Molecular target therapy for gastrointestinal stromal tumors
Introduction
Gastrointestinal stromal tumors (GIST) initially named by Mazur and Clark in 1983 (1) are the most common mesenchymal tumors of the GI tract with a specific mutation and a suitable targeted treatment. They occur most commonly in the stomach (60-70%) and small intestine (25-35%) and rarely in the colorectal region (5%), esophagus (<2%), appendix, omentum, mesentery or retroperitoneum (2) occurring most commonly in the 5th to 6th decade of life. Various risk stratification schemes have been identified for prognostication of GIST. As an addition to these factors, tumor genotyping is being studied extensively and in future may also be incorporated into the risk stratification schemes. Tumor genotyping involves the identification of the causative genetic alteration and tailored therapy catering to that particular genetic abnormality (3). Here we present a comprehensive review of targeted therapy used in the management of GIST.
Pathology
On histo-pathology, GISTs are made of fascicles of spindle cells with eosinophilic cytoplasm, nuclear palisading, inconspicuous nucleoli and extracellular collagen. They can be of three types: spindle (70%), epitheloid or mixture of both (2). On immunohistochemistry (IHC), along with diffuse CD117 positivity (about 95%), other markers which are useful diagnostically are BCL-2 (80%) and CD34 positivity (70%), variable expression of smooth muscle actins (20-30%) and S100 protein (10%) and desmin negativity (2-4% positive). DOG-1 (discovered on GIST) is a novel marker, which is a calcium dependent protein and is positive in GIST irrespective of the mutation status (4). Most of the tumors have low rate of mitoses. These tumor cells are admixed with lymphocytes and apoptotic debris giving a false impression of high mitotic index. Calculation of mitoses is one of the major tasks in calculation of the recurrence risk and the loophole is the difficulty in calculating mitotic count, as most pathologists tend to over count it due to the miscount of lymphocytes and apoptotic and karyorrhectic bodies as part of active mitotic figures (5). The dilemma lies in where exactly the mitotic count has to be assessed and how large the 50 high power fields (HPF) must be. While the area of each HPF has varied from 5 to 12 mm2, the ESMO recommendation is an area of 10 mm2. Moreover, whether the areas should be consecutive or randomly selected in highly cellular parts has not been standardized.
Molecular basis and mutations driving therapy
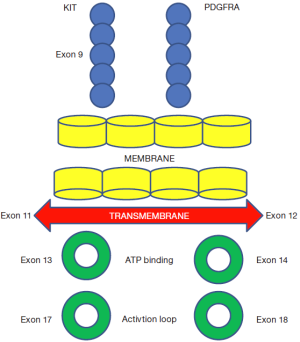
CD117 (KIT) mutation is the most common mutation seen in GIST (80-85%) (6). It was discovered by Hirota et al. in 1998 (7). It is encoded by the KIT proto-oncogene which is present on chromosome 4 (8). In physiologic conditions, the ligand for KIT receptor called the stem cell factor (SCF) (steel factor) binds at the receptor and then after homodimerisation results in a cascade of events causing cell survival and proliferation. Under malignant conditions, this cascade gets activated due to activating mutations and the same cycle is continued irrespective of ligand binding and results in tumorigenesis (9). The pathways activated are the Ras/Raf/MAPK pathway, JAK-STAT pathway, IGF pathway, PI3K/AKT and mTOR pathways (10,11). CD117 is expressed on the interstitial cells of Cajal which are responsible for GI peristalsis, thus hypothesized to be the cell of origin for GIST (7). Exon 11 KIT mutations are the most common (65-70%), which happens usually in the juxtamembrane domain (Figure 1). These could be point mutations, deletions or duplications and are more common in gastric GIST and show good response to imatinib, whereas exon 9 mutations (5-10%) usually are 2-codon 502-503 duplications in the extracellular domain (made up of five immunoglobulin like molecules) and these occur predominantly in intestinal versus gastric GISTs and are less responsive to imatinib. Other mutations could occur in the ATP binding domain of exon 13 and 14 or exon 17 at the activation loop of the kinase domain (12). In patients with KIT negative tumors (15%), 30-40% will be positive for platelet derived growth factor receptor alpha (PDGFRA), the gene for which is also situated on chromosome 4 and these tumors are usually of epitheloid variant and gastric in location (8,13). PDGFRA mutations could be in exon 18 (most common) (activation loop domain), 12 (juxtamembrane domain) or 14 (ATP binding domain) (11,13). A minority of the cases especially in pediatric age group will be wild type GISTs. KIT negative tumors have a better prognosis than KIT positive tumors (14). Patients with KIT mutation have a poor prognosis especially those with deletions affecting codons 557-558 (15,16). Presently studies are undergoing to study the genetic expression of GIST. Stomach and small bowel GISTs have varying genetic expression. High gene expression of vascular endothelial growth factor (VEGF), Macrophage colony stimulating factor, and BCL2 was noticed in the wild-type group, and Mesothelin in exon 9 mutation group (17). AKT3 and Ezrin was expressed more in KIT exon 11 and 9 mutations and less in PDGFRA mutated GISTs whereas MEK and T Cell receptor signaling genes were found to be high in PDGFRA mutated tumors (18). In addition to the above mutations, loss of tumor suppressor genes present on chromosome 14 and 22q have also been seen (19). Other GISTs could be familial (mutation in exon 8, 11, 13 or 17) or associated with neurofibromatosis 1, Carney’s triad or Carney-Stratakis syndrome (20).
Management of non-metastatic GIST
Neoadjuvant therapy and surgery
The success of imatinib in metastatic GIST led its entry into neo-adjuvant and adjuvant setting. Surgical resection with clear margins should be the main goal while treating GISTs with curative intent. While the median survival post complete resection is approximately 66 months, it gets reduced to 22 months if the disease is unresectable (9). Tumors more than 2 cm should be resected and lymph node dissection is not recommended as metastases to nodes are rare (21). Imatinib when used in borderline resectable locally advanced cases can reduce the tumor bulk and make the tumor amenable for surgery with clear margins especially in critical sites like rectum (22,23). In a study of 46 patients, they found that all eleven patients with locally advanced disease could undergo complete surgical resection after a median of 11.9 months of neoadjuvant imatinib (24). The duration of neoadjuvant imatinib is not clearly defined. In a study they found that post neoadjuvant imatinib the 2-year recurrence-free survival (RFS) was 85% and 44% and overall survival (OS) was 97% and 73% for primary and recurrent/metastatic disease, respectively. Moreover on univariate analysis, the duration of neoadjuvant therapy of more than 365 days (P=0.02) was associated with a higher risk of recurrence (25). In a pooled database of ten EORTC STBSG sarcoma centers, patients with locally advanced GIST who received neo-adjuvant imatinib were studied. After a median 40 weeks of imatinib, the rate of R0 resection was 83% and the 5-year disease free survival (DFS) was 65% with median OS of 104 months (26). In a study done at Tata Memorial hospital in India, after a median duration of 8.5 months of neo-adjuvant imatinib, the response rate was 79% with a manageable post-operative complication rate of 14% and a 3-year OS of 100% (27).
Risk stratification and adjuvant therapy
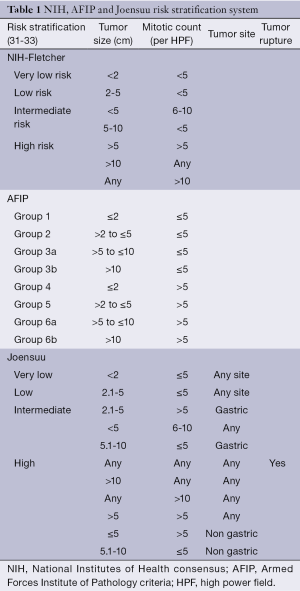
In the absence of adjuvant therapy, 50% patients recurred, especially in the first 5 years (28). Patients with high risk of recurrence are recommended to take adjuvant imatinib after complete gross resection (28-30). There are a number of risk stratification systems to predict the recurrence of GIST after complete surgical resection. The important ones being (Table 1):
Full table
- The National Institutes of Health (NIH) consensus criteria (Fletcher’s criteria);
- The Armed Forces Institute of Pathology (AFIP) criteria (Miettinen’s criteria);
- Joensuu’s modified NIH classification (J-NIHC) (the two modifications were):
- Tumor rupture was added;
- Non-gastric tumors in the intermediate risk were converted to high risk;
- The American Joint Committee on Cancer staging system (AJCCS);
- The Japanese modified NIH criteria.
The NIH, AFIP and Joensuu’s criteria are the most commonly used. Bases on good to poor prognosis the site predilection is as follows: gastric, small intestine, colorectal, extra GI GISTs. Based on size, the 10-year recurrence rate for <1 cm (micro GIST), 5-10 cm and 10-15 cm tumors is 0%, 50% and 70% respectively. Based on mitosis, the 10-year recurrence rate for <5 and >5 mitoses/HPF is 25% and 70% respectively (34). According to the modified NIH criteria, the 10-year RFS for very low, low, intermediate and high risk is 95%, 90%, 85% and 35% respectively (34). Of 127 patients were analyzed at the MSKCC with localized primary GIST who underwent complete gross surgical resection of disease. After a median follow-up of 4.7 years, RFS was 83%, 75%, and 63% at 1, 2, and 5 years, respectively. Factors predictive of increased recurrence were ≥5 mitoses/50 HPF, tumor size 10 cm, and patients with small intestine tumors did worse. While KIT exon 11 point mutations and insertions had a good prognosis, KIT exon 9 mutations or exon 11 deletions involving amino acid W557 and/or K558 had a bad prognosis and wild type GISTs had intermediate outcome (35). A nomogram to predict RFS based on tumor size, location and mitotic index (<5 or ≥5/HPF) after surgery in the absence of adjuvant imatinib was proposed by Gold et al. The concordance probability was 0.78 (standard error ±0.02). Moreover this nomogram was better than the NIH staging system and equivalent to the AFIP staging system for recurrence prediction (36). Yanagimoto et al. analyzed 712 GIST patients after surgery and compared the above systems. They found that the factors significant on multivariate analysis were size >5 cm, mitotic count >5/50 HPF, non-gastric location, and the presence of rupture and/or macroscopic invasion. They also found out that the J-NIHC and AJCCS were respectively the most sensitive and accurate tools to predict recurrence (37). Zhao et al. further classified the high risk group into very high risk group which included tumors having mitoses count >10/50 HPF and serosal invasion. Specifically in tumors with serosal invasion, despite adjuvant imatinib the recurrence rates were high, thus stressing the importance of neoadjuvant imatinib so that serosal invasion is reduced (38). In another study by the same authors, they found that Ki67 index >8% also was a poor prognostic factor (39).
In the ACOSOG Z9000 phase II trial, 107 high risk recurrence (tumor size >10 cm, tumor rupture, or <5 peritoneal metastases) patients received 1 year of imatinib 400 mg as adjuvant therapy and was compared with placebo. The 1-, 3- and 5-year RFS was 96%, 60% and 40% and OS was 99%, 97% and 83% respectively. While the median RFS was 4 years, the median OS had not been reached (40). In the subsequent phase III trial (ACOSOG Z9001) patients with tumor >3 cm were randomized to adjuvant imatinib versus placebo for 1 year. The RFS was 98% in the imatinib arm and 83% in the placebo arm [hazard ratio (HR), 0.35; 95% confidence interval (CI), 0.22-0.53; P<0.0001], especially better in patients with high (size ≥10 cm) and intermediate (≥6 to <10 cm) risk. However there was no difference in OS which could be as a result of crossover to imatinib arm on progression (41). In this study, 28% patients discontinued imatinib due to toxicity. Based on these results, adjuvant imatinib was granted accelerated FDA approval in the year 2008 which in 2012 was converted to full approval. In a recent publication, in the same study they showed that large tumor size, small bowel location and high mitotic rate had lower RFS irrespective of the tumor genotype. Moreover, adjuvant imatinib improved RFS in KIT exon 11 deletions but not in KIT exon 11 insertions or point mutations, KIT exon 9 mutations, PDGFRA mutation or wild type GIST (41).
In the subsequent phase III Scandinavian Sarcoma Group/Arbeitsgemeinschaft Internistische Onkologie trial XVIII (SSG XVIII/AIO) trial, patients at high risk for recurrence (with at least one of the following: longest tumor diameter >10 cm, mitotic count >10/50 HPF, tumor diameter >5 cm, and mitotic count >5 or tumor rupture) after surgical removal, were randomly assigned to either 1 or 3 years of adjuvant imatinib. The 5-year RFS and OS were 66% versus 48% (HR, 0.46; 95% CI, 0.32-0.65; P<0.0001) and 92% versus 82% (HR, 0.45; 95% CI, 0.22-0.89; P=0.02), respectively in the 3- and 1-year group (29). 13.6% of patients in the 3-year arm discontinued imatinib due to adverse events than 7.5% in the 1-year arm. In another study 900 patients with intermediate- or high-risk resected GIST were randomized to 2 years of adjuvant imatinib versus no adjuvant therapy. The 3- and 5-year RFS was 84% versus 66% (P<0.001) and 69% versus 65% (P<0.001) in the imatinib versus no adjuvant therapy arms, respectively (42). In the phase II PERSIST-5 trial (Post resection Evaluation of Recurrence-free Survival for gastrointestinal Stromal Tumors) the benefit of 5 years of adjuvant imatinib will be studied. The current recommendation is to give 3 years of adjuvant imatinib for tumors with high risk of recurrence after complete gross resection (30,43).
Management of metastatic GIST
Surgery in metastatic GIST
The role of surgery after imatinib pre-treatment in metastatic patients is controversial. Cheng et al. studied the significance of pathological complete response (pCR) post imatinib in metastatic GIST and found out that patients with pCR had better PFS and OS than those without pCR [2-year PFS and OS: 82.5% and 100% versus 35.6% and 49.4%, (P=0.014 and P=0.004) respectively]. They also found that patients with pCR had lesser secondary mutations (44). In another study, patients with recurrent or metastatic GIST who had stable disease after 6 months of imatinib were randomized to surgery followed by imatinib continuation versus surgery alone and found that the surgery group had better PFS (HR, 2.326; 95% CI, 1.034-5.236; P=0.0412) and OS (HR, 5.464; 95% CI, 1.460-20.408; P=0.0117) (45). In a study done in China, the 2-year PFS was 88.4% in the surgery arm and 57.7% in the imatinib alone arm (P=0.089) while the median OS was not reached in the surgery arm and was 49 months in patients with Imatinib-alone arm (P=0.024) (46). In spite of all these data, surgery in metastatic patients is not recommended as a guideline and may be decided on an individual patient’s basis based on patient symptoms. The indications for surgery recommended by the NCCN in recurrent or metastatic GIST are (21):
- Disease that is stable or shrinking on TKI therapy when complete gross resection is possible;
- Isolated clones progressing on TKI therapy after initial response while other sites of disease remain stable;
- Emergencies like hemorrhage, perforation, obstruction or abscess.
First line TKI therapy for metastatic GIST
With the use of imatinib, the survival of advanced GIST can extend up to 5 years (47). Imatinib produces response rate of 67% in exon 11 KIT mutation and 40% in exon 9 mutation (48). Before the advent of imatinib, the OS of GIST patients varied from 10 to 20 months. The initial studies of imatinib in metastatic GIST were phase II trials which showed response rate of 82% with time to treatment progression (TTP) of 24 months and OS of 57 months (49,50). These benefits were later reconfirmed with phase III randomized trials (51,52). In the The EORTC Soft Tissue and Bone Sarcoma Group phase III randomised trial, 946 patients were randomised to receive 400 or 800 mg once daily imatinib. On progression on 400 mg, patients were allowed to crossover to the 800 mg arm. After a median follow-up of 2 years, the response rate in both groups was around 50% and OS at 1 and 2 years was 85% and 70% in the 400 mg and 800 mg groups respectively with many patients in the 800 mg arm requiring dose reductions (52). Even in the North American Sarcoma Intergroup study (S0033) 746 patients were randomised in a similar manner as the EORTC study. Even in this study the objective response rate (ORR), PFS and OS was similar in both the groups (53). Meta-analysis of these trials showed that the median OS was 4 years and both the doses were equivalent, however patients with exon 9 required 800 mg imatinib (54).
In metastatic patients, imatinib has to be continued until disease progression. In the French Sarcoma Group trial, 58 patients were randomised to imatinib continuation versus interruption after 1 year of treatment. Most of the patients in the interrupted group progressed, however majority of them responded to reintroduction of imatinib and no difference was seen in OS, resistance patterns or quality of life (55). The phase III Intergroup trial proved that KIT exon 11-mutant GIST had a better ORR of 71% and OS of 60 months, versus 45% (P=0.01) for both exon 9-mutant and KIT/PDGFRA wild-type tumors with OS of 39 and 49 months (P=0.049) (56).
Masitinib mesylate is another TKI with greater selectivity than imatinib especially in exon 11 mutation which has shown promising results in phase II trials when used as 1st line in metastatic GIST with a PFS of approximately 41 months and is currently being studied in phase III trials (57,58).
Response assessment to imatinib in GIST
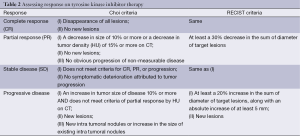
RECIST which is used for response assessment in most solid tumors is not a very good criterion for assessing response to TKI in GIST, as due to necrosis and cystic degeneration, only calculation of tumor size may not be accurate. Choi et al. proposed different criteria (Table 2) in which along with size, tumor density is also taken into account (59). While routine CT scan is sufficient for assessing response, 18FDG-PET can be used for (59):
Full table
- Staging and detecting metastases that may otherwise not be apparent;
- Detecting an otherwise unknown primary site;
- Monitoring response to TKI therapy especially if quick responses need to be assessed for planning early surgery (PET response post imatinib appears as early as 24 hours);
- Detecting primary and secondary resistance to TKI;
- When the CT findings are inconclusive or inconsistent with clinical findings.
Second line therapy for metastatic GIST
Sunitinib is recommended as the second line agent in metastatic GIST patients who have progressed on imatinib or are intolerant to imatinib (60). Sunitinib is a TKI which acts against the stem cell-factor receptor (KIT), PDGFR—VEGF receptor, glial cell line-derived neurotrophic factor receptor [rearranged during transfection (RET)], colony-stimulating factor-1 receptor (CSF1R), and Fms-like tyrosine kinase-3 receptor (FLT3) (61). In a phase III trial, the PFS was 24 versus 6 months for patients on sunitinib versus placebo respectively (60). In another phase III trial, the PFS with sunitinib was 7, 9 and 13 months in patients who progressed after 1, 3 and 5 years of imatinib respectively (62). In a study by Demetri et al., once daily sunitinib 50 mg was given for 4 weeks with a 2-week break and was compared with placebo (patients on placebo arm could cross over to sunitinib arm on disease progression). Although there was no significant difference in OS due to crossover, TTP was 27 weeks in the sunitinib arm versus 6 weeks in the placebo arm. Patients in the placebo arm had a 3-fold greater risk of disease progression (HR, 0.339; 95% CI, 0.244-0.472; P≤0.001) (63). The side effects most commonly encountered with sunitinib are fatigue, anorexia, stomatitis, diarrhea, hand foot syndrome, thrombocytopenia, hypertension and hypothyroidism (64). When sunitinib is used in imatinib failure patients, it is more sensitive in patients with exon 9 mutation and wild type GISTs (65). The mechanisms proposed for sunitinib resistance are increased expression of interleukin-8, AMFR gene expression which is involved in angiogenesis and extracellular matrix metalloproteinase inducer (EMMPRIN), however most of these resistance mechanisms have been studied in renal cell carcinoma patients (66-68).
Third line therapy for metastatic GIST
Regorafenib which is structurally similar to sorafenib, is recommended once patients have progressed on imatinib and sunitinib. It is a pan-TKI which has multiple targets: KIT, RET, RAF1, BRAF, VEGFR1-3, TEK, PDGFR and fibroblast growth factor receptor (FGFR) (69,70). The dose recommended is 160 mg oral tablet once daily for 21 days, with cycle of 28 days each. In the initial multicentre phase II study, regorafenib as 3rd line agent showed a PFS of 10 months (71). In the subsequent phase III randomized study (GRID), 199 patients were randomized to third-line regorafenib versus placebo. Patients on progression in the placebo arm were allowed to cross over to the regorafenib arm. At 3 and 6 months, PFS was 60% versus 11% and 38% versus 0% in the regorafenib versus placebo arm respectively. The median PFS was 4.8 versus 0.9 months in the regorafenib versus placebo arms respectively (HR, 0.27, 95% CI, 0.19-0.39; P<0.0001), whereas the disease control rate was 53% versus 9% (P<0.0001). However as expected, due to crossover OS was not statistically different (72). Moreover the benefit of regorafenib was less if the patient had received less than 6 months of imatinib. Toxicity greater than grade 3 or more (HFS, mucositis, diarrhea, hypertension, fatigue) was seen in about 60%, with half the patients requiring dose reductions, however only 2% discontinued treatment due to toxicity. Based on the GRID study, the FDA in 2013 approved regorafenib as a third-line agent (progressed or intolerant to imatinib and sunitinib) in metastatic GIST.
Another option for patients in third-line setting is to rechallenge the patient with imatinib after progression on imatinib and sunitinib, however the patients should have initially shown some response to imatinib. This was studied in a randomized manner in the phase III RIGHT study, in which 81 patients were randomized to imatinib 400 mg daily or placebo as 3rd line agent. The disease control rate with imatinib was 32% and the PFS was 1.8 versus 0.9 months in the imatinib versus placebo arms respectively, however there was no OS benefit (73).
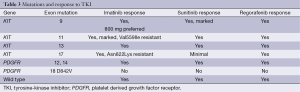
Table 3 shows the response of various TKIs based on the mutation. Table 4 summarizes the key phase III trials.
Full table
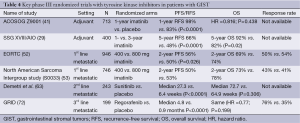
Full table
Mechanism/drivers of resistance to molecular therapy
In metastatic patients, after a few years of imatinib, the tumors become resistant. However most of the times this resistance is partial, i.e., only few clones become resistant and grow, while few other clones are still sensitive. Imatinib resistance could be either primary or secondary. Primary imatinib resistant tumors progress within the initial few months of therapy whereas secondary resistance happens later due to the development of new secondary mutations, which prevent the binding of imatinib to the KIT receptor (74,75). Some of the mechanisms proposed for Imatinib resistance are (76):
- Development of secondary mutations in KIT and PDGFRA which are resistant to imatinib (77);
- Amplification and over expression of the KIT genome (irrespective of mutation);
- Activation of alternate receptor tyrosine kinases;
- Functional resistance—activation of other sites in the KIT apparatus (other than usual juxtamembrane site).
Primary resistance is seen in mutations in the activating loop of PDGFRA such as D842V in which imatinib is unable to bind to the ATP-binding site of the tyrosine kinase receptor (78,79) and in 15% of KIT exon 9 mutations. Secondary mutations usually affect KIT exons 13 to 17 (11). In tumors with mutations in exons 13 and 14 which corresponds to the ATP-binding region of the kinase domain, competitive inhibition of imatinib is impaired, where as in exons 17 and 18 mutations the activation loop is affected. Hence in the former more potent TKIs like sunitinib may be beneficial whereas the latter are equally resistant to most TKIs (80).
D842V mutations are usually also resistant to 2nd and 3rd line agents like sunitinib and regorafenib (65,71,79,81) while exon 9 mutations may benefit with higher dose (800 mg) imatinib.
In an EORTC study, factors predictive of early and late resistance were studied. While the presence of lung metastases and absence of liver metastases predicted early resistance, late resistance was predicted by high baseline granulocyte count, a non-stomach primary tumor, large primary size, and low initial imatinib dose (76). Imatinib causes cell death by apoptosis, however some cells escape this due to quiescence, during which the cells are sent to resting phase and hence they escape death by imatinib. This process of quiescence is further enhanced by DREAM complex, hence in imatinib resistant cases, targeting this DREAM complex is also an active part of research in the recent times (82).
In phase II studies sunitinib as third-line therapy had a response rate of 10% with PFS of 5 months (80,83). Another TKI which has got significant benefit in chronic myeloid leukemia called nilotinib failed to demonstrate any benefit in GIST both as 1st line and 3rd line agent in phase III trial (84,85). Ponatinib in another TKI which was initially studied in CML is now being probed in GIST also (86). Heat shock protein (HSP) prevents the proteosomal degradation of KIT, hence the new area of interest in the management of GIST is HSP90 inhibitors (87), especially in imatinib resistant cases. Presently the HSP90 inhibitors which are under clinical trials are STA-9090, AT-13387 and AUY922 (88-90). In imatinib resistant clones the PI3K/AKT pathway plays an important role in cell survival and hence targeting this pathway with PI3K inhibitors looks promising (91,92). The MAPK pathway stabilizes ETS translocation variant 1 (ETV1), which is a transcription factor responsible for tumorigenesis. The transcription factor ETV1 involved in the MAPK pathway is also expressed on GIST cells, which has led to the study of MEK 162 which is a MEK inhibitor along with imatinib in GIST (93).
Studies have shown that in wild type GISTs with imatinib resistance, there is deficiency in succinate dehydrogenase (SDH) activity which is most often the result of up regulation of IGF1 receptor (10). Hence in wild type GIST the IGF1 receptor inhibitor linsitinib is being studied in phase II trials currently. Other targets which are being studied in imatinib resistance are the downstream signaling pathway molecules like m TOR inhibitors (everolimus and temsirolimus), AKT inhibitor (perifosine), CDK inhibitor (flavopiridol) (11), IGF1 and BRAF inhibitors. Crenolanib is an oral benzimidazole which is a selective and potent inhibitor of PDGFRA and PDGFRB. It is found to be 135 fold more potent than imatinib in PDGFRA D842V mutated GISTs (94). Recently, an anti-KIT monoclonal antibody called SR1 has been identified which is active in both imatinib sensitive and resistant cell lines. SR1 reduces cell surface KIT expression and also enhances macrophagic phagocytosis of cancer cells causing immunologic cell mediated tumor clearance (95). The development of so many molecules is the proof that imatinib resistance is an active field in current medical research and like the recent approval of regorafenib we are hopeful to have many approvals in the near future.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol 1983;7:507-19. [PubMed]
- Miettinen M, Majidi M, Lasota J. Pathology and diagnostic criteria of gastrointestinal stromal tumors (GISTs): a review. Eur J Cancer 2002;38:S39-51. [PubMed]
- Wozniak A, Rutkowski P, Schöffski P, et al. Tumor genotype is an independent prognostic factor in primary gastrointestinal stromal tumors of gastric origin: a European multicenter analysis based on ConticaGIST. Clin Cancer Res 2014;20:6105-16. [PubMed]
- West RB, Corless CL, Chen X, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol 2004;165:107-13. [PubMed]
- Agaimy A. Gastrointestinal stromal tumors (GIST) from risk stratification systems to the new TNM proposal: more questions than answers? A review emphasizing the need for a standardized GIST reporting. Int J Clin Exp Pathol 2010;3:461-71. [PubMed]
- Andersson J, Bumming P, Meis-Kindblom JM, et al. Gastrointestinal stromal tumors with KIT exon 11 deletions are associated with poor prognosis. Gastroenterology 2006;130:1573-81. [PubMed]
- Hirota S, Isozaki K, Moriyama Y, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 1998;279:577-80. [PubMed]
- Stenman G, Eriksson A, Claesson-Welsh L. Human PDGFA receptor maps to the same region on chromosome 4 as the KIT oncogene. Genes Chromosomes Cancer 1989;1:155-8. [PubMed]
- DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51-8. [PubMed]
- Songdej N, von Mehren M. GIST treatment options after tyrosine kinase inhibitors. Curr Treat Options Oncol 2014;15:493-506. [PubMed]
- Lasota J, Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology 2008;53:245-66. [PubMed]
- Corless CL, Barnett CM, Heinrich MC. Gastrointestinal stromal tumors: origin and molecular oncology. Nat Rev Cancer 2011;11:865-78. [PubMed]
- Heinrich MC, Corless CL, Duensing A, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science 2003;299:708-10. [PubMed]
- Kontogianni-Katsarou K, Lariou C, Tsompanaki E, et al. KIT-negative gastrointestinal stromal tumors with a long term follow-up: A new subgroup does exist. World J Gastroenterol 2007;13:1098-102. [PubMed]
- Taniguchi M, Nishida T, Hirota S, et al. Effect of c-kit mutation on prognosis of gastrointestinal stromal tumors. Cancer Res 1999;59:4297-300. [PubMed]
- Martín J, Poveda A, Llombart-Bosch A, et al. Deletions affecting codons 557-558 of the c-KIT gene indicate a poor prognosis in patients with completely resected gastrointestinal stromal tumors: a study by the Spanish Group for Sarcoma Research (GEIS). J Clin Oncol 2005;23:6190-8. [PubMed]
- Antonescu CR, Viale A, Sarran L, et al. Gene expression in gastrointestinal stromal tumors is distinguished by KIT genotype and anatomic site. Clin Cancer Res 2004;10:3282-90. [PubMed]
- Subramanian S, West RB, Corless CL, et al. Gastrointestinal stromal tumors (GISTs) with KIT and PDGFRA mutations have distinct gene expression profiles. Oncogene 2004;23:7780-90. [PubMed]
- El-Rifai W, Sarlomo-Rikala M, Andersson LC, et al. DNA sequence copy number changes in gastrointestinal stromal tumors: tumor progression and prognostic significance. Cancer Res 2000;60:3899-903. [PubMed]
- Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumor. Lancet 2013;382:973-83. [PubMed]
- Demetri GD, Benjamin RS, Blanke CD, et al. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)--update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw 2007;5 Suppl 2:S1-29; quiz S30.
- Eisenberg BL, Harris J, Blanke CD, et al. Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol 2009;99:42-7. [PubMed]
- McAuliffe JC, Hunt KK, Lazar AJ, et al. A randomized, phase II study of preoperative plus postoperative imatinib in GIST: evidence of rapid radiographic response and temporal induction of tumor cell apoptosis. Ann Surg Oncol 2009;16:910-9. [PubMed]
- Andtbacka RH, Ng CS, Scaife CL, et al. Surgical resection of gastrointestinal stromal tumors after treatment with imatinib. Ann Surg Oncol 2007;14:14-24. [PubMed]
- Bednarski BK, Araujo DM, Yi M, et al. Analysis of prognostic factors impacting oncologic outcomes after neoadjuvant tyrosine kinase inhibitor therapy for gastrointestinal stromal tumors. Ann Surg Oncol 2014;21:2499-505. [PubMed]
- Rutkowski P, Gronchi A, Hohenberger P, et al. Neoadjuvant imatinib in locally advanced gastrointestinal stromal tumors (GIST): the EORTC STBSG experience. Ann Surg Oncol 2013;20:2937-43. [PubMed]
- Shrikhande SV, Marda SS, Suradkar K, et al. Gastrointestinal stromal tumors: case series of 29 patients defining the role of imatinib prior to surgery. World J Surg 2012;36:864-71. [PubMed]
- Dematteo RP, Ballman KV, Antonescu CR, et al. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumor: a randomised, doubleblind, placebo-controlled trial. Lancet 2009;373:1097-104. [PubMed]
- Joensuu H, Eriksson M, Sundby Hall K, et al. One vs. three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 2012;307:1265-72. [PubMed]
- National Comprehensive Cancer Network (NCCN). (2013) NCCN Clinical Practice Guidelines in Oncology: soft tissue sarcoma. Available online: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Version I. 2013.
- Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 2002;33:459-65. [PubMed]
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83. [PubMed]
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411-19. [PubMed]
- Joensuu H, Vehtari A, Riihimaki J, et al. Risk of recurrence of gastrointestinal stromal tumor after surgery: An analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265-74. [PubMed]
- Dematteo RP, Gold JS, Saran L, et al. Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer 2008;112:608-15. [PubMed]
- Gold JS, Gonen M, DeMatteo RP. Development and Validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localized, primary gastrointestinal stromal tumor (GIST): A retrospective analysis. Lancet Oncol 2009;10:1045-52. [PubMed]
- Yanagimoto Y, Takahashi T, Muguruma K, et al. Re-appraisal of risk classifications for primary gastrointestinal stromal tumors (GISTs) after complete resection: indications for adjuvant therapy. Gastric Cancer 2015;18:426-33. [PubMed]
- Zhao WY, Xu J, Wang M, et al. Evaluation of high-risk clinicopathological indicators in gastrointestinal stromal tumors for prognosis and imatinib treatment outcome. BMC Gastroenterology 2014;14:105-12. [PubMed]
- Zhao WY, Xu J, Wang M, et al. Prognostic value of Ki67 index in gastrointestinal stromal tumors. Int J Clin Exp Pathol 2014;7:2298-304. [PubMed]
- DeMatteo RP, Ballman KV, Antonescu CR, et al. Long-term results of adjuvant imatinibmesylate in localized, high-risk, primary gastrointestinal stromal tumor: ACOSOG Z9000 (Alliance) intergroup phase 2 trial. Ann Surg 2013;258:422-9. [PubMed]
- Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol 2014;32:1563-70. [PubMed]
- Casali PG, Le Cesne A, Velasco AP, et al. Imatinib failure-free survival (IFS) in patients with localized gastrointestinal stromal tumors (GIST) treated with adjuvant imatinib (IM): The EORTC/AGITG/FSG/GEIS/ISG randomized controlled phase III trial. J Clin Oncol 2013;31 suppl:abstr 10500.
- The ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 suppl 7:vii49-55. [PubMed]
- Cheng CT, Tsai CY, Yeh CN, et al. Clinical significance of pathological complete response in patients with metastatic gastrointestinal stromal tumors after imatinib mesylate treatment - lessons learned. Anticancer Res 2014;34:6617-25. [PubMed]
- Park SJ, Ryu MH, Ryoo BK, et al. The role of surgical resection following imatinib treatment in patients with recurrent or metastatic gastrointestinal stromal tumors: results of propensity score analyses. Ann Surg Oncol 2014;21:4211-7. [PubMed]
- Du CY, Zhou Y, Song C, et al. Is there a role of surgery in patients with recurrent or metastatic gastrointestinal stromal tumours responding to imatinib: a prospective randomised trial in China. Eur J Cancer 2014;50:1772-8. [PubMed]
- Blanke CD, Joensuu H, Demetri GD, et al. Outcome of advanced gastrointestinal stromal tumor (GIST) patients treated with imatinib mesylate: four-year follow-up of a phase II randomized trial. ASCO Gastrointestinal Cancers Symposium, 2006:Abstract 7.
- Heinrich MC, Shoemaker JS, Corless CL, et al. Correlation of target kinase genotype with clinical activity of imatinib mesylate (IM) in patients with metastatic GI stromal tumors (GISTs) expressing KIT (KIT1). J Clin Oncol 2005;23:7.
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80. [PubMed]
- Blanke CD, Demetri GD, Von Mehren M, et al. Long-term results from a randomized phase II trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 2008;26:620-5. [PubMed]
- Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626-32. [PubMed]
- Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumors with high-dose imatinib: randomised trial. Lancet 2004;364:1127-34. [PubMed]
- Benjamin RS, Rankin C, Fletcher C, et al. Phase III dose-randomized study of imatinib mesylate (STI571) for GIST: Intergroup S0033 early results. Proceedings of the American Society of Clinical Oncology 2003;22:A-3271.
- Gastrointestinal Stromal Tumor Meta-Analysis Group. Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol 2010;28:1247-53. [PubMed]
- Blay JY, Le Cesne A, Ray-Coquard I, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol 2007;25:1107-13. [PubMed]
- Heinrich MC, Owzar K, Corless CL, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol 2008;26:5360-7. [PubMed]
- Le Cesne A, Blay JY, Bui BN, et al. Phase II studyof oral masitinib mesilate in imatinib-naive patients with locally advanced or metastatic gastro-intestinalstromal tumor (GIST). Eur J Cancer 2010;46:1344-51. [PubMed]
- Clinical Trials identifier: NCT00812240. A Phase 3 Study to Evaluate Efficacy and Safety of Masitinib in Comparison to Imatinib in Patients With Gastro-Intestinal Stromal Tumor in First Line Medical Treatment. (Recruiting).
- Choi H, Charnsangavej C, Faria SC, et al. Correlation of computed tomography (CT) and positron emission tomography (PET) in patients with metastatic GIST treated at a single institution with imatinibmesylate: proposal of new CT response criteria. J Clin Oncol 2007;25:1753-9. [PubMed]
- Younus J, Verma S, Franek J, et al. Sunitinib malate for gastrointestinal stromal tumor in imatinib mesylate-resistant patients: recommendations and evidence. Curr Oncol 2010;17:4-10. [PubMed]
- Sweeney CJ, Chiorean EG, Verschraegen CF, et al. A phase I study of sunitinib plus capecitabine in patients with advanced solid tumors. J Clin Oncol 2010;28:4513-20. [PubMed]
- Bertucci F, Ray-Coquard IL, Nguyen BB, et al. Effect of five years of imatinib on cure for patients with advanced GIST: Updated survival results from the prospective randomized phase III BFR14 trial. J Clin Oncol 2012;30:abstr 10095.
- Demetri GD, Garrett CR, Schoffski P, et al. Complete longitudinal analyses of the randomized, placebo controlled,phase III trial of sunitinib in patients with gastrointestinal stromal tumor following imatinib failure. Clin Cancer Res 2012;18:3170-9. [PubMed]
- Kim S, Ding W, Zhang L, et al. Clinical response to sunitinib as a multitargeted tyrosine-kinase inhibitor (TKI) in solid cancers: a review of clinical trials. Onco Targets Ther 2014;7:719-28. [PubMed]
- Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol 2008;26:5352-9. [PubMed]
- Shojaei F, Lee JH, Simmons BH, et al. HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res 2010;70:10090-100. [PubMed]
- Thodima VJ. Molecular classification of sunitinib response in metastatic renal cell carcinoma (mRCC) patients by gene expression profiling. 2011 ASCO Annual Meeting. Chicago, USA, 2011:abtr 4556.
- Sato M, Nakai Y, Nakata W, et al. EMMPRIN promotes angiogenesis, proliferation, invasion and resistance to sunitinib in renal cell carcinoma, and its level predicts patient outcome. PLoS One 2013;8:e74313. [PubMed]
- Serrano C, George S. Recent advances in the treatment of gastrointestinal stromal tumors. Ther Adv Med Oncol 2014;6:115-27. [PubMed]
- Ferraro D, Zalcberg J. Regorafenib in gastrointestinal stromal tumors: clinical evidence and place in therapy. Ther Adv Med Oncol 2014;6:222-8. [PubMed]
- George S, Wang Q, Heinrich MC, et al. Efficacy and safety of regorafenib in patients with metastatic and/or unresectable GI stromal tumor after failure of imatinib and sunitinib: a multicenter phase II trial. J Clin Oncol 2012;30:2401-7. [PubMed]
- Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumors after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:295-302. [PubMed]
- Kang YK, Ryu MH, Ryoo BY, et al. Randomized phase III trial of imatinib (IM) rechallenge versus placebo in patients (pts) with metastatic and/or unresectable gastrointestinal stromal tumor (GIST) after failure of at least both IM and sunitinib (SU): Right study. J Clin Oncol 2013;31:LBA10502.
- Debiec-Rychter M, Cools J, Dumez H, et al. Mechanisms of resistance to imatinibmesylate in gastrointestinal stromal tumors and activity of the PKC412 inhibitor against imatinib-resistant mutants. Gastroenterology 2005;128:270-9. [PubMed]
- Heinrich MC, Corless C, Blanke C, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol 2006;24:4764-74. [PubMed]
- Fletcher JA, Corless CL, Dimitrijevic S, et al. Mechanisms of resistance to imatinibmesylate (IM) in advanced gastrointestinal stromal Tumor (GIST). Proc Am Soc Clin Oncol 2003;22.
- Antonescu CR, Besmer P, Guo T, et al. Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res 2005;11:4182-90. [PubMed]
- Corless CL, Schroeder A, Griffith D, et al. PDGFRA mutations in gastrointestinal stromal tumors: frequency, spectrum and in vivo sensitivity to imatinib. J Clin Oncol 2005;23:5357-64. [PubMed]
- Cassier PA, Fumagalli E, Rutkowski P, et al. Outcome of patients with platelet-derived growth factor receptor alpha-mutated gastrointestinal stromal tumors in the tyrosine kinase inhibitor era. Clin Cancer Res 2012;18:4458-64. [PubMed]
- Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib resistant gastrointestinal stromal tumor. J Clin Oncol 2008;26:5352-9. [PubMed]
- Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumor after failure of imatinib: a randomised controlled trial. Lancet 2006;368:1329-38. [PubMed]
- DeCaprio JA, Duensing A. The DREAM complex in antitumor activity of imatinibmesylate in gastrointestinal stromal tumors. Curr Opin Oncol 2014;26:415-21. [PubMed]
- Park SH, Ryu MH, Ryoo BY, et al. Sorafenib in patients with metastatic gastrointestinal stromal tumors who failed two or more prior tyrosine kinase inhibitors: a phase II study of Korean gastrointestinal stromal tumors study group. Invest New Drugs 2012;30:2377-83. [PubMed]
- Reichardt P, Blay JY, Gelderblom H, et al. Phase III study of nilotinib versus best supportive care with or without a TKI in patients with gastrointestinal stromal tumors resistant to or intolerant of imatinib and sunitinib. Ann Oncol 2012;23:1680-7. [PubMed]
- Blay JY, Shen L, Kang YK, et al. Phase III trial of nilotinib versus imatinib as first-line targeted therapy of advanced gastrointestinal stromal tumors (GIST). J Clin Oncol 2013;31:abstr 10501.
- Lierman E, Smits S, Cools J, et al. Ponatinib is active against imatinib-resistant mutants of FIP1L1-PDGFRA and KIT, and against FGFR1-derived fusion kinases. Leukemia 2012;26:1693-5. [PubMed]
- Bauer S, Yu LK, Demetri GD, et al. Heat shock protein 90 inhibition in imatinib resistant gastrointestinal stromal tumor. Cancer Res 2006;66:9153-61. [PubMed]
- Clinical Trials Identifier: NCT01039519. A Study Evaluating STA-9090 in Patients With Metastatic and/or Unresectable Gastrointestinal Stromal Tumor (GIST). (Completed).
- Clinical Trials identifier: NCT01294202. A Study to Investigate the Safety and Efficacy of AT13387, Alone or in Combination with Imatinib, in Patients with GIST. (Completed).
- Clinical Trials identifier: NCT01404650. Study of Hsp90 Inhibitor AUY922 for the Treatment of Patients with Refractory Gastrointestinal Stromal Tumor. (Ongoing).
- Bauer S, Duensing A, Demetri GD, et al. KIT oncogenic signaling mechanisms in imatinib-resistant gastrointestinal stromal tumor: PI3-kinase/AKT is a crucial survival pathway. Oncogene 2007;26:7560-8. [PubMed]
- Floris G, Wozniak A, Sciot R, et al. A potent combination of the novel PI3K Inhibitor, GDC-0941, with imatinib in gastrointestinal stromal tumor xenografts: long-lasting responses after treatment withdrawal. Clin Cancer Res 2013;19:620-30. [PubMed]
- Chi P, Chen Y, Zhang L, et al. ETV1 is a lineage survival factor that cooperates with KIT in gastrointestinal stromal tumors. Nature 2010;467:849-53. [PubMed]
- Heinrich MC, Griffith D, McKinley A, et al. Crenolanib inhibits the drug-resistant PDGFRA D842V mutation associated with imatinib-resistant gastrointestinal stromal tumors. Clin Cancer Res 2012;18:4375-84. [PubMed]
- Edris B, Willingham SB, Weiskopf K, et al. Anti-KIT monoclonal antibody inhibits imatinib-resistant gastrointestinal stromal tumor growth. Proc Natl Acad Sci U S A 2013;110:3501-6. [PubMed]


