Laparoscopic radical gastrectomy for gastric cancer
Laparoscopy-assisted distal gastrectomy for gastric cancer was first reported by Kitano et al. in 1994, but only for early gastric cancer. In 1997, laparoscopy-assisted D2 radical gastrectomy was performed for the first time by Goh et al. for the treatment of advanced gastric cancer and achieved good short-term efficacy, thus dramatically expanded its indications from early gastric cancer to advanced gastric cancer. Up until 2004, roughly 7,800 gastric cancer patients had received laparoscopy-assisted surgery in Japan. Currently, the role of laparoscopic techniques for early gastric cancer has been widely recognized by surgeons. In China, the low early diagnosis rate of gastric cancer has limited the application of laparoscopic radical operations for gastric carcinoma. However, the development of minimally invasive techniques in recent years has facilitated the application of laparoscope in gastrointestinal surgery, and laparoscopic radical gastrectomy has become a promising procedure for gastric cancer. Compared with the conventional open surgery, this approach offers bigger visual fields and larger local magnifications. However, because the gastrointestinal tract is easy to move, it is relatively hard to dissect the lymph nodes and reconstruct the gastrointestinal tract. Therefore, laparoscopic radical gastrectomy remains the most difficult procedure among laparoscopic gastrointestinal operations and therefore can not be widely applied.
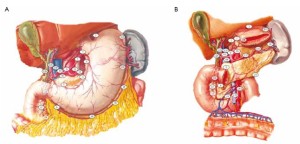
Radical resection for gastric cancer includes the following three aspects: (I) Complete resection of the primary tumors and the adjacent tissues involved by these tumors; (II) standardized dissection of the involved lymph nodes; and (III) clearance of the exfoliated cancer cells in the abdominal cavity. Using the high-intensity focused ultrasound (HIFU), the laparoscopic surgery can easily remove the primary tumors and their surrounding tissues. As the stomach is a hollow organ attached with flaky greater and lesser omentums, the gastric tumors and their adjacent tissues can be lifted outside the body via a 4-6 cm abdominal incision and resected under direct vision. Gastrointestinal tract reconstruction is generally completed in vitro. During laparoscopic surgery, lymph nodes are often dissected en bloc, together with their surrounding connective tissues, in order to maintain the integrity of lymph nodes and their lymphatic vessels and reduce cancer cell exfoliation and seeding. As the perigastric lymphatic drainage patterns generally go in parallel with the main arteries of the stomach and lymph nodes of perigastric group 16 (station 3) are mainly distributed adjacent to the various gastric blood vessels, the contextualized dissection of arteries is equally important in laparoscopic surgery. Laparoscopic surgery also demonstrates mange advantages in effectively eliminating exfoliated cancer cells in the abdominal cavity: wide field of vision, polytropical angles of view, and its effectiveness in flushing the various parts of the abdominal cavity. Laparoscopic surgery is comparable and even superior to the open surgery in terms of the distance between the surgical margin and the reactive zone of the tumor and number of lymph nodes (stations) dissected.
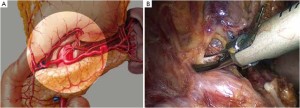
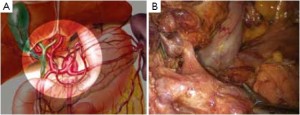
Distribution of gastric lymph nodes
Figure 1 is the distribution of gastric lymph nodes.
Indication
Early and locally advanced gastric antral cancers.
Contraindications
(I) Patients with advanced gastric antral cancer, on whom the metastatic lymph nodes can not be easily dissected.
(III) Patients with a history of upper abdominal surgery and/or extensive upper abdominal adhesion.
Surgical instruments
HIFU Ace’s exambusters, linear cutter stapler, and circular stapler (diameter: 28-31 mm).
Preoperative preparations
(I) Treat anemia and hypoproteinemia, supplement
vitamins, and adjust water and electrolyte balance.
(II) Perform normal saline enema once one night before
surgery.
(III) Indwel the gastric canal and urinary canal in the
morning on the operation day.
Anesthesia
Tracheal intubation under general anesthesia.
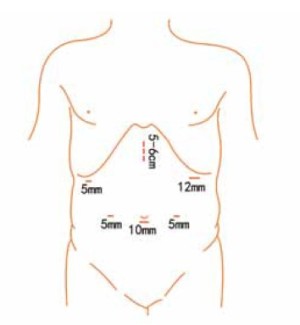
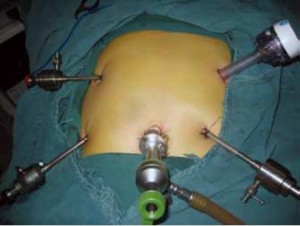
Position and cannula placement
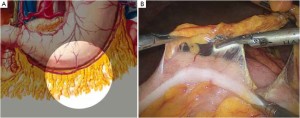
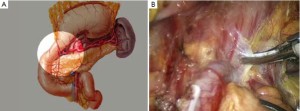
The patient lies on the table in the supine position, with legs apart, or in the modified lithotomy position. A 10-mm cannula is placed at the level of the inferior umbilical margin as the observation hole, and the intra-abdominal pressure is maintained at 13 mmHg. A 12-mm cannula is inserted 1cm to the left anterior axillary line under the costal margin as the main operation hole, and a 5-mm cannula is placed in the left midclavicular line at the level of umbilicus as an adjunct hole. Two 5-mm cannulas are inserted in the symmetrical position on the right side of the abovementioned operation cannulas. The operator stands on the left of the patient, assistant on the right, and scrub nurse between the legs of the patient (Figures 2, 3).
Surgical procedures
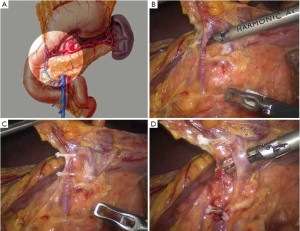
Surgical procedures of laparoscopic radical gastrectomy for gastric antral cancer(Figure 4; Videos 1, 2, 3, 4, 5, 6, 7, 8).
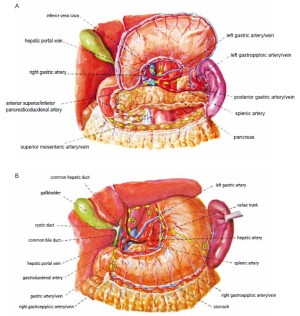
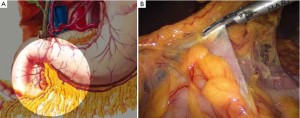
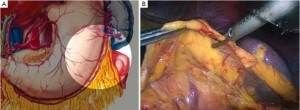
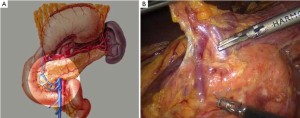
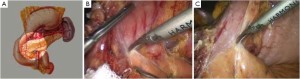
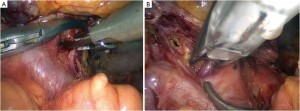
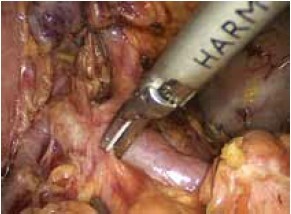
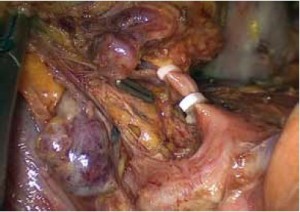
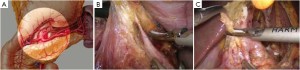
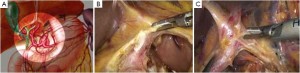
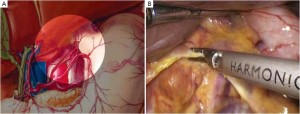
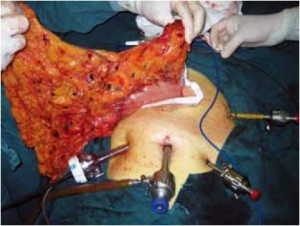
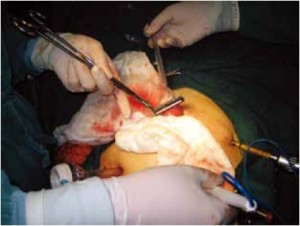
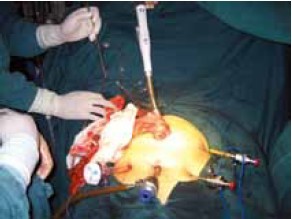
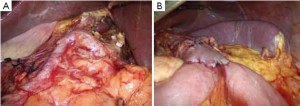
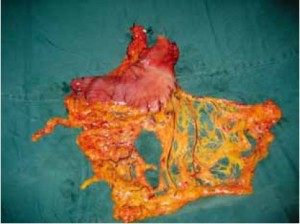
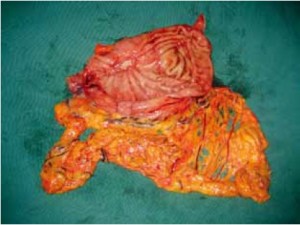
Open the greater omentum to the head side; divide the gastrocolic ligament along the avascular zone at the upper border of the transverse colon (Figure 5), rightward to the hepatic flexure of colon (Figure 6) and leftward to the splenic flexure of colon; ligate and divide left gastroepiploic vessels (Figure 7) and dissociate the greater curvature of stomach to the second vascular branch of the left gastroepiploic artery. Expose the middle colic artery to dissect the anterior lobe of the transverse mesocolon until the lower border of the pancreas. Divide the superior mesenteric vein at the lower border of the pancreas and then dissect group 14v lymph nodes (Figure 8). Divide and expose right gastroepiploic artery and vein along the surface of the head of the pancreas for ligation and transection at the root, and then dissect group 6 lymph nodes (Figure 9). Divide the loose tissue among the duodenum, gastric antrum and pancreas along the root of the right gastroepiploic artery to expose the gastroduodenal artery (Figure 10). Dissect the encapsulated structure that envelops the pancreas from right to left and from bottom to top till the upper border of the pancreas (Figure 11). Peel off the retroperitoneal membrane along the upper border of the pancreas to expose the coronary vein, which is ligated and transected near its basilar part (Figure 12). Expose the common hepatic artery along the upper border of the pancreas (Figure 13) and then divide it along the arterial sheath; meanwhile, dissect the group 8 lymph nodes (Figure 14). Expose the celiac trunk, proximal splenic artery and left gastric artery, and then ligate and transect the left gastric artery at the root; meanwhile, dissect group 7, 9 and 11p lymph nodes (Figure 15, 16, 17). Upward along the gastroduodenal artery, ligate and transect the root of the right gastric artery to dissect group 5 lymph nodes (Figure 18). Open the hepatoduodenal ligament to expose the proper hepatic artery and dissect group 12a lymph nodes (Figure 19). Dissociate the lesser omentum along the lower border of the liver to the right side of the cardia (Figure 20), and then downward along the lesser curvature to 3-4 cm above the tumor to dissect group 1 and 3 lymph nodes (Figure 21). Dissociate the duodenal bulb to 2 cm under the pylorus, and then transect the duodenum using a linear cutter stapler (Figure 22). Lift the transverse colon upward to find the beginning part of the jejunum (Treitz ligament) and mark the jejunum 12 cm to the Treitz ligament with a cloth (Figure 23). Make a 5-6 cm median longitudinal incision on upper abdomen (Figure 24) and then drag the stomach and greater and lesser omentums out from the abdominal cavity after the placement of the incision protective film (Figure 25) and remove the tumor at the scheduled plane. Lift the proximal jejunum out of the abdomen and place the stapler anvil (diameter 28-29 mm) of the circular stapler at the marking site to perform Billroth II (B-II) IIgastrojejunostomy in the posterior surface of stomach via the gastric cavity using the stapler (Figures 26, 27); then, inspect the anastomotic bleeding (if any) and stop bleeding by suture if necessary. Place the gastric canal into the afferent loop of the jejunam via anastomosis from the gastral cavity, and then close the gastral cavity by suturing or using the linear cutter stapler 2 cm outside anastomosis. Gastrointestinal tract reconstruction can also be performed using B-I anastomosis; namely, put the stapler anvil of the circular stapler into the duodenal stump to perform end-to-side anastomosis with the duodenum in the posterior wall of gastric body via the gastric cavity using the stapler. Suture the small incision to reconstruct pneumoperitoneum, and then inspect the abdominal cavity for bleeding or anastomosis (Figure 28). Rinse the surgical wound thoroughly with distilled water. Routinely place the drainage tube at the surgical wound for drainage from the upper left cannula. Exhaust pneumoperitoneum and pull out the cannula to gradually suture the cannula mouth at the level of umbilicus layer by layer to end the procedure. Specimens are shown in figures as follows (Figures 29, 30).
Comments
(I) The scrub nurse must be well prepared during a laparoscopic surgery for gastric cancer due to the wide resection range and long operation interval. When the telescope moves along the operation sites, the The general view of the operative field and the quick detail view should be appropriately navigated. Meanwhile, such navigation should be performed in a smooth way to avoid the seasick sense due to unstable lens. When moving the lens in a large angle, retract the lens to the opening of its casing tube, and then slowly turn it.
(II) The inverted visual fields of local sites should be considered. An inverted visual field refers to the visual angle between laparoscopic view and equipment operation when the operator is standing at the left side of a patient to treat the greater omentum in the region of the splenic flexure. The operator must adapt to this situation and adjust his/her sensations to ensure the accuracy of operation. The assistant standing on the right side of the patient also faces the same problems when treating the hepatic flexure of colon.
(III) Unlike an open operation, the laparoscopic surgery has a visual line virtually horizontal with the anatomical layer of abdominal organs. During the surgery, tissues are often lifted upward to expose the surgical field; therefore, the relationship between local anatomy and visual direction must be properly addressed. During the laparoscopic surgery, when the lower border of the greater gastric curvature is lifted upwards to expose the posterior wall of stomach and the head of the pancreas, divide and skeletonize the right gastroepiploic artery and vein along the surface of the pancreatic head; the vessels are ligated at or near their roots to dissect the group 6 lymph node. On the contrary, the right gastroepiploic artery and vein are treated under pylorus in open surgeries. Therefore, if the vascular sources can not be determined during the laparoscopic operations, bring the stomach back into its original position; compare the change of its positions before and after lifting, so as to identify its vascular anatomy and avoid any faulty penetration.
(IV) The assistant plays an important role during the laparoscopic operations for gastric cancer. Since stomach and its gastroepiploic tissues are easy to move, the assistant must prevent their movement and assist to maintain tissue tension at local sites, so as to facilitate the surgical operation. When dividing the anterior lobe of transverse mesocolon, the assistant should exert traction on the larger omentum to its head side using bowel clamp in his/her left hand and lift the omentum near the transverse colon; meanwhile, the assistant lifts the omentum on another side of the intestine, allowing the avascular zone in the mesentery of the upper edge of the anterior lobe of transverse colon expanded thoroughly. Thus, this region can be easily divided using an ultrasonic scalpel. Also, when removing the capsule in front of pancreas, the assistant must grasp the lesser curvature and lifts it upwards to expose the pancreatic surface. Since a radical stomach surgery often involves a large region, the tubular visual field of laparoscope has a contradiction with the overall operative field, which requires the assistant to be able to operate skillfully outside the visual field of laparoscope. During the operation, the visual field is mainly focused on the instrument for the operator and only few shots are available for the assistant; therefore, the assistant must be able to operate skillfully in the “indirect visual field” based on his/her good endoscopic sensations. Otherwise, operations without visual field can easily cause damage and even death.
(V) The stomach has a rich blood supply. Its small blood vessels distributes irregularly, and can easily bleed during division. Therefore, the operator must have solid knowledge about the vascular anatomy around the stomach. Most perigastric lymph nodes are attached around the main artery and must be thoroughly dissected. It is usually a challenging task to dissect lymph nodes in arterial sheath with HIFU, because the operator must carefully maintain the integrity of the arterial wall when dissecting the lymph nodes. Another difficult task is to dissect lymph nodes near the venous wall, such as dissections of lymph node along the superior mesenteric vein (No. 14v) and along the portal vein (No. 8). The vein wall is thin and fragile, and difficult to be repaired. The injury of the above key veins means the failure of the whole laparoscopic surgery, and the patient must be transferred for open surgery. Therefore, the vein walls must be clearly identified during lymph node dissection before division.
Post-operational management
(I) Gastrointestinal decompression: continuous decompression through a gastric tube is conducted until anal exsufflation happens.
(II) Analgesia: the pain can be relieved through continuous epidural catheter infusion. Injection of analgesics may be applied when appropriate.
(III) Position: After the patient fully wakes up from anesthesia and his/her blood pressure becomes stable, a semirecumbent position is recommended to facilitate breathing and reduce risk of respiratory infection.
(IV) Massage: Venous return to heart from lower limbs is often affected during the long-duration pneumoperitoneum surgery. Interrupted limb massage after surgery can prevent deep vein thrombosis.
(V) Supportive care: patients after radical gastrectomy often have varying degrees of malnutrition. Overall parenteral nutritional support should be provided after surgery. Meanwhile, adjust the water and electrolyte balance and use antibiotics to carry out symptomatic management for gastrointestinal discomforts.
(VI) Diets: Avoid drinking water during the first three days following surgery. Drink warm water (10-20 mL every two hours) until anal exsufflation happens. Increase the water consumption on a daily basis, gradually followed by salt water/sugar water, rice soup, and broth. Give liquid diet 8 days after surgery and semi-liquid diet 9 days after surgery.
Complications and their management
(I) Bleeding: The stomach has complex blood supply and highly variable anatomy. The vessels are often injured during surgery due to unclear anatomical structures or laparoscopic vision. The larger arteties and veins should be ligated using ligature clamps, and the smaller vessels should coagulated and dissected using HIFU. A clear vision should be maintained during surgery. Avoid rude lifting/division or violent traction; the operation must be gentle.
(II) Spleen injury: during the dissociation of the greater curvature of stomach, violent maneuvers can tear the lower pole of the spleen. If the spleen bleeding can not be effectively controlled, splenectomy should be performed. Open surgery may be performed when necessary.
(III) Patients with bile duct or portal vein injury should also be transferred for open surgery.
(IV) Post-operative complications: Duodenal stump fistula or anastomotic leakage often occurs 4-6 days after surgery. Fistulas occurring 1-2 days after surgery are often due to anastomotic technique, whereas those occurring 4-6 days after surgery are often due to poor blood supply in local tissues, increased tension, and edema. In either condition, whether surgical exploitation and/or drain cleaning is required is mainly based on the clinical signs of peritonitis, volume of fistula drainage, and presence of uncontrollable fever.
(V) Long-duration pneumoperitoneum surgery may increase the risk of deep vein thrombosis. The lower limbs should be lifted appropriately after surgery. Wearing stockings or interrupted massage can also be helpful.
(VI) Veress needle or puncture cannula can cause the damage of intestinal canal and/or tissues, causing intra- and post-operative bleeding and intestinal fistula. Standardized procedures can minimize the occurrence of these complications.
Acknowledgements
Disclosure: The authors declare no conflict of interest.