Ghrelin: a journey from GH secretagogue to regulator of metabolism
Introduction
In the last 40 years, the human lifestyle has been suffering radical changes, where the availability and accessibility to food as well as food advertising and marketing contribute to create an obesogenic environment. A ‘side effect’ of our modern lifestyle is that the incidence of obesity has grown to pandemic proportions. According to the National Health and Nutrition Examination Study (NHANES), 33.8% of adults are obese in the United States (1). Moreover, the prevalence of obesity worldwide has more than doubled over the past three decades (2). Obesity is associated with a range of metabolic diseases, cardiovascular diseases and the development and progression of several cancers (3,4). To prevent the development of obesity it is crucial to understand the mechanisms that regulate food intake and energy expenditure.
Feeding and energy metabolism is essential for species survival. The complex homeostatic mechanism regulating body weight involves interactions between peripheral organs, such as white adipose tissue (WAT), the gastrointestinal system (GIS) and the central nervous system (CNS), through signals, that inform brain centers of the nutritional and metabolic status of the animal (5).
A primary site for controlling energy balance is the hypothalamus; a region that is intimately associated with the regulation of basic functions such as reproduction, temperature, hormonal balances and biological rhythms. Hypothalamic nuclei and areas that are associated with regulation of energy balance include the arcuate nucleus of the hypothalamus (ARH), ventromedial hypothalamus (VMH), dorsomedial hypothalamus (DMH), paraventricular hypothalamus (PVH) and lateral hypothalamus (LH) area (6). These hypothalamic areas form interconnected neuronal circuits that respond to changes in energy status by altering the expression of specific molecules, especially neuropeptides, resulting in changes in energy intake and expenditure. These neurons also project to other regions of the brain, like the brainstem and spinal cord. Apart from the hypothalamus, the brainstem in particular, plays an important role in the regulation of feeding behavior.
A large number of peripheral signals, particularly peptides, have been identified in the last three decades. Such peptides have been shown to regulate food intake and energy expenditure. Most of the peptides (hormones) that regulate appetite are anorexigenic “satiety” factors (5). However, there is an exception; Ghrelin, a hormone secreted mainly by the gut, displays an exclusive characteristic; and so far, it is a unique known orexigenic hormone, which confers a predominant role on feeding control (7).
In this review, we will provide an overview of the current knowledge in the literature regarding the integration between the brain and gastrointestinal signals to regulate energy homeostasis. We will also highlight the role of ghrelin in maintaining energy and glucose homeostasis.
Signals to the brain from GIS
To maintain energy homeostasis, the brain must closely monitor the peripheral energy state within the body. Since the brain does not store energy, it is dependent on a continuous supply of nutrients from the general circulation to survive. The brain receives signals via vagal afferents or through the circulation. The enteric nervous system that interconnects with the autonomic nervous system transmits information of mechanical (distension, contraction), chemical (presence of nutrients in the gut lumen) and neurohumoral stimuli (gut hormones, neurotransmitters and neuromodulators) to the CNS through vagal and sympathetic nerves (8). The signal peptides released locally from enteroendocrine cells of the GIS activate receptors of the vagal afferent fibers, informing the brainstem of luminal composition (9,10).
The hindbrain (caudal brainstem) contains neurons and circuits that involve autonomic control of ingestion, digestion, and absorption of food (8) independently of the forebrain (11). The nucleus of the solitary (NTS) tract is one of the major processors of vagal afferent signals that conveys messages to higher neural centers involved in appetite control, such as the ARH, PVH and DMH (12). The integration of all these afferent signals related to food presence in the gut in turn regulates the meal size of individuals (8,10,11). Therefore, vagal afferent nerves are the major conduit by which nutrients signal to the brain and influence motility and secretion, as well as hunger and satiety (8-10).
Hormones secreted from WAT and the GIS can influence several specific brain regions and neurons via the circulation. Among these regions, the NTS—area postrema (AP) complex in the hindbrain and the ARH in the forebrain are two of the major targets of those hormones.
The gastrointestinal tract secretes several satiety signals, such as Cholecystokinin (CCK), Bombesin, Glucagon-like peptide-1 and 2 (GLP-1 and GLP2), Amylin, Peptide YY (PYY), Oxyntomodulin, somatostatin and enterostatin in response to gut nutrient content and most of them play an important role in the control of energy homeostasis (13,14).
The circulating signals are often categorized as long-acting adiposity signals, and short-acting gastrointestinal factors (Figure 1). Long-acting signals characteristically reflect the levels of energy stores and regulate body weight and the amount of energy stored as fat over time. The two adiposity signals that are best known are insulin and leptin. The functional ability of insulin and leptin as adiposity signals to the brain have been reviewed several times and are beyond the scope of the current review [see (15,16) for review]. Several hormones are a representation of short-acting signals that regulate appetite and the majority of them decrease food intake.
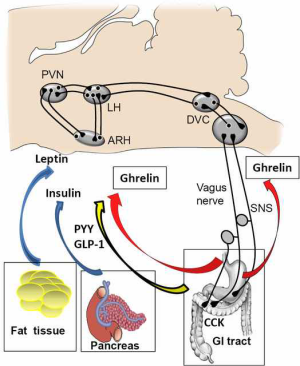
Neural circuits within the hypothalamus and the brainstem can regulate both food intake as well as integrate short and long-term signals of energy balance (17-19). Both types of signalling are able to act together. For example, leptin enhances the satiation effect of GLP-1 at vagal afferent fibers and the hindbrain (20). Similarly, the activation of GLP-1R-expressing neurons of the NTS, which project to hypothalamic areas involved in appetite regulation, can modulate the activity of those areas (12,14).
Ghrelin acts in the brain to increase food intake via activation of its ghrelin receptors (GHS-R). These receptors are expressed in several caudal brainstem nuclei, including area postrema, NTS and the dorsal motor nucleus of the vagus nerve, forming the dorsal vagal complex (DVC) (21). Stimulation of GHS-R in the caudal brainstem leads to a hyperphagic response (22). A direct action in the same area (on the dorsal vagal complex) that increases food intake was demonstrated using microinjections of ghrelin (23). Moreover, fourth-ventricle ghrelin delivery increases Fos in the NTS but not in the hypothalamic areas involved in food regulation such as ARH and LH. This suggests the existence of hypothalamic circuits within the forebrain and the hindbrain that respond independently to ghrelin (24).
In the hypothalamus, the ARH is the most studied neural circuit that regulates energy balance. ARH and PVH are two critical brain centers that transmit the orexigenic action of ghrelin. Several lines of evidence have demonstrated that ghrelin induces feeding by stimulating an orexigenic population of neurons in the ARH; the neuropeptide Y and agouti-related peptide neurons (NPY/AgRP neurons) (25-33). More than 90% of NPY/AgRP neurons express the GHS-R1a in the ARH (34). The GHS-R1a is also expressed on growth hormone releasing neurons (34,35) and tyrosine hydroxylase neurons (21,36) in the ARH. The GHS-R1a-expressing neurons of the PVH are also activated by ghrelin to increase food intake and to promote the intake of carbohydrate rich diets and increase adiposity (37).
The ghrelin axis: peptides, receptors and enzymes
Ghrelin synthesis and structure
Ghrelin is a peptide that was discovered as an endogenous ligand for the growth hormone secretagogue receptor (GHSR-1a) in 1999 (38) through which it stimulates GH release from the anterior pituitary.
Ghrelin is predominantly synthesized in the stomach and secreted into the circulation. The X/A-like cells of gastric oxyntic glands of the stomach are the most abundant source of circulating ghrelin. The small intestine also synthesizes ghrelin to a lesser extent, with the amount of ghrelin produced diminishing with an increased distance to the pylorus (39). Additionally, it has been demonstrated that ghrelin is expressed in many tissues such as the lung, heart, pancreas, kidney, testis, pituitary, and hypothalamus (40).
Ghrelin has a unique structure with 28 amino acids and a n-octanoyl ester at its third serine residue, which is essential for its potent biological activity at the GHS-R1a. Several steps involving different enzymes detail the process from gene synthesis to the final structure. The ghrelin gene is transcribed as a pre pro-ghrelin mRNA isoform, which leads to the translation of a 117 amino-acid peptide. This prohormone is cleaved into pro-ghrelin, a 94 amino acid signalling peptide. Pro-ghrelin then requires posttranslational acylation with n-octanoic acid or n-decanoic acid at the third serine residue for its biological activity at the GHS-R1a. Ghrelin O-Acyltransferase (GOAT) is the enzyme responsible for pro-ghrelin acylation (41) and it is also found predominantly in the stomach and digestive tract (41,42). In the stomach and duodenum, GOAT co-localizes with ghrelin expressing cells (43), where it readily acylates newly synthesized pro-ghrelin. GOAT can also acylate pro-ghrelin with other fatty acid substrates besides octanoate (44). Intake of diet enriched with either medium chain fatty acids or medium-chain triglycerides could lead to a modification in the proportions of octanoyl or decanoyl ghrelin stored in the same granules in gastric cells, suggesting that GOAT is likely to use the most available substrate to perform ghrelin acylation (45). Pro-ghrelin is further processed by prohormone convertase (PC 1/3) to produce the 28 amino-acid mature ghrelin peptide (46,47). Other forms of ghrelin (truncated peptides) have also been described; more of them arise from alternatively spliced variants that may act on autocrine/paracrine pathways (48). It has been suggested that these truncated peptides could play multiple roles in several diseases, particularly in breast and prostate cancer (46).
Circulating ghrelin
Ghrelin exists as two forms in the plasma, acylated ghrelin and des-acylated ghrelin. In humans, approximately 80-90% of plasma ghrelin is des-acyl ghrelin (49) however the mechanisms that control the rate at which acyl-ghrelin converts into des-acylated ghrelin is unknown. Recently a ghrelin deacetylation enzyme; acyl-protein thioesterase-1 (APT1) has been described, and it can des-acylate ghrelin in the plasma (50). The high des-acyl to acyl ghrelin ratio in the circulation can be explained by the shorter half-life of ghrelin compared to des-acyl ghrelin (51,52). The proportion of acyl to des-acyl ghrelin seems to be physiologically important since des-acyl ghrelin is unable to activate GHS-R1a. While most des-acyl ghrelin circulates as a free peptide, the vast majority of acyl-ghrelin is bounded to larger molecules such as lipoproteins (53).
Ghrelin levels exhibit a circadian rhythm and closely follow feeding schedules; peripheral ghrelin levels rise sharply before main meals in a scheduled meal-fed sheep and decrease once the animal has been fed (54). Under fasting conditions, plasma ghrelin peaks match the previous pattern of daily meals in humans (55).
Ghrelin levels show a marked gender distribution, with higher levels in women as compared to men and the levels decline with age, BMI, hypertension and others markers of metabolic syndrome (56).
A large number of studies that evaluate ghrelin levels do not distinguish between total ghrelin, acyl ghrelin, and des-acyl ghrelin levels. Appropriate sample collection and storage strategies are necessary to limit the acyl ghrelin degradation in order to elucidate the physiological and pathophysiological roles of ghrelin (see Delporte’s review) (57).
Ghrelin receptors
Two isoforms of Ghrelin receptors have been identified; GHS-R1a is a 366 amino acid protein belonging to the G-protein coupled receptor (GPCR) superfamily and is characterized by seven transmembrane receptor domains (58), whereas GHS-R1b is a truncated form that lacks part of transmembrane domains 6 and 7. GHS-R1a is the functional ghrelin receptor required to elicit growth hormone release or food intake in response to exogenous administered ghrelin. On the contrary, GHS-R1b is regarded as a non-functional receptor due to its inability to bind to acyl or des-acyl ghrelin (58).
Recent evidence has unravelled some of the important regulatory functions of GHS-R1b. For example, GHS-R1b forms heterodimers with GHS-R1a to decrease the constitutive activity of GHS-R1a (59,60). Both isoforms of the GHSR are widely expressed. GHS-R1a expression was first identified in the pituitary and hypothalamus, where it is highly expressed (58). GHS-R1a expression has also been demonstrated in a number of brain regions and in a wide range of peripheral tissues, including the stomach, intestine, pancreas, spleen, thyroid gland, adrenal gland, kidney, heart, lung, liver, lymphocytes and adipose tissue (58-61).
Functional activity of ghrelin
Ghrelin acylation is required to bind GHS-R1a and exert its biological activity. Interestingly, acylated ghrelin is also able to produce some endocrine effects in tissues where there is no expression of GHS-R1a (9), suggesting the presence of an unknown alternate ghrelin receptor.
Even though des-acyl ghrelin is unable to bind to GHS-R1a, it is not functionally inactive. Extensive studies have demonstrated that des-acyl ghrelin can modulate cell proliferation, apoptosis, metabolism and glucose homeostasis (9,57,62-65), indicating the presence of an alternative receptor. Nevertheless, further studies are required to unveil these pathways to understand the potential biological importance of des-acyl ghrelin, the most abundant isoform in circulation.
Hypothalamic circuits that are involved in ghrelin-mediated feeding.
It is believed that neural circuits within the ARH regulate the effects of ghrelin on feeding. Two neuronal populations in the ARH are considered ‘first-order’ sensory neurons in the control of food intake. One population expresses the appetite-suppressing peptides, α-MSH [derived from the proopiomelanocortin (POMC) precursor] and cocaine- and amphetamine-regulated transcript (CART). The other co-expresses two appetite-stimulating peptides, neuropeptide Y (NPY) and agouti-related peptide (AgRP) (66). Both NPY/AgRP and POMC neurons project to the PVH and other hypothalamic nuclei (67).
Importantly, these neurons receive different metabolic signals such as glucose, hormones and fatty acids, and subsequently project them to second-order hypothalamic nuclei to regulate food intake. In addition, using novel human diphtheria toxin targeted therapy, the conditional deletion of NPY/AgRP neurons results in a rapid decrease in food consumption and body weight (68,69). Various methods including electrophysiology (25,26), or c-fos immunoreactivity (25,27,28), peptide secretion (29), or gene expression (30-33) have all revealed that ghrelin robustly stimulates NPY and AgRP to mediate feeding. To further bolster the role of ghrelin in energy homeostasis, selective ablation of AgRP in adult mice abrogates the orexigenic effects of ghrelin entirely (70) and double NPY/AgRP knockout mice fail to increase food intake in response to ghrelin (30). Collectively, these studies suggest that the orexigenic effects of ghrelin are mainly exerted through the ARH.
Upon the stimulation of NPY/AgRP neuronal activity with Ghrelin, POMC neurons are concurrently suppressed through the inhibitory γ-aminobutyric acid (GABA)-eric inputs from activated NPY/AgRP neurons (26). The inhibitory tone on POMC neurons is reversed when the vesicular GABA transporter was genetically ablated in AgRP neurons, causing subsequent anorexia following activation of the melanocortin system (71). Ghrelin increases GABA-mediated inhibitory inputs from NPY/AgRP neurons and alters POMC neuronal synaptic plasticity by increasing the number of inhibitory perikaryal synapses to POMC neurons, thus lowering POMC neuronal activation and results in a decrease in energy consumption (25).
Recent evidence has shed some light on a unique intracellular signaling modality that unravels how ghrelin activates NPY neurons to initiate changes in feeding behavior. However in this review, the molecular mechanisms are only discussed in brief. Of note, both intraperitoneal or intracerebroventricular delivery of ghrelin increases AMP-activated protein kinase (AMPK) phosphorylation and activity in the hypothalamus and consequently leads to increased calorie intake (25,72,73). AMPK plays an important role in energy homeostasis as central compound C abolishes AMPK activity and suppresses ghrelin-mediated food intake (25,74). In a nutshell, ghrelin binds to the GHSR and initiates a signal transduction cascade that begins with Ca2+ influx in identified NPY neurons (75-77). Ca2+ subsequently interacts with calmodulin (CaM) to activate CaM-dependent protein kinase kinases (CaMKK), which in turn leads to AMPK phosphorylation and increases NPY messenger RNA and protein expression (78,79).
The downstream intracellular actions after ghrelin-induced AMPK activation involve phosphorylation of acetyl CoA carboxylase (ACC), which causes the suppression of malonyl CoA and disinhibition of carnitine palmitoyl transferase 1 (CPT1) (25,74). The notion that CPT1 mediates ghrelin-induced food intake is strengthened by data showing that inhibition of CPT1 prevented ghrelin’s ability to increase NPY and AgRP mRNA expression in the hypothalamus (25). The downstream mechanism is unraveled when ghrelin stimulates palpitate-driven uncoupled respiration in isolated hypothalamic mitochondria in an UCP2-dependent manner. In essence, upon binding to GHS-R1a, ghrelin initiates AMPK-CPT1-UCP2 axis and mitochondrial respiration that necessitates mitochondrial biogenesis in NPY/AgRP neurons, depolarization of NPY/AgRP neurons and ghrelin-mediated synaptic plasticity of POMC neurons. The activation of GHS-R1a also triggers the opening of calcium channels, causing an intracellular influx of Ca2+ through the adenylate cyclase-protein kinase A (PKA) pathway (75). Altogether, both molecular mechanisms converge to increase mRNA expression of NPY and ultimately resulting in increased food intake.
Ghrelin and obesity
In line with the notion that ghrelin stimulates food intake, ghrelin levels are reported to be elevated in fasted mice and rats, and decreased in obesity (80,81). It is believed that the increased ghrelin levels during the fasting/starvation state serve to restore neutral energy balance and combat hunger (82). However in diet induced obesity (DIO), peripheral ghrelin does not stimulate food intake (83), ghrelin transport across the blood brain barrier is impeded (84), NPY/AgRP feeding circuits are perturbed (85) and basal hypothalamic AMPK activity is suppressed (86). In addition, DIO mice display ghrelin resistance in NPY/AgRP neurons at the level of the ARH (29,87) but not in other hypothalamic nuclei such as the PVH (6). An example of a similar condition is when mice are fed on a high-density diet; they develop leptin resistance in the ARH neurons while leptin sensing is preserved in second order neuronal region like the VMH (6,88). To date, it is still unclear how this phenomenon can impact obesity. However, studies have suggested that ghrelin resistance could be a factor in the decline of cognitive functions, as ghrelin signalling in the brain is essential for memory, learning and neuroprotection (89-92).
Interestingly, central ghrelin signaling in the brain can selectively modulate body weight without affecting food intake, because RNA interference of GHSR expression in the PVH significantly reduces body weight and blood ghrelin levels independent of food intake (93). Moreover, chronic central ghrelin infusion in pair-fed animals significantly increases respiratory quotient (RQ), which is indicative of increased fat deposition (94). The evident increase in mRNA of lipogenic enzymes lipoprotein lipase (LPL), acetyl-CoA carboxylase α (ACC), fatty acid synthase (FAS), and stearoyl-CoA desaturase-1 (SCD1) in pair-fed animals further substantiates that the effects on WAT is independent of changes in food intake (94). Importantly, there are studies in the literature showing that ghrelin administration in NPY-deficient mice increases body weight (95), overexpression of NPY in PVH induces obesity (96) and NPY deficiency decreases body weight when mice are fed on a high-density diet (97). Therefore, in terms of energy homeostasis, the actions of ghrelin in the hypothalamus are likely to be mediated by either ghrelin acting on NPY/AgRP neurons in the ARH to increase food consumption and body weight or the direct actions of ghrelin on GHSR-expressing neurons in the PVH to increase adiposity and body weight.
Ghrelin and lipogenesis
Besides the actions of ghrelin in the brain, it also has direct effects on lipogenesis in peripheral adipose tissue. Recent in vitro studies have provided compelling evidence that both ghrelin isoforms (acyl and des-acyl ghrelin) trigger the activation of adipogenic factors such as PPAR gamma and C/EBPα to induce cell proliferation, and adipocyte differentiation in 3T3-L1 preadipocytes (98-101). In human visceral adipocytes, both ghrelin isoforms stimulate lipid accumulation (102) and in cultured rat adipocytes, ghrelin directly increases leptin production (103). In vivo studies have revealed that ghrelin regulates adipogenesis by binding directly to the GHSR, which is expressed in adipose tissue (102,104,105). Ghrelin increases triglyceride content in adipose tissues (106) and Davies et al. (104) demonstrated that acyl-ghrelin increases abdominal adipose tissue in a depot-specific manner. In contrast, the GHSR-independent effect is proposed to be centrally mediated when in vivo studies with ghrelin delivered iv, ip or via minipumps altered adipogenesis (9,94). Even though it was mentioned previously that both ghrelin isoforms could stimulate adipogenesis in cultured adipocytes, studies have emerged to discriminate the differential effects of acyl and des-acyl ghrelin in whole animals. Des-acyl ghrelin is shown to increase adiposity (9), or have no effect on adiposity (104) or decrease adiposity and improve insulin sensitivity (107). More recent studies support the idea that des-acyl ghrelin maintains insulin sensitivity and prevents adipogenesis (65). There is no doubt that ghrelin stimulates weight gain by increasing food intake and adiposity. However, there are many equivocal issues that need to be addressed in the future. For example, does ghrelin primarily influence adiposity through central or peripheral mechanisms? How do acyl ghrelin, des-acyl and the ratio of acyl/des acyl ghrelin affect adipogenesis and insulin sensitivity? Understanding these critical issues will help design specific therapies to combat obesity and diabetes.
As there is a wealth of literature showing that exogenous ghrelin administration increases food intake and adiposity, it is noteworthy that studies from knockout models suggest that ghrelin signaling has only modest effects on body weight and food intake. There are reports showing that GHSR-/- mice have significantly reduced body weight on a regular chow diet (108,109), while others did not observe any change in body weight on a regular diet, possibly due to the employment of different GHSR-/- mouse lines (110,111).
Due to the equivocal nature of these knockout studies, it remains possible that animals could develop compensatory mechanisms to counteract ghrelin deletion. Thus, to understand the true physiological role of ghrelin in regulation of food intake and body weight, it will be useful to generate temporal ghrelin knockout mouse models (i.e., ghrelin ablation in adult mice).
Ghrelin regulates glucose homeostasis
Knockout models illustrate that ghrelin plays a major role in glucose homeostasis. Tschöp and colleagues were the first to document a possible interaction between ghrelin and glucose by demonstrating that a single subcutaneous ghrelin injection reduces serum ghrelin levels in rats (80).
Although the functionality of knockout models in evaluating ghrelin-mediated energy homeostasis is limited, they are beneficial to dissociate ghrelin’s actions in regulating glucose homeostasis. In fact, ghrelin gene deletion prevents the development of glucose intolerance when mice were fed on a high fat diet despite having similar body weight and food intake between ghrelin KO mice and wild type littermates (112). Furthermore, disruption of the ghrelin gene in leptin deficient (ob/ob) mice ameliorates glucose intolerance and augmented insulin secretion (113). Despite the fact that Pfluger and colleagues observed no difference in glucose disposal between chow fed mice after deleting both ghrelin and its receptors (111), we believe that it is beyond one’s imagination to improve a normal condition further and that may elucidate the clearer effects of ghrelin on glucose homeostasis seen in mice on an obesogenic HFD.
It is valuable to note that ghrelin potently stimulates GH secretion, and GH increases plasma glucose concentrations, free fatty acid uptake and suppresses glucose transport in skeletal muscles (38). It was proposed that the effect of ghrelin on glucose metabolism is driven by increased GH secretion. However, ghrelin infusion in humans given GHR antagonist (pegvisomant) increased plasma glucose concentration and decreased insulin sensitivity, proving the theory that ghrelin regulates blood glucose through a GH-independent mechanism (114,115).
Possible mechanistic action of ghrelin
Ghrelin is primarily produced in the stomach, as well as in the hypothalamus and other peripheral tissues albeit at low levels. On that note, it remains feasible that paracrine, endocrine, and neural pathways are all possible alternatives to explain ghrelin’s effects on glucose. In order to achieve normal glucose balance, it requires the activation of glucose sensing mechanisms in the hypothalamus and peripheral tissues. We will discuss some of them in the section below. For more in depth information, refer to the reviews of Sangiao-Arvarellos et al. (116) and Heppner et al. (117).
Ghrelin activates glucose sensing neurons in the brain
Emerging studies have identified glucosensing neurons within the hypothalamus and brainstem that are involved in orchestrating the activity of the autonomic nervous system, hormone secretion, and changes in fuel metabolism, which include glucose production and glucose uptake and utilization (118). It is plausible that hormonal signals such as ghrelin could converge and act directly on melanocortin circuits to modulate peripheral glucose balance, because POMC neuronal activity is triggered by glucose (119) whereas, NPY neuronal activity seems to be suppressed by glucose (120,121). It was also proposed that ghrelin is synthesized by a group of neurons close to the third ventricle between the dorsal, ventral, paraventricular and ARH (26). These ghrelin expressing neurons project to key hypothalamic circuits that synthesizes neuropeptides such as NPY, AgRP, POMC and CRH, clearing the uncertainty that hypothalamic ghrelin contributes to whole body glucose regulation. Importantly, the activation of NPY neurons in the hypothalamus affects peripheral glucose regulation by increasing hepatic glucose production and gluconeogenic enzymes such as Glucose-6-phophatase, consequently inducing hepatic insulin resistance (122).
Peripheral targets of ghrelin to modulate glucose homeostasis
Peripheral glucose responsive tissues such as the pancreas, liver, skeletal muscle and WAT are vital to ensure lipid and glucose balance in our body. Here, we will outline some of the pertinent studies about the interaction between ghrelin and these tissues to achieve glucose balance.
Pancreas
Ghrelin and its receptor are expressed in the islets (α-, β- and ε cells) of rodents and human pancreas (123,124). β-cells sense glucose through its metabolism and the resulting increase in ATP and the activation of ATP-sensitive K+ channels and Ca2+ influx stimulates insulin secretion. UCP2 is a key component in glucose sensing in pancreatic β cells because UCP2 mediates mitochondrial proton leak, decreasing ATP production and consequently decreases insulin secretion. The importance of UCP2 as a negative regulator of insulin secretion was demonstrated by the fact that UCP2-deficient mice display higher islet ATP levels and increased glucose-stimulated insulin (125,126). Moreover, the generation of ob/ob mice lacking UCP2 restores first-phase insulin secretion (acute insulin response to glucose) and ameliorates the hyperglycemia condition (125,126).
According to the in vitro study in rat pancreatic islets, ghrelin inhibits glucose-stimulated insulin secretion in a dose dependent manner (124). Interestingly, the ablation of ghrelin reduces expression of UCP2 mRNA in the pancreas, which contributes toward enhanced glucose-induced insulin secretion (113). Hence, ghrelin may play a part in glucose homeostasis by chronically regulating pancreatic UCP2 expression and subsequently glucose stimulated insulin secretion.
Whilst animal models demonstrate an important effect of ghrelin on insulin secretion, it is analogous to studies in humans as Tong et al. recently assessed the effect of continuous infusion of ghrelin on dynamic insulin secretion and glucose metabolism. By and large, they showed that exogenous ghrelin markedly reduced the first-phase insulin response to intravenous glucose in healthy humans (7). All together, these studies strongly suggest an inhibitory effect of ghrelin on insulin secretion.
Liver
Phosphorylation of AKT is associated with the suppression of hepatic gluconeogenesis, as both in vitro and in vivo studies in rats demonstrate that ghrelin reduces insulin-mediated AKT signalling in the liver (127,128), resulting in potential concomitant blood glucose increments (127,128). On the contrary, ghrelin infusion studies done in humans did not affect hepatic glucose production despite inducing peripheral resistance (115). It is important to take into account the differential effects of the nutritional status on ghrelin regulation of glucose metabolism, as neutralization of ghrelin with a specific Spiegelmer compound during the fasting-refeeding cycle decreases hepatic glucose and lipid metabolism in rats (129).
Skeletal muscle
There are reports showing that ghrelin administration in rats increased insulin sensitivity in skeletal muscle by enhancing AKT-dependent insulin signalling selectively in oxidative muscle (128). However, studies in human subjects showed otherwise. It is worth mentioning that high doses of ghrelin may cause undesired secretions of many pituitary hormones, such as GH, Prolactin and ACTH that may exert influence on systemic glucose homeostasis (130). To circumvent these interferences, Vestergaard and colleagues compared a population of human subjects with hypopituitarism to healthy individuals and showed that ghrelin infusion during a hyperinsulinemic-euglycemic clamp acutely reduces insulin sensitivity in skeletal muscle together with stimulation of lipolysis, indicating the direct effects of ghrelin on skeletal muscle is independent of GH (115).
In summary, most of the literature support the idea that ghrelin acts to impair insulin sensitivity, particularly in the muscle; nevertheless the underlying mechanism remains to be elucidated.
White adipose tissue
In terms of adipocytes, there has been quite a disagreement in the literature regarding the impact of ghrelin on insulin sensitivity of white adipose tissue. Some studies showed that ghrelin acts directly on isolated epididymal adipocytes to enhance insulin-stimulated glucose uptake (131) and adipocyte differentiation (9). On the other hand, others showed that ghrelin increases lipolysis in WAT (106,115). This inconsistency may potentially be explained by the use of different modes of ghrelin exposure and analysis of adipocytes from different parts of the body. Consistent with this, there are reports demonstrating fat-depot specific sensitivity to ghrelin, which utilizes different signal transduction pathways through GHS-R1a (104). For the understanding of the physiological relevance of ghrelin on glucose uptake in adipocytes, additional in vitro and in vivo studies are required.
In brief, the above studies have highlighted the main effects of ghrelin on peripheral tissues; (I) increases glucose production in the liver, (II) attenuates glucose-stimulated insulin release from the pancreas, (III) impairs insulin sensitivity in skeletal muscle and lastly (IV) differential effects of ghrelin on specific fat-depots. Under non-physiological conditions, all these undesired events may converge into the development of diabetes (113). Even so, it is imperative we consider this action of ghrelin in a physiological context as metabolic status has been implicated in the regulation of ghrelin’s influence in the brain and circulating ghrelin levels (87). Furthermore, ghrelin levels are elevated in calorie restricted mice, rats and humans and that may aid in the future development of anti-obesity and anti-diabetic therapies to combat the metabolic dysregulation(29,81,92,132-135).
Ghrelin and cancer
Concurrent with the discovery of ghrelin, GHSR1a and ghrelin expression was uncovered in the pituitary and neuroendocrine tumors (136), suggesting a role in pituitary pathogenesis (137). However, due to the important effects of ghrelin in the stimulation of food intake and adiposity; attention was shifted to its effect on regulating of body weight.
Recently, ghrelin has been “re-discovered’ in relation to its role in the dynamics of cellular proliferation. Accumulating literatures has implicated ghrelin in a number of processes in relation to cancer, including cell proliferation, apoptosis, cell invasion and migration, and angiogenesis, presumably through an autocrine/paracrine mechanism (46).
To date, it is widely known that ghrelin and its receptor are expressed in a range of peripheral tumour types. It is thought that ghrelin and its receptor may function as autocrine/paracrine growth factors, which underlies the development of a number of cancers. However, it is imperative to be mindful of the complex relationship between ghrelin and cancer. Some studies have shown that colorectal carcinoma cells secrete excessive ghrelin in vitro and it promotes cell proliferation in an autocrine/paracrine manner (138,139), similar to the results observed in human pancreatic and hepatoma cell lines (140,141). On the contrary, others studies have showed that ghrelin induces both, proliferative and anti-proliferative effects, which are often dependent of the cell type and dose of ghrelin administration (46).
Throughout the literatures, there are scant reports that examined the relationship between serum ghrelin levels and gastrointestinal cancer. However, to emphasize on the role of serum ghrelin in gastric cancers, a prospective epidemiological study has demonstrated that individuals with lower baseline concentration of ghrelin have a significant increase risks of both gastric and esophagogastric adenocarcinomas (142). This finding is recently validated by Sadjadi et al. (143); suggesting atrophic changes of ghrelin-producing cells.
In light of the limited information on the role of ghrelin in the aetiology of gastrointestinal cancers, further studies are required to define the interaction between ghrelin and carcinogenesis in different organs.
Conclusions
To recapitulate our knowledge based on the above studies, ghrelin is produced in the stomach and its unique structure requires acylation for its biological activity. Earlier studies have identified ghrelin as an endogenous agonist for the growth hormone secretagogue receptor involved in growth hormone secretion. As the research of ghrelin progresses, there is increasing evidence that hypothalamic circuits are implicated in ghrelin-mediated feeding. Presently ghrelin appears to have a greater importance for the regulation of blood glucose during starvation.
Since the initial discovery of ghrelin, the range of physiological and pathophysiological functions attributed to this hormone has grown rapidly. Ghrelin not only functions as a regulator for inflammation and the cardiovascular system, it also governs a number of processes in relation to cancer, including cell proliferation, apoptosis, cell invasion and migration, and angiogenesis. Whether or not ghrelin plays a role in the progression of cancer remains to be determined.
Acknowledgements
This work was supported by Monash University, and NHMRC grant. We thank Zachary Chow for his kind help in editing this manuscript.
Disclosure: The authors declare no conflict of interest.
References
- Ogden CL, Carroll MD, Kit BK, et al. Prevalence of obesity among adults: United States, 2011-2012. NCHS Data Brief 2013;(131):1-8.
- Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384:766-81. [PubMed]
- Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569-78. [PubMed]
- Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 2000;21:697-738. [PubMed]
- Yi CX, Tschöp MH. Brain-gut-adipose-tissue communication pathways at a glance. Dis Model Mech 2012;5:583-7. [PubMed]
- Enriori PJ, Evans AE, Sinnayah P, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 2007;5:181-94. [PubMed]
- Tong J, Prigeon RL, Davis HW, et al. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes 2010;59:2145-51. [PubMed]
- Berthoud HR. The neurobiology of food intake in an obesogenic environment. Proc Nutr Soc 2012;71:478-87. [PubMed]
- Thompson NM, Gill DA, Davies R, et al. Ghrelin and des-octanoyl ghrelin promote adipogenesis directly in vivo by a mechanism independent of the type 1a growth hormone secretagogue receptor. Endocrinology 2004;145:234-42. [PubMed]
- Näslund E, Hellström PM. Appetite signaling: from gut peptides and enteric nerves to brain. Physiol Behav 2007;92:256-62. [PubMed]
- Grill HJ, Hayes MR. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab 2012;16:296-309. [PubMed]
- Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science 2005;307:1909-14. [PubMed]
- Begg DP, Woods SC. The endocrinology of food intake. Nat Rev Endocrinol 2013;9:584-97. [PubMed]
- Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest 2007;117:13-23. [PubMed]
- Sohn JW, Elmquist JK, Williams KW. Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci 2013;36:504-12. [PubMed]
- Enriori PJ, Evans AE, Sinnayah P, et al. Leptin resistance and obesity. Obesity (Silver Spring) 2006;14 Suppl 5:254S-258S. [PubMed]
- Hisadome K, Reimann F, Gribble FM, et al. Leptin directly depolarizes preproglucagon neurons in the nucleus tractus solitarius: electrical properties of glucagon-like Peptide 1 neurons. Diabetes 2010;59:1890-8. [PubMed]
- Williams KW, Zsombok A, Smith BN. Rapid inhibition of neurons in the dorsal motor nucleus of the vagus by leptin. Endocrinology 2007;148:1868-81. [PubMed]
- Schwartz GJ, Moran TH. Leptin and neuropeptide y have opposing modulatory effects on nucleus of the solitary tract neurophysiological responses to gastric loads: implications for the control of food intake. Endocrinology 2002;143:3779-84. [PubMed]
- Zhao S, Kanoski SE, Yan J, et al. Hindbrain leptin and glucagon-like-peptide-1 receptor signaling interact to suppress food intake in an additive manner. Int J Obes (Lond) 2012;36:1522-8. [PubMed]
- Zigman JM, Jones JE, Lee CE, et al. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 2006;494:528-48. [PubMed]
- Lawrence CB, Snape AC, Baudoin FM, et al. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology 2002;143:155-62. [PubMed]
- Faulconbridge LF, Cummings DE, Kaplan JM, et al. Hyperphagic effects of brainstem ghrelin administration. Diabetes 2003;52:2260-5. [PubMed]
- Faulconbridge LF, Grill HJ, Kaplan JM, et al. Caudal brainstem delivery of ghrelin induces fos expression in the nucleus of the solitary tract, but not in the arcuate or paraventricular nuclei of the hypothalamus. Brain Res 2008;1218:151-7. [PubMed]
- Andrews ZB, Liu ZW, Walllingford N, et al. UCP2 mediates ghrelin's action on NPY/AgRP neurons by lowering free radicals. Nature 2008;454:846-51. [PubMed]
- Cowley MA, Smith RG, Diano S, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003;37:649-61. [PubMed]
- Hewson AK, Dickson SL. Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. J Neuroendocrinol 2000;12:1047-9. [PubMed]
- Wang L, Saint-Pierre DH, Taché Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y - synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett 2002;325:47-51. [PubMed]
- Briggs DI, Enriori PJ, Lemus MB, et al. Diet-induced obesity causes ghrelin resistance in arcuate NPY/AgRP neurons. Endocrinology 2010;151:4745-55. [PubMed]
- Chen HY, Trumbauer ME, Chen AS, et al. Orexigenic action of peripheral ghrelin is mediated by neuropeptide Y and agouti-related protein. Endocrinology 2004;145:2607-12. [PubMed]
- Kamegai J, Tamura H, Shimizu T, et al. Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology 2000;141:4797-800. [PubMed]
- Kamegai J, Tamura H, Shimizu T, et al. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and Agouti-related protein mRNA levels and body weight in rats. Diabetes 2001;50:2438-43. [PubMed]
- Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature 2001;409:194-8. [PubMed]
- Willesen MG, Kristensen P, Rømer J. Co-localization of growth hormone secretagogue receptor and NPY mRNA in the arcuate nucleus of the rat. Neuroendocrinology 1999;70:306-16. [PubMed]
- Tannenbaum GS, Lapointe M, Beaudet A, et al. Expression of growth hormone secretagogue-receptors by growth hormone-releasing hormone neurons in the mediobasal hypothalamus. Endocrinology 1998;139:4420-3. [PubMed]
- Shuto Y, Shibasaki T, Otagiri A, et al. Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. J Clin Invest 2002;109:1429-36. [PubMed]
- Patterson ZR, Parno T, Isaacs AM, et al. Interruption of ghrelin signaling in the PVN increases high-fat diet intake and body weight in stressed and non-stressed C57BL6J male mice. Front Neurosci 2013;7:167. [PubMed]
- Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656-60. [PubMed]
- Ariyasu H, Takaya K, Tagami T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 2001;86:4753-8. [PubMed]
- Ghelardoni S, Carnicelli V, Frascarelli S, et al. Ghrelin tissue distribution: comparison between gene and protein expression. J Endocrinol Invest 2006;29:115-21. [PubMed]
- Yang J, Brown MS, Liang G, et al. Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 2008;132:387-96. [PubMed]
- Gutierrez JA, Solenberg PJ, Perkins DR, et al. Ghrelin octanoylation mediated by an orphanlipid transferase. Proc Natl Acad Sci U S A 2008;105:6320-5. [PubMed]
- Sakata I, Yang J, Lee CE, et al. Colocalization of ghrelin O-acyltransferase and ghrelin in gastric mucosal cells. Am J Physiol Endocrinol Metab 2009;297:E134-41. [PubMed]
- Kirchner H, Gutierrez JA, Solenberg PJ, et al. GOAT links dietary lipids with the endocrine control of energy balance. Nat Med 2009;15:741-5. [PubMed]
- Nishi Y, Mifune H, Yabuki A, et al. Changes in Subcellular Distribution of n-Octanoyl or n-Decanoyl Ghrelin in Ghrelin-Producing Cells. Front Endocrinol (Lausanne) 2013;4:84. [PubMed]
- Chopin LK, Seim I, Walpole CM, et al. The ghrelin axis--does it have an appetite for cancer progression? Endocr Rev 2012;33:849-91. [PubMed]
- Zhu X, Cao Y, Voogd K, et al. On the processing of proghrelin to ghrelin. J Biol Chem 2006;281:38867-70. [PubMed]
- Jeffery PL, Duncan RP, Yeh AH, et al. Expression of the ghrelin axis in the mouse: an exon 4-deleted mouse proghrelin variant encodes a novel C terminal peptide. Endocrinology 2005;146:432-40. [PubMed]
- Murakami N, Hayashida T, Kuroiwa T, et al. Role for central ghrelin in food intake and secretion profile of stomach ghrelin in rats. J Endocrinol 2002;174:283-8. [PubMed]
- Satou M, Nakamura Y, Ando H, et al. Understanding the functional significance of ghrelin processing and degradation. Peptides 2011;32:2183-90. [PubMed]
- Hosoda H, Doi K, Nagaya N, et al. Optimum collection and storage conditions for ghrelin measurements: octanoyl modification of ghrelin is rapidly hydrolyzed to desacyl ghrelin in blood samples. Clin Chem 2004;50:1077-80. [PubMed]
- De Vriese C, Gregoire F, Lema-Kisoka R, et al. Ghrelin degradation by serum and tissue homogenates: identification of the cleavage sites. Endocrinology 2004;145:4997-5005. [PubMed]
- De Vriese C, Hacquebard M, Gregoire F, et al. Ghrelin interacts with human plasma lipoproteins. Endocrinology 2007;148:2355-62. [PubMed]
- Sugino T, Hasegawa Y, Kikkawa Y, et al. A transient ghrelin surge occurs just before feeding in a scheduled meal-fed sheep. Biochem Biophys Res Commun 2002;295:255-60. [PubMed]
- Natalucci G, Riedl S, Gleiss A, et al. Spontaneous 24-h ghrelin secretion pattern in fasting subjects: maintenance of a meal-related pattern. Eur J Endocrinol 2005;152:845-50. [PubMed]
- Ingelsson E, Larson MG, Yin X, et al. Circulating ghrelin, leptin, and soluble leptin receptor concentrations and cardiometabolic risk factors in a community-based sample. J Clin Endocrinol Metab 2008;93:3149-57. [PubMed]
- Delporte C. Structure and physiological actions of ghrelin. Scientifica (Cairo) 2013;2013:518909.
- Howard AD, Feighner SD, Cully DF, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 1996;273:974-7. [PubMed]
- Leung PK, Chow KB, Lau PN, et al. The truncated ghrelin receptor polypeptide (GHS-R1b) acts as a dominant-negative mutant of the ghrelin receptor. Cell Signal 2007;19:1011-22. [PubMed]
- Chu KM, Chow KB, Leung PK, et al. Over-expression of the truncated ghrelin receptor polypeptide attenuates the constitutive activation of phosphatidylinositol-specific phospholipase C by ghrelin receptors but has no effect on ghrelin-stimulated extracellular signal-regulated kinase 1/2 activity. Int J Biochem Cell Biol 2007;39:752-64. [PubMed]
- Gnanapavan S, Kola B, Bustin SA, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab 2002;87:2988. [PubMed]
- De Vriese C, Delporte C. Influence of ghrelin on food intake and energy homeostasis. Curr Opin Clin Nutr Metab Care 2007;10:615-9. [PubMed]
- Ariyasu H, Takaya K, Iwakura H, et al. Transgenic mice overexpressing des-acyl ghrelin show small phenotype. Endocrinology 2005;146:355-64. [PubMed]
- Granata R, Baragli A, Settanni F, et al. Unraveling the role of the ghrelin gene peptides in the endocrine pancreas. J Mol Endocrinol 2010;45:107-18. [PubMed]
- Delhanty PJ, Sun Y, Visser JA, et al. Unacylated ghrelin rapidly modulates lipogenic and insulin signaling pathway gene expression in metabolically active tissues of GHSR deleted mice. PLoS One 2010;5:e11749. [PubMed]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci 2005;8:571-8. [PubMed]
- Elmquist JK, Coppari R, Balthasar N, et al. Identifying hypothalamic pathways controlling food intake, body weight, and glucose homeostasis. J Comp Neurol 2005;493:63-71. [PubMed]
- Gropp E, Shanabrough M, Borok E. er al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci 2005;8:1289-91. [PubMed]
- Luquet S, Perez FA, Hnasko TS, et al. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 2005;310:683-5. [PubMed]
- Luquet S, Phillips CT, Palmiter RD. NPY/AgRP neurons are not essential for feeding responses to glucoprivation. Peptides 2007;28:214-25. [PubMed]
- Tong Q, Ye CP, Jones JE, et al. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci 2008;11:998-1000. [PubMed]
- Andersson U, Filipsson K, Abbott CR, et al. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 2004;279:12005-8. [PubMed]
- Kola B, Hubina E, Tucci SA, et al. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem 2005;280:25196-201. [PubMed]
- López M, Lage R, Saha AK, et al. Hypothalamic fatty acid metabolism mediates the orexigenic action of ghrelin. Cell Metab 2008;7:389-99. [PubMed]
- Kohno D, Gao HZ, Muroya S, et al. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes 2003;52:948-56. [PubMed]
- Kohno D, Sone H, Minokoshi Y, et al. Ghrelin raises [Ca2+]i via AMPK in hypothalamic arcuate nucleus NPY neurons. Biochem Biophys Res Commun 2008;366:388-92. [PubMed]
- Kohno D, Nakata M, Maekawa F, et al. Leptin suppresses ghrelin-induced activation of neuropeptide Y neurons in the arcuate nucleus via phosphatidylinositol 3-kinase- and phosphodiesterase 3-mediated pathway. Endocrinology 2007;148:2251-63. [PubMed]
- Woods A, Dickerson K, Heath R, et al. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab 2005;2:21-33. [PubMed]
- Anderson KA, Ribar TJ, Lin F, et al. Hypothalamic CaMKK2 contributes to the regulation of energy balance. Cell Metab 2008;7:377-88. [PubMed]
- Tschöp M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 2000;407:908-13. [PubMed]
- Tschöp M, Weyer C, Tataranni PA, et al. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001;50:707-9. [PubMed]
- Andrews ZB, Erion DM, Beiler R, et al. Uncoupling protein-2 decreases the lipogenic actions of ghrelin. Endocrinology 2010;151:2078-86. [PubMed]
- Perreault M, Istrate N, Wang L, et al. Resistance to the orexigenic effect of ghrelin in dietary-induced obesity in mice: reversal upon weight loss. Int J Obes Relat Metab Disord 2004;28:879-85. [PubMed]
- Banks WA. The blood-brain barrier as a cause of obesity. Curr Pharm Des 2008;14:1606-14. [PubMed]
- Bouret SG, Gorski JN, Patterson CM, et al. Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab 2008;7:179-85. [PubMed]
- Martin TL, Alquier T, Asakura K, et al. Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J Biol Chem 2006;281:18933-41. [PubMed]
- Briggs DI, Andrews ZB. Metabolic status regulates ghrelin function on energy homeostasis. Neuroendocrinology 2011;93:48-57. [PubMed]
- Münzberg H. Differential leptin access into the brain--a hierarchical organization of hypothalamic leptin target sites? Physiol Behav 2008;94:664-9. [PubMed]
- Diano S, Farr SA, Benoit SC, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci 2006;9:381-8. [PubMed]
- Zhang W, Lin TR, Hu Y, et al. Ghrelin stimulates neurogenesis in the dorsal motor nucleus of the vagus. J Physiol 2004;559:729-37. [PubMed]
- Andrews ZB, Erion D, Beiler R, et al. Ghrelin promotes and protects nigrostriatal dopamine function via a UCP2-dependent mitochondrial mechanism. J Neurosci 2009;29:14057-65. [PubMed]
- Lutter M, Sakata I, Osborne-Lawrence S, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci 2008;11:752-3. [PubMed]
- Shrestha YB, Wickwire K, Giraudo S. Effect of reducing hypothalamic ghrelin receptor gene expression on energy balance. Peptides 2009;30:1336-41. [PubMed]
- Theander-Carrillo C, Wiedmer P, Cettour-Rose P, et al. Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest 2006;116:1983-93. [PubMed]
- Tschöp M, Statnick MA, Suter TM, et al. GH-releasing peptide-2 increases fat mass in mice lacking NPY: indication for a crucial mediating role of hypothalamic agouti-related protein. Endocrinology 2002;143:558-68. [PubMed]
- Tiesjema B, la Fleur SE, Luijendijk MC, et al. Viral mediated neuropeptide Y expression in the rat paraventricular nucleus results in obesity. Obesity (Silver Spring) 2007;15:2424-35. [PubMed]
- Patel HR, Qi Y, Hawkins EJ, et al. Neuropeptide Y deficiency attenuates responses to fasting and high-fat diet in obesity-prone mice. Diabetes 2006;55:3091-8. [PubMed]
- Kim MS, Yoon CY, Jang PG, et al. The mitogenic and antiapoptotic actions of ghrelin in 3T3-L1 adipocytes. Mol Endocrinol 2004;18:2291-301. [PubMed]
- Liu J, Lin H, Cheng P, et al. Effects of ghrelin on the proliferation and differentiation of 3T3-L1 preadipocytes. J Huazhong Univ Sci Technolog Med Sci 2009;29:227-30. [PubMed]
- Zwirska-Korczala K, Adamczyk-Sowa M, Sowa P, et al. Role of leptin, ghrelin, angiotensin II and orexins in 3T3 L1 preadipocyte cells proliferation and oxidative metabolism. J Physiol Pharmacol 2007;58 Suppl 1:53-64. [PubMed]
- Miegueu P, St Pierre D, Broglio F, et al. Effect of desacyl ghrelin, obestatin and related peptides on triglyceride storage, metabolism and GHSR signaling in 3T3-L1 adipocytes. J Cell Biochem 2011;112:704-14. [PubMed]
- Rodríguez A, Gómez-Ambrosi J, Catalán V, et al. Acylated and desacyl ghrelin stimulate lipid accumulation in human visceral adipocytes. Int J Obes (Lond) 2009;33:541-52. [PubMed]
- Giovambattista A, Piermaría J, Suescun MO, et al. Direct effect of ghrelin on leptin production by cultured rat white adipocytes. Obesity (Silver Spring) 2006;14:19-27. [PubMed]
- Davies JS, Kotokorpi P, Eccles SR, et al. Ghrelin induces abdominal obesity via GHS-R-dependent lipid retention. Mol Endocrinol 2009;23:914-24. [PubMed]
- Papotti M, Ghè C, Cassoni P, et al. Growth hormone secretagogue binding sites in peripheral human tissues. J Clin Endocrinol Metab 2000;85:3803-7. [PubMed]
- Tsubone T, Masaki T, Katsuragi I, et al. Ghrelin regulates adiposity in white adipose tissue and UCP1 mRNA expression in brown adipose tissue in mice. Regul Pept 2005;130:97-103. [PubMed]
- Zhang W, Chai B, Li JY, et al. Effect of des-acyl ghrelin on adiposity and glucose metabolism. Endocrinology 2008;149:4710-6. [PubMed]
- Sun Y, Butte NF, Garcia JM, et al. Characterization of adult ghrelin and ghrelin receptor knockout mice under positive and negative energy balance. Endocrinology 2008;149:843-50. [PubMed]
- Sun Y, Wang P, Zheng H, et al. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci U S A 2004;101:4679-84. [PubMed]
- Zigman JM, Nakano Y, Coppari R, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest 2005;115:3564-72. [PubMed]
- Pfluger PT, Kirchner H, Günnel S, et al. Simultaneous deletion of ghrelin and its receptor increases motor activity and energy expenditure. Am J Physiol Gastrointest Liver Physiol 2008;294:G610-8. [PubMed]
- Dezaki K, Sone H, Koizumi M, et al. Blockade of pancreatic islet-derived ghrelin enhances insulin secretion to prevent high-fat diet-induced glucose intolerance. Diabetes 2006;55:3486-93. [PubMed]
- Sun Y, Asnicar M, Saha PK, et al. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab 2006;3:379-86. [PubMed]
- Muller AF, Janssen JA, Hofland LJ, et al. Blockade of the growth hormone (GH) receptor unmasks rapid GH-releasing peptide-6-mediated tissue-specific insulin resistance. J Clin Endocrinol Metab 2001;86:590-3. [PubMed]
- Vestergaard ET, Gormsen LC, Jessen N, et al. Ghrelin infusion in humans induces acute insulin resistance and lipolysis independent of growth hormone signaling. Diabetes 2008;57:3205-10. [PubMed]
- Sangiao-Alvarellos S, Cordido F. Effect of ghrelin on glucose-insulin homeostasis: therapeutic implications. Int J Pept 2010;2010.
- Heppner KM, Tong J, Kirchner H, et al. The ghrelin O-acyltransferase-ghrelin system: a novel regulator of glucose metabolism. Curr Opin Endocrinol Diabetes Obes 2011;18:50-5. [PubMed]
- Sandoval D, Cota D, Seeley RJ. The integrative role of CNS fuel-sensing mechanisms in energy balance and glucose regulation. Annu Rev Physiol 2008;70:513-35. [PubMed]
- Parton LE, Ye CP, Coppari R, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 2007;449:228-32. [PubMed]
- Murphy BA, Fioramonti X, Jochnowitz N, et al. Fasting enhances the response of arcuate neuropeptide Y-glucose-inhibited neurons to decreased extracellular glucose. Am J Physiol Cell Physiol 2009;296:C746-56. [PubMed]
- van den Top M, Lyons DJ, Lee K, et al. Pharmacological and molecular characterization of ATP-sensitive K(+) conductances in CART and NPY/AgRP expressing neurons of the hypothalamic arcuate nucleus. Neuroscience 2007;144:815-24. [PubMed]
- Singhal NS, Lazar MA, Ahima RS. Central resistin induces hepatic insulin resistance via neuropeptide Y. J Neurosci 2007;27:12924-32. [PubMed]
- Date Y, Nakazato M, Hashiguchi S, et al. Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes 2002;51:124-9. [PubMed]
- Dezaki K, Hosoda H, Kakei M, et al. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: implication in the glycemic control in rodents. Diabetes 2004;53:3142-51. [PubMed]
- Zhang CY, Baffy G, Perret P, et al. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell 2001;105:745-55. [PubMed]
- Zhang CY, Parton LE, Ye CP, et al. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets. Cell Metab 2006;3:417-27. [PubMed]
- Murata M, Okimura Y, Iida K, et al. Ghrelin modulates the downstream molecules of insulin signaling in hepatoma cells. J Biol Chem 2002;277:5667-74. [PubMed]
- Barazzoni R, Zanetti M, Cattin MR, et al. Ghrelin enhances in vivo skeletal muscle but not liver AKT signaling in rats. Obesity (Silver Spring) 2007;15:2614-23. [PubMed]
- Sangiao-Alvarellos S, Helmling S, Vázquez MJ, et al. Ghrelin neutralization during fasting-refeeding cycle impairs the recuperation of body weight and alters hepatic energy metabolism. Mol Cell Endocrinol 2011;335:177-88. [PubMed]
- Lim CT, Kola B, Korbonits M, et al. Ghrelin's role as a major regulator of appetite and its other functions in neuroendocrinology. Prog Brain Res 2010;182:189-205. [PubMed]
- Patel AD, Stanley SA, Murphy KG, et al. Ghrelin stimulates insulin-induced glucose uptake in adipocytes. Regul Pept 2006;134:17-22. [PubMed]
- Barazzoni R, Zanetti M, Stebel M, et al. Hyperleptinemia prevents increased plasma ghrelin concentration during short-term moderate caloric restriction in rats. Gastroenterology 2003;124:1188-92. [PubMed]
- Redman LM, Veldhuis JD, Rood J, et al. The effect of caloric restriction interventions on growth hormone secretion in nonobese men and women. Aging Cell 2010;9:32-9. [PubMed]
- Yukawa M, Cummings DE, Matthys CC, et al. Effect of aging on the response of ghrelin to acute weight loss. J Am Geriatr Soc 2006;54:648-53. [PubMed]
- Zhao TJ, Liang G, Li RL, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc Natl Acad Sci U S A 2010;107:7467-72. [PubMed]
- Korbonits M, Jacobs RA, Aylwin SJ, et al. Expression of the growth hormone secretagogue receptor in pituitary adenomas and other neuroendocrine tumors. J Clin Endocrinol Metab 1998;83:3624-30. [PubMed]
- Kim K, Arai K, Sanno N, et al. Ghrelin and growth hormone (GH) secretagogue receptor (GHSR) mRNA expression in human pituitary adenomas. Clin Endocrinol (Oxf) 2001;54:759-68. [PubMed]
- Waseem T. Role of ghrelin axis in colorectal cancer: a novel association. Peptides 2008;29:1369-76. [PubMed]
- Waseem T, Duxbury M, Ashley SW, et al. Ghrelin promotes intestinal epithelial cell proliferation through PI3K/Akt pathway and EGFR trans-activation both converging to ERK 1/2 phosphorylation. Peptides 2014;52:113-21. [PubMed]
- Cassoni P, Ghé C, Marrocco T, et al. Expression of ghrelin and biological activity of specific receptors for ghrelin and des-acyl ghrelin in human prostate neoplasms and related cell lines. Eur J Endocrinol 2004;150:173-84. [PubMed]
- Duxbury MS, Waseem T, Ito H, et al. Ghrelin promotes pancreatic adenocarcinoma cellular proliferation and invasiveness. Biochem Biophys Res Commun 2003;309:464-8. [PubMed]
- Murphy G, Kamangar F, Dawsey SM, et al. The relationship between serum ghrelin and the risk of gastric and esophagogastric junctional adenocarcinomas. J Natl Cancer Inst 2011;103:1123-9. [PubMed]
- Sadjadi A, Yazdanbod A, Lee YY, et al. Serum ghrelin; a new surrogate marker of gastric mucosal alterations in upper gastrointestinal carcinogenesis. PLoS One 2013;8:e74440. [PubMed]


