Treatment of capecitabine-induced enterocolitis with cholestyramine
Introduction
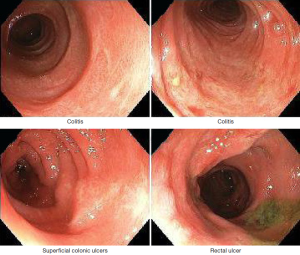
Non-neutropenic enterocolitis is an uncommon side effect of chemotherapeutic agents for solids tumors. Its incidence is unknown. Capecitabine is a cytotoxic agent and an oral prodrug of 5-Fluorouracil (5-FU). It is known to cause diarrhea in up to 67% of patients [11% of which are, common terminology criteria (CTC) grade 3 to 4, Table 1] which often necessitates dose limitation or discontinuing the medication (2). Cytotoxic chemotherapy agents have a direct effect on gastrointestinal (GI) mucosa causing inflammation, edema, ulceration and atrophy (3). Chemotherapy induced diarrhea can cause depletion of fluids and electrolytes, malnutrition, dehydration and hospitalization, all of which can lead to cardiovascular compromise and death. Current recommendations for treatment are mainly limited to IV hydration, opioids, and octreotide (2). Cholestyramine does not appear to have been reported previously as a potential treatment option for chemotherapy induced enterocolitis or diarrhea. Here we report one case of capecitabine induced non-neutropenic enterocolitis that was treated effectively using cholestyramine.
Case
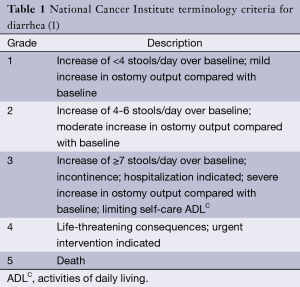
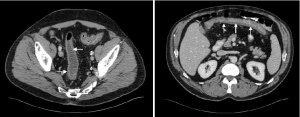
Our patient is a 61-year-old male with stage IIIB (T3N1M0) rectal carcinoma who was started on neoadjuvant therapy with capecitabine and radiation. His medical history was significant for dyslipidemia and peripheral sensory neuropathy. Two weeks after starting his chemotherapy he developed frequent episodes of nonbloody diarrhea with more than 20 greenish bowel movements per day, associated with abdominal cramping and vomiting. The patient was admitted to the hospital for progressive weakness and dehydration. Physical examination was remarkable only for mild diffuse abdominal tenderness without rebound. Laboratory studies were notable for a normal renal function panel and mild leukopenia (WBC 3.05 K/mm3 with 79% polymorphonuclear cells (PMNs) and 10.8% lymphocytes). The patient was therefore not neutropenic. An abdominal computed tomography scan showed moderate circumferential wall thickening of the entire colon consistent with pancolitis (Figure 1). The patient was initially started on ceftriaxone and metronidazole for possible infectious colitis, which were discontinued after stool cultures were negative. Colonoscopy was performed and showed evidence of pancolitis and ileitis with mucosal edema with superficial ulcerations (Figure 2). Histopathological evaluation showed nonspecific acute and chronic inflammation throughout his colonic mucosa with ulceration. Immunohistochemistry for cytomegalovirus was negative. Initial treatment with loperamide, diphenoxylate/atropine, dicyclomine and IV fluids provided minimal symptom control. One week into his admission, he was placed on total bowel rest and started on total parenteral nutrition, but the diarrhea persisted. Given the persistent symptoms on medical therapy, he was then started on a trial of oral cholestyramine 4 grams twice a day. Subsequently his stool frequency decreased to 4-8 the first day, 4 on the second and 1-2 on the third. He was then restarted on a regular diet which he tolerated. Of note dihydropyrimidine dehydrogenase (DPD) level was checked and was positive for one copy of the IVS14+1G>A mutation. This is the most common deficiency allele in the DPD gene. Patients with this deficiency are known to have an increased risk of adverse events from capecitabine or 5-FU (1,4).
Discussion
Capecitabine is used in the treatments of different types of tumors including breast, larynx, genitourinary and GI tumors including metastatic colorectal cancer (5). Capecitabine releases 5-FU for extended durations delivering a higher drug concentrations at tumor sites which likely causes a range of GI side effects. These include nausea, vomiting, mucositis diarrhea, GI hemorrhage, ileus, necrotizing enterocolitis, and toxic dilatation of the intestine. The incidence of diarrhea has been reported to be (47-67%), and is graded 1-4 (Table 1) depending on severity (6). Clinical factors predictive of fluoropyrimidine-induced diarrhea are female sex, and Caucasian race, which are associated with DPD deficiency, and diabetes. DPD deficiency is thought to be a risk factor because of decreased drug clearance and prolonged exposure to the drug (7).
Non-neutropenic capecitabine-induced enterocolitis is rare and to our knowledge, this case is the fourth reported in the literature. The first two cases were reported by Gordon et al. in 2011 (8). Pathobiology of 5-FU related diarrhea is not understood although data from animal studies suggests that mitotic arrest of intestinal crypt cells and decrease of the relative fraction of villous enterocytes and the surface area for resorption could be responsible (9). The median time to first occurrence of grade 2-4 diarrhea has been reported to be 34 days and had a median duration of about 5 days (7). Grade 3-4 diarrhea had a reported incidence of 11.5% and 1.5%, respectively (6). Clinical management requires stopping therapy with subsequent dose reduction or discontinuing capecitabine. In general, treatment is conservative and limited to IV hydration, electrolyte replacement, and medications such as opioids, loperamide and octreotide (10). To our knowledge, there are no previous reports demonstrating the use of cholestyramine for 5-FU or capecitabine-induced enterocolitis or diarrhea. Cholestyramine is a bile resin that is FDA approved for hypercholesterolemia and pruritus associated with excess bile acids. The use of cholestyramine for diarrhea associated with excess fecal bile acids such as post-cholecystectomy or bile acid malabsorption syndromes is common but remains an off label indication. The patient’s computed tomography scan didn’t show any abnormality in his liver, gallbladder or bile duct.
The mechanism of action of cholestyramine in this situation is unclear. However bile acid malabsorption in chemotherapy induced diarrhea is possible and through forming a complex with the bile acids, cholestyramine could shield the already sensitized colonic and ileal mucosa from inadvertent stimulation or injury by bile acids. Further reports and studies are needed to validate the possible benefit of cholestyramine in chemotherapy induced diarrhea.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Van Kuilenburg AB, Meinsma R, Zoetekouw L, et al. High prevalence of the IVS14 + 1G>A mutation in the dihydropyrimidine dehydrogenase gene of patients with severe 5-fluorouracil-associated toxicity. Pharmacogenetics 2002;12:555-8. [PubMed]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. Bethesda: NIH Publication, 2010.
- Andreyev HJ, Davidson SE, Gillespie C, et al. Practice guidance on the management of acute and chronic gastrointestinal problems arising as a result of treatment for cancer. Gut 2012;61:179-92. [PubMed]
- Loganayagam A, Arenas-Hernandez M, Fairbanks L, et al. The contribution of deleterious DPYD gene sequence variants to fluoropyrimidine toxicity in British cancer patients. Cancer Chemother Pharmacol 2010;65:403-6. [PubMed]
- Walko CM, Lindley C. Capecitabine: a review. Clin Ther 2005;27:23-44. [PubMed]
- Cassidy J, Twelves C, Van Cutsem E, et al. First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann Oncol 2002;13:566-75. [PubMed]
- Capecitabine/Concerns related to adverse effects. Available online: Uptodate.com
- Gordon VL, Harding GA, Czaykowski P. Capecitabine-induced, nonneutropenic enterocolitis. J Gastrointest Cancer 2011;42:278-81. [PubMed]
- Siber GR, Mayer RJ, Levin MJ. Increased gastrointestinal absorption of large molecules in patients after 5-fluorouracil therapy for metastatic colon carcinoma. Cancer Res 1980;40:3430-6. [PubMed]
- Hoff PM, Saragiotto DF, Barrios CH, et al. Randomized phase III trial exploring the use of long-acting release octreotide in the prevention of chemotherapy-induced diarrhea in patients with colorectal cancer: the LARCID trial. J Clin Oncol 2014;32:1006-11. [PubMed]