Cytokeratin 18 for chemotherapy efficacy in gastric cancer
Introduction
Although the overall incidence and mortality of gastric cancer have dramatically declined over the last few decades, it remains a major health problem and the second leading cause of cancer deaths worldwide (1,2). Radical surgery is the most efficient treatment for operable cancer, but recurrences are common, being detected in approximately 60% of patients (3). Therefore, identifying of poor prognostic factors that may predict the tumor recurrence and prognosis of patients is an important for selection appropriate treatment protocols. Several clinicopathological parameters such as tumor size, histological type, tumor differentiation, depth of tumor invasion, regional lymph node involvement, distant metastasis and tumor stage, have been reported as important prognostic factors (4). Recently, new prognostic biological and molecular indicators have been documented including oncogenes, cell-cycle regulators, DNA repair genes, c-erbB-2 (5,6). Thus, understanding of the importance of these markers remains an important challenge in translational research.
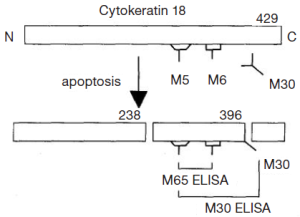
Cytokeratin 18 (CK18) is an intermediate filament that released from cells into the circulation during both necrotic and apoptotic cell death. Caspase-cleaved CK18 (M30) and total CK18 (M65) are measured in the circulation by enzyme-linked immunoabsorbent assays (ELISA) (Figure 1). It is believed that to act as a quantitative biomarker of total cell death (8,9). Prognostic importance of both M30 and M65 assays have previously been evaluated and it has been shown that they may have an important prognostic and predictive biomarkers in several malignancies (10-15). Scott et al. indicated that these assays may be potential markers of tumor response to chemotherapy and were associated with increased risk of recurrence in gastrointestinal malignancy (16).
The anticancer activity of chemotherapeutic agents is directly associated with the induction of apoptosis in tumors. Whilst the apoptotic pathway is complex, the intrinsic mitochondrial pathway is the predominant apoptotic pathway in cancer cells. Among the several cellular substrates of the capsases, members of the cytokeratin family, including CK18, contribute to cellular collapse and apoptosis. Most cytotoxic drugs induce apoptosis, which increase significantly 24 hours after chemotherapy (17). Therefore, apoptosis can be measured in serum by several biomarkers by which the efficacy of cytotoxic chemotherapy can be detected (11,18). de Haas et al. showed that both serum M30 and M65 levels were increased after chemotherapy. These assays could also reflect chemotherapy induced cell-death in patients with testicular cancer (19). Recently, we analyzed prognostic significance of the increase in the serum M30 and M65 values after chemotherapy in two studies for patients advanced-non-small cell lung cancer (NSCLC) and gastric cancer (20,21). In addition, we showed that increased serum M65 levels after chemotherapy could be predicted tumor response in advanced-gastric cancer patients (21). This article reviews the importance of CK18 for the efficacy of chemotherapy in patients with gastric cancer in the light of recent advances.
Prognostic significance of CK18 in cancer
The majority of chemotherapeutic agents kill tumor cells by some mechanisms including apoptosis and necrosis. Apoptosis may be an important mechanism for the evaluation of the efficacy of the anti-cancer therapy (7,22,23). Some biomarkers such as CK18 have been used for the detection of apoptosis of epithelial cells. M30 is a caspase-cleaved form of CK18 that it is released into circulation during apoptosis, whereas necrosis is supposed to release M65 that is an intact or total form of CK18 (8,9,24). Two sandwiched ELISA assays, M30 and M65, can be used to determine different circulating forms of CK18 in either plasma or serum, and have been proposed to be surrogate biomarkers of different mechanisms of cell death (8,25). The M30 ELISA assay uses the M5 antibody as a catcher and the M30 antibody to detect caspase-cleaved CK18 produced during the early stages of apoptosis (25). The M65 assay also detects cleaved fragments but uses a different detection antibody from M30, which does not distinguish between the full-length protein and its fragments. The M65 assay theoretically measures both caspase cleavage and cellular release of intact CK18 (8). Serum M65 and M30 levels were shown to be elevated in patients with different types of carcinoma (10,12,14,16). The measurement of caspase-cleaved or total CK18 from epithelial-derived tumors could be a simple, noninvasive way to monitor or predict tumor progression (26) and prognosis (10,12,26).
de Haas et al. showed that serum M30 level was an important prognostic factor in testicular cancer (19). In a study performed by Dive et al., they reported that the median M65 levels in patients with metastatic pancreas cancer were higher compared to patients with locally advanced or resectable pancreas cancer. Moreover, they found that M65 levels were associated with poor overall survival (OS) in the univariate analysis (27). Koelink et al. indicated that M65 levels were related to disease stage and tumor diameter in colorectal cancer patients (26). The caspase-cleaved/CK18 ratio, which decreased with tumor progression, was also predictive of disease-free survival (DFS), with a low ratio associated with worse DFS. In a study of patients with advanced gastric carcinoma, Yaman et al. found that both serum M30 and M65 levels were significantly increased in patients with advanced gastric cancer compared to control group (10). In addition, the patient population with higher M30 levels had significantly shorter median survival rates than the population with lower serum M30 levels, whereas there was no impact of serum M65 levels on survival. In contrast to their study, we showed that increased plasma levels of both M30 and M65 could predict progression-free survival (PFS) in patients with advanced-gastric cancer in our study (28).
Recently, Yildiz et al. analyzed the serum M30 and M65 levels in patients with epithelial ovarian cancer (EOC) (29). They found that the median M30 and M65 serum levels were significantly elevated in the EOC patients compared with the healthy controls. Furthermore, patients with higher M65 levels had shorter PFS, both M30 and M65 serum levels were significantly higher for serous-type histology and increased M65 serum levels were associated with advanced disease and higher grade. M65 levels were higher for chemotherapy-resistant patients and in the multivariate analysis an elevated serum M65 level was found to be only significant independent prognostic factor (29). Similarly, in our study including advanced-staged NSCLC, our findings demonstrated that serum 65 levels elevated in patients population compared to a healthy control group and increased M65 level could predict PFS (30). On the other hand, it found that there were discrepancies in other studies. Although M30 and M65 levels were detected to be increased in patients with breast cancer, pancreatic cancer and nasopharyngeal carcinoma compared to healthy control, their predictive and prognostic roles on survival were not proved (14,31,32). Selected trials evaluating prognostic significance of CK18 in solid tumors are summarized in Table 1.
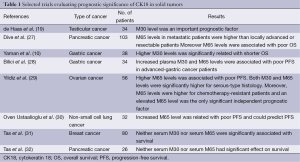
Full table
Changing of CK18 after chemotherapy in solid tumors
M30 and M65 levels have been previously found to be increased within 1 to 3 days after the chemotherapy in patients with breast and prostate cancer (13,33). However, de Haas et al. observed that most significant changes occurred 7 days after the chemotherapy in patients with testicular cancer, which may reflect the cumulative effect of the 5-day dosing regimen used in testicular cancer (19). Demiray et al. evaluated the serum M30 levels before and 24 and 48 hours after neoadjuvant chemotherapy in 42 patients with breast cancer (11). The authors found that the serum M30 levels increased significantly at 24 and 48 hours after chemotherapy and that this change was a predictor of the tumor response. Similar findings for M30 levels were reported for patients with breast cancer in Ulukaya et al.’s study (18).
We previously reported that the serum M30 and M65 levels were increased significantly after chemotherapy in patients with NSCLC (20). In addition, the elevated M30 values were an independent prognostic factor for both PFS and OS after chemotherapy. de Haas et al. showed that both serum M30 and M65 levels were significantly increased up to 7 days after chemotherapy in patients with testicular cancer (19). Furthermore, they found a significant decrease in the serum M30 and M65 levels during the first two weeks of chemotherapy compared to baseline values. Thus, the authors indicated that the overall decrease in M30 and M65 values may be indicative for response to treatment and reflect a decrease in tumor load because of chemotherapy-induced cell death.
Greystoke et al. explored the utility pf serum total CK18 (M65) and caspase cleaved CK18 (M30) as a pharmacodynamic biomarker in patients treated with chemotherapy for metastatic colorectal cancer (34). They showed that in patients with progressive disease on therapy repeated sampling revealed profiles with high pre-treatment and progressive upwardly after one cycle of chemotherapy.
Prognostic and predictive significance of CK18 after chemotherapy for patients with gastric cancer
Firstly, the serum M30 and M65 levels were analyzed in a study carried out by Yaman et al. in patients with advanced-gastric cancer (10). They showed that the serum M30 and M65 values were significantly higher in patient population compared with healthy control group. In addition, only M30 levels were associated with worse survival. Thereafter, we also evaluated plasma M30 and M65 levels for advanced-gastric cancer patients (28). We found that plasma 65 levels for patients with gastric cancer were significantly higher those of healthy controls. Furthermore, both elevated plasma M30 and M65 levels were related with worse PFS and OS, but this relationship could not be proved in the multivariate analysis.
In our recent study, the significance of changes in the serum M30 and M65 levels after chemotherapy in patients with advanced-gastric cancer (21). We showed that both serum M30 and M65 serum levels were significantly increased 48 hours after start of chemotherapy. A multivariate analysis showed that only the increase of M30 values was an independent prognostic indicator for PFS, but not for OS. Although the increased M65 value was an important prognostic marker for both PFS and OS in the univariate analysis, it’ prognostic significance could not be confirmed by multivariate analysis. Moreover, patients with increased both serum M30 and M65 values after chemotherapy had better objective response rate compared to patients with low M30 and M65 levels. However, only the changing of M65 after chemotherapy was significantly found to be an independent factor in predicting response to chemotherapy. In other words, patients who responded to chemotherapy had 1.4-fold higher increase in serum M65 values compared with the non-responder.
Previous studies showed that CK18 assays may be beneficial in early assessment of treatment-related tumor death and subsequent prediction of response to therapy (35,36). On the other hand, early both M30 and M65 changes during chemotherapy may not be helpful, because of overlap with host toxicity (37). We found significant changes of both serum M30 and M65 levels within 48 hours after the first chemotherapy cycle in patients with advanced-gastric cancer (21). This was in contrast to the results of Greystoke et al. (34), who did not show that any significant changes in the serum levels of both M30 and M65 within the first 48 hours, but they have indicated that for many patients there was a decrease in serum M65 levels, and to a lesser extent M30, from 1 week after chemotherapy. These findings were compatible with de Haas et al.’s study (19).
The effect of taxanes on mitotic catastrophe, characterized by the occurence of aberrant mitosis has been demonstrated. Although mitotic catastrophe is not a type of cell death, it will result in cell death either by apoptosis or necrosis (38,39). Kramer et al. also reported that serum caspase-cleaved CK18 (M30) levels were increased during docetaxel treatment in patients with hormone refractory prostate cancer (33). Hence, the authors concluded that docetaxel induced apoptosis in vivo. The majority of patients with metastatic gastric cancer are treated with a combination chemotherapy including docetaxel. Therefore, significant changes in serum M30 and M65 levels for patients with advanced-gastric cancer receiving docetaxel are reasonable. There is an initial effect of chemotherapy on the population of cells that are chemo-sensitive leading to an initial reduction in overall tumour burden, with the later increases in circulating CK18 reflecting subsequent growth in the population of chemo-resistant cells. Therefore, both serum M30 and M65 levels could be used as surrogates of treatment response to monitor the development of chemo-resistance and lead to early changes in therapy. Table 2 shows selected trials that analyzed prognostic and predictive significance of CK18 after chemotherapy for patients with gastric cancer and other cancer.
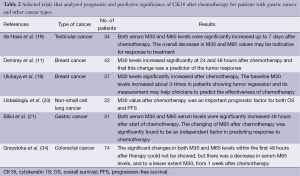
Full table
Conclusions and future direction
CK18 may modulate intracellular signaling and apoptosis via interactions with various related proteins. There is evidence to show that CK18 is involved in the invasive or growth properties of tumors. Caspase-cleaved CK18 (M30) and total CK18 (M65) are measured in the circulation by enzyme-linked immunoabsorbent assays (ELISA). The measurement of M30 or M65 from epithelial-derived tumors could be a simple, noninvasive way to monitor or predict tumor progression and prognosis. However, there are conflicting results in vitro and in vivo. These discrepancies may be due to differences among the carcinoma types, therefore the precise roles of CK18 are currently unknown. But, particularly, in patients with gastric cancer, testicular cancer and colorectal cancer, serum M30 and/or M65 levels could be used as biomarkers to evaluate treatment response and they might guide in determining of the most appropriate combination chemotherapy regimen.
These assays may be useful for evaluating treatment effects and survival in patients with gastric cancer, but in combination with other cell death markers, their importance should be tested after multiple chemotherapy sessions in larger prospective studies with long follow-up time in future.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol 2006;12:354-62. [PubMed]
- Buzzoni R, Bajetta E, Di Bartolomeo M, et al. Pathological features as predictors of recurrence after radical resection of gastric cancer. Br J Surg 2006;93:205-9. [PubMed]
- Hohenberger P, Gretschel S. Gastric cancer. Lancet 2003;362:305-15. [PubMed]
- Lee KE, Lee HJ, Kim YH, et al. Prognostic significance of p53, nm23, PCNA and c-erbB-2 in gastric cancer. Jpn J Clin Oncol 2003;33:173-9. [PubMed]
- Lee HK, Lee HS, Yang HK, et al. Prognostic significance of Bcl-2 and p53 expression in gastric cancer. Int J Colorectal Dis 2003;18:518-25. [PubMed]
- Ueno T, Toi M, Linder S. Detection of epithelial cell death in the body by cytokeratin 18 measurement. Biomed Pharmacother 2005;59:S359-62. [PubMed]
- Kramer G, Erdal H, Mertens HJ, et al. Differentiation between cell death modes using measurements of different soluble forms of extracellular cytokeratin 18. Cancer Res 2004;64:1751-6. [PubMed]
- Schutte B, Henfling M, Kolgen W, et al. Keratin 8/18 breakdown and reorganization during apoptosis. Exp Cell Res 2004;297:11-26. [PubMed]
- Yaman E, Coskun U, Sancak B, et al. Serum M30 levels are associated with survival in advanced gastric carcinoma patients. Int Immunopharmacol 2010;10:719-22. [PubMed]
- Demiray M, Ulukaya EE, Arslan M, et al. Response to neoadjuvant chemotherapy in breast cancer could be predictable by measuring a novel serum apoptosis product, caspase-cleaved cytokeratin 18: a prospective pilot study. Cancer Invest 2006;24:669-76. [PubMed]
- Ulukaya E, Yilmaztepe A, Akgoz S, et al. The levels of caspase-cleaved cytokeratin 18 are elevated in serum from patients with lung cancer and helpful to predict the survival. Lung Cancer 2007;56:399-404. [PubMed]
- Olofsson MH, Ueno T, Pan Y, et al. Cytokeratin-18 is a useful serum biomarker for early determination of response of breast carcinomas to chemotherapy. Clin Cancer Res 2007;13:3198-206. [PubMed]
- Ozturk B, Coskun U, Sancak B, et al. Elevated serum levels of M30 and M65 in patients with locally advanced head and neck tumors. Int Immunopharmacol 2009;9:645-8. [PubMed]
- Ausch C, Buxhofer-Ausch V, Olszewski U, et al. Caspase-cleaved cytokeratin 18 fragment (M30) as marker of postoperative residual tumor load in colon cancer patients. Eur J Surg Oncol 2009;35:1164-8. [PubMed]
- Scott LC, Evans TR, Cassidy J, et al. Cytokeratin 18 in plasma of patients with gastrointestinal adenocarcinoma as a biomarker of tumour response. Br J Cancer 2009;101:410-7. [PubMed]
- Hickman JA, Beere HM, Wood AC, et al. Mechanism of cytotoxicity caused by antitumor drugs. Toxicol Lett 1992;64-65:553-61. [PubMed]
- Ulukaya E, Karaagac E, Ari F, et al. Chemotherapy increases caspase-cleaved cytokeratin 18 in the serum of breast cancer patients. Radiol Oncol 2011;45:116-22. [PubMed]
- de Haas EC, di Pietro A, Simpson KL, et al. Clinical evaluation of M30 and M65 ELISA cell death assays as circulating biomarkers in a drug-sensitive tumor, testicular cancer. Neoplasia 2008;10:1041-8. [PubMed]
- Ustaalioglu BB, Bilici A, Ercan S, et al. The prognostic importance of changing serum M30 and M65 values after chemotherapy in patients with advanced-stage non-small-cell lung cancer. Med Oncol 2013;30:551. [PubMed]
- Bilici A, Ustaalioglu BB, Ercan S, et al. The prognostic significance of the increase in the serum M30 and M65 values after chemotherapy and relationship between these values and clinicopathological factors in patients with advanced gastric cancer. Tumour Biol 2012;33:2201-8. [PubMed]
- Renz A, Berdel WE, Kreuter M, et al. Rapid extracellular release of cytochrome c is specific for apoptosis and marks cell death in vivo. Blood 2001;98:1542-8. [PubMed]
- Green AM, Steinmetz ND. Monitoring apoptosis in real time. Cancer J 2002;8:82-92. [PubMed]
- Caulín C, Salvesen GS, Oshima RG. Caspase cleavage of keratin 18 and reorganization of intermediate filaments during epithelial cell apoptosis. J Cell Biol 1997;138:1379-94. [PubMed]
- Leers MP, Kölgen W, Björklund V, et al. Immunocytochemical detection and mapping of a cytokeratin neo-epitope exposed during early apoptosis. J Pathol 1999;187:567-72. [PubMed]
- Koelink PJ, Lamers CB, Hommes DW, et al. Circulating cell death products predict clinical outcome of colorectal cancer patients. BMC Cancer 2009;9:88. [PubMed]
- Dive C, Smith RA, Garner E, et al. Considerations for the use of plasma cytokeratin 18 as a biomarker in pancreatic cancer. Br J Cancer 2010;102:577-82. [PubMed]
- Bilici A, Ustaalioglu BB, Ercan S, et al. Is there any impact of plasma M30 and M65 levels on progression-free survival of patients with advanced gastric cancer? Cancer Chemother Pharmacol 2011;68:309-16. [PubMed]
- Yildiz I, Tas F, Kilic L, et al. A high serum level of M65 is associated with tumour aggressiveness and an unfavourable prognosis for epithelial ovarian cancer. Cancer Chemother Pharmacol 2013;72:437-44. [PubMed]
- Oven Ustaalioglu B, Bilici A, Ercan S, et al. Serum M30 and M65 values in patients with advanced stage non-small-cell lung cancer compared with controls. Clin Transl Oncol 2012;14:356-61. [PubMed]
- Tas F, Karabulut S, Yildiz I, et al. Clinical significance of serum M30 and M65 levels in patients with breast cancer. Biomed Pharmacother 2014;68:1135-40. [PubMed]
- Tas F, Karabulut S, Bilgin E, et al. Clinical significance of serum M30 and M65 levels in metastatic pancreatic adenocarcinoma. Tumour Biol 2013;34:3529-36. [PubMed]
- Kramer G, Schwarz S, Hägg M, et al. Docetaxel induces apoptosis in hormone refractory prostate carcinomas during multiple treatment cycles. Br J Cancer 2006;94:1592-8. [PubMed]
- Greystoke A, Dean E, Saunders MP, et al. Multi-level evidence that circulating CK18 is a biomarker of tumour burden in colorectal cancer. Br J Cancer 2012;107:1518-24. [PubMed]
- Dean E, Jodrell D, Connolly K, et al. Phase I trial of AEG35156 administered as a 7-day and 3-day continuous intravenous infusion in patients with advanced refractory cancer. J Clin Oncol 2009;27:1660-6. [PubMed]
- Gandhi L, Camidge DR, Ribeiro de Oliveira M, et al. Phase I study of navitoclax (ABT-263), a novel bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumours. J Clin Oncol 2011;29:909-16. [PubMed]
- Greystoke A, O’Connor JP, Linton K, et al. Assessment of circulating biomarkers for potential pharmacodynamic utility in patients with lymphoma. Br J Cancer 2011;104:719-25. [PubMed]
- Blajeski AL, Kottke TJ, Kaufmann SH. A multistep model for paclitaxel-induced apoptosis in human breast cancer cell lines. Exp Cell Res 2001;270:277-88. [PubMed]
- Ricci MS, Zong WX. Chemotherapeutic approaches for targeting cell death pathways. Oncologist 2006;11:342-57. [PubMed]


