, Sasako M, Ponti A, et al. Survival results of a multicentre phase II study to evaluate D2 gastrectomy for gastric cancer. Br J Cancer 2004;90:1727-32.
- Jatzko GR, Lisborg PH, Denk H, et al. A 10-year experience with Japanese-type radical lymph node dissection for gastric cancer outside of Japan. Cancer 1995;76:1302-12.
- Sierra A, Regueira FM, Hernández-Lizoáin JL, et al. Role of the extended lymphadenectomy in gastric cancer surgery: experience in a single institution. Ann Surg Oncol 2003;10:219-26.
- Enzinger PC, Benedetti JK, Meyerhardt JA, et al. Impact of hospital volume on recurrence and survival after surgery for gastric cancer. Ann Surg 2007;245:426-34.
- Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Cooperative Group. Br J Cancer 1999;79:1522-30.
- Bonenkamp JJ, Songun I, Hermans J, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet 1995;345:745-8.
- Hartgrink HH, van de Velde CJ, Putter H, et al. Extended lymph node dissection for gastric cancer: who may benefit? Final results of the randomized Dutch gastric cancer group trial. J Clin Oncol 2004;22:2069-77.
- Degiuli M, Sasako M, Calgaro M, et al. Morbidity and mortality after D1 and D2 gastrectomy for cancer: interim analysis of the Italian Gastric Cancer Study Group (IGCSG) randomised surgical trial. Eur J Surg Oncol 2004;30:303-8.
- Wu CW, Hsiung CA, Lo SS, et al. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol 2006;7:309-15.
- Sasako M, Sano T, Yamamoto S, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 2008;359:453-62.
- Wang JY, Huang TJ, Chen FM, et al. A comparative study of pancreatectomy and pancreas-preserving gastrectomy in advanced gastric carcinomas. Hepatogastroenterology 2004;51:1229-32.
- Sano T, Yamamoto S, Sasako M. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma: Japan clinical oncology group study JCOG 0110-MF. Jpn J Clin Oncol 2002;32:363-4.
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20.
- Persiani R, Rausei S, Pozzo C, et al. 7-Year survival results of perioperative chemotherapy with epidoxorubicin, etoposide, and cisplatin (EEP) in locally advanced resectable gastric cancer: up-to-date analysis of a phase-II study. Ann Surg Oncol 2008;15:2146-52.
- Yoshikawa T, Sasako M, Yamamoto S, et al. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg 2009;96:1015-22.
- Ott K, Sendler A, Becker K, et al. Neoadjuvant chemotherapy with cisplatin, 5-FU, and leucovorin (PLF) in locally advanced gastric cancer: a prospective phase II study. Gastric Cancer 2003;6:159-67.
- Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851-6.
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 200;345:725-30.
- Sasako M, Sakuramoto S, Katai H, et al. Five-Year Outcomes of a Randomized Phase III Trial Comparing Adjuvant Chemotherapy With S-1 Versus Surgery Alone in Stage II or III Gastric Cancer. J Clin Oncol 2011;29:4387-93.
- Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 2008;9:215-21.
- Van Cutsem E, Kang Y, Chung H, et al. Efficacy results from the ToGA trial: A phase III study of trastuzumab added to standard chemotherapy (CT) in first-line human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer (GC). J Clin Oncol 2009;27:18s
- Boige V, Pignon J, Saint-Aubert B, et al. Final results of a randomized trial comparing preoperative 5-fluorouracil (F)/cisplatin(P) to surgery alone in adenocarcinoma of the stomach and lower esophagus (ASLE): FNLCC ACCORD07-FFCD 9703 trial. J Clin Oncol 2007;25:4510.