Are CTCs metastatic precursors?
Yu et al. (1) recently described the use of microfluidic technology to capture circulating tumor cells (CTCs) labeled with green fluorescent protein (GFP) from a mouse pancreatic cancer model. Single-molecule RNA sequencing demonstrated that WNT2 is selectively highly expressed in the CTCs. Expression of WNT2 in human pancreatic cancer cells was found to suppress anoikis (apoptosis induced by cell detachment), enhance anchorage-independent sphere formation, and increase metastatic potential. Fibronectin was also upregulated by WNT2. The authors tested a panel of inhibitors of WNT-related pathways to identify small-molecule inhibitors capable of suppressing the WNT2 effect on anoikis. Among these, 5Z-7-oxozeaenol, an inhibitor of MAP3K7, also known as TAK1 (TGF-b activated kinase 1) 11, was remarkable in completely abrogating WNT2-induced tumor-sphere growth, without suppressing baseline sphere formation form human pancreatic cancer cells. In addition, pancreatic CTCs were enriched for WNT expression in 5 out of 11 pancreatic cancer patient cases (1). These results suggest that WNT signaling pathways may contribute to metastasis in human pancreatic cancer and ideutify a potential drug target for metastasis suppression. Yu et al. (1) concluded that overexpression of WNT contributes to the metastatic potential of CTCs.
We originally demonstrated the malignant potential of CTCs, in a novel dual-color imaging orthotopic model of PC-3 human prostate cancer metastasis in nude mice. This model was made by co-injection of an equivalent mixture of cultured GFP-expressing CTC isolated from the mouse model and parental red fluorescent protein (RFP)-expressing PC-3 prostate carcinoma cells. In the dual-color model, the selected GFP-labeled CTCs had an increased metastatic propensity relative to the RFP -labeled parental cells. The identification and isolation of highly malignant CTCs from orthotopic, but not ectopic models, indicated the metastatic potential of CTCs (2).
We then showed that the PC-3-GFP CTCs have increased resistance to anoikis. Using gene silencing and gene transfer techniques, we showed that increased expression of the apoptosis inhibitory protein XIAP contributed to anoikis resistance of the CTCs (3). We subsequently demonstrated that prostate cancer CTCs overexpress both BMI1 and Ezh2 Polycomb-group proteins associated with metastasis (4).
In another study in our laboratory, CTCs were isolated from the GFP-expressing PC-3 orthotopic model using immunomagnetic beads coated with anti-epithelial cell adhesion molecule (EpCAM) and anti-prostate specific-membrane antigen (PSMA). GFP-expressing CTCs were isolated within 15 minutes and were readily visualized by GFP fluorescence. It was possible to immediately place the immunomagnetic-bead-captured GFP-expressing PC-3 CTCs in 3-dimensional sponge cell culture, where they proliferated. The combination of GFP expression and the use of immunomagnetic beads is a very powerful method to obtain CTCs for either immediate analysis or for biological characterization in vivo or in 3-dimensional culture (Figure 1) (5).
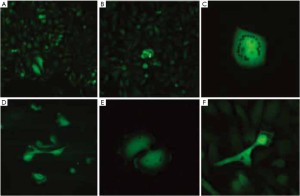
The PC-3-GFP CTCs were then expanded in culture in parallel with the parental PC-3-GFP cell line. Both cell types were then inoculated onto the chorioallentoic membrane (CAM) of chick embryos. Inoculation of embryos with PC-3-GFP CTCs resulted in a 3 to 10-fold increase in brain metastasis when compared to those with the parental PC-3-GFP cells. This is further evidence that CTCs have increased metastatic potential compared to their parental counterparts (6).
The chemosensitivity of PC-3 prostate cancer CTCs was compared to that of the parental PC-3 cells. PC-3 CTC cells exhibited a significantly increased sensitivity to both cisplatin and docetaxel when compared to PC-3 parental cells, with docetaxel having the greater efficacy (7).
We subsequently demonstrated that C-MET, h-TERT, CK20, and CEA are expressed in CTC of pancreatic patients and that there genes could be used as an indicator for circulating cancer cells. The combined assay of the four genes improved the specificity and sensitivity for detecting cancer to 100%, which may be attributable to the use of immuno-magnetic separation and enrichment of the circulating pancreatic cancer cells (8).
A new approach to visually detect cancer-patient-CTCs, was developed using a telomerase-specific adenovirus expressing GFP. Infection with this virus selectively labeled CTCs with GFP. This GFP-expressing virus-based method is an effective and highly sensitive method to detect and quantify CTCs from patients (9).
The Yu et al. (1) paper is thus an additional contribution to our knowledge of the metastatic potential of CTCs, originally demonstrated by our laboratory, indicating that WNT2 expression is yet another gene contributing to the CTC phenotype. The evidence presented here strongly suggest that CTCs are metastatic precursors. With the technology described in this report CTCs can be prime targets for cancer diagnosis and therapy.
Acknowledgements
Research in the author’s laboratories is supported in part by grants from the National Cancer Institute CA142669 and CA132971 (to M.B. and AntiCancer, Inc).
Disclosure: The author declares no conflict of interest.
References
- Yu M, Ting DT, Stott SL, et al. RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature 2012;487:510-3.
- Glinskii AB, Smith BA, Jiang P, et al. Viable circulating metastatic cells produced in orthotopic but not ectopic prostate cancer models. Cancer Res 2003;63:4239-43.
- Berezovskaya O, Schimmer AD, Glinskii AB, et al. Increased expression of apoptosis inhibitor protein XIAP contributes to anoikis resistance of circulating human prostate cancer metastasis precursor cells. Cancer Res 2005;65:2378-86.
- Berezovska OP, Glinskii AB, Yang Z, et al. Essential role for activation of the Polycomb group (PcG) protein chromatin silencing pathway in metastatic prostate cancer. Cell Cycle 2006;5:1886-901.
- Kolostova K, Pinterova D, Hoffman RM, et al. Circulating human prostate cancer cells from an orthotopic mouse model rapidly captured by immunomagnetic beads and imaged by GFP expression. Anticancer Res 2011;31:1535-9.
- Menen RS, Pinney E, Kolostova K, et al. A rapid imageable in vivo metastasis assay for circulating tumor cells. Anticancer Res 2011;31:3125-8.
- Menen R, Zhao M, Zhang L, et al. Comparative chemosensitivity of circulating human prostate cancer cells and primary cancer cells. Anticancer Res 2012;32:2881-4.
- Zhou J, Hu L, Yu Z, et al. Marker expression in circulating cancer cells of pancreatic cancer patients. J Surg Res 2011;171:631-6.
- Kojima T, Hashimoto Y, Watanabe Y, et al. A simple biological imaging system for detecting viable human circulating tumor cells. J Clin Invest 2009;119:3172-81.


