Chinese guidelines for diagnosis and treatment of gastric cancer (2011 edition)
Introduction
Gastric cancer is one of the most common malignant tumors in China. According to 2010 Health Statistics Yearbook, gastric cancer is the third leading cause of cancer-related death in China in 2005. The incidence of gastric cancer which is considered to be a consequence of long-term effects of multiple factors, varies significantly by region. Host factors are subordinate to Environment in the occurrence of gastric cancer. Studies have shown that Helicobacter pylori (H.pylori) infection, diet, smoking, and genetic susceptibility of the host are the important factors in the development of gastric cancer.
The guideline is developed to standardize and improve the diagnosis and treatment of gastric cancer in medical institutions in China, ameliorate the prognosis of gastric cancer patients, insure the quality and safety of healthcare services. Gastric cancer, in the guideline, which means gastric adenocarcinoma (hereinafter referred to as gastric cancer, GC), includes gastroesophageal junction cancer.
Diagnosis
Diagnosis and differential diagnosis of gastric cancer should be based on the clinical presentation, endoscopic and histopathologic findings, and imaging results.
Clinical manifestations
There are no specific clinical symptoms of GC. Patients with early gastric cancer are often asymptomatic. Common symptoms include upper abdominal discomfort or pain, anorexia, weight loss, fatigue, nausea, vomiting, hematemesis or melena, diarrhea, constipation, and fever.
Signs
In general, no obvious signs can be found in the early stage or some of the locally advanced cancer.
An abdominal mass may be palpable in patients with advanced gastric cancer. If there is distant metastasis, local manifestations could be found according to metastatic sites. There may be corresponding signs when upper gastrointestinal perforation, gastrointestinal hemorrhage or obstruction and other symptoms are present.
Accessory examination
Endoscopy
Endoscopy: required for diagnosis of gastric cancer , determine the location of the tumor and obtain tissue samples for pathological examination. If necessary, chromoendoscopy or magnifying endoscopy may be needed.
Ultrasound endoscopy: recommended for preoperative staging of gastric cancer, useful in assessing the depth of tumor invasion and lymph node metastasis. This procedure is required for patients undergoing minimally invasive surgery, including proposed endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD).
Laparoscopy: considered when peritoneal metastasis or intra-abdominal dissemination is suspected.
Histopathological examination
The confirmation and treatment of gastric cancer is based on histopathological findings. Biopsy confirmed invasive cancer needs standardized treatment. For patients with precancerous lesions or suspected invasion, repeated biopsy or review of imaging findings is recommended when the depth of invasion is undetermined due to biopsy limitations. The therapeutic strategy can be designed when cancer is confirmed.
Endoscopic biopsy specimen treatment
Preprocessing of specimen: biopsy specimens, when obtained, should be immediately flattened so that the basal mucous membrane is attached to the filter paper.
Fixation: 10-13% formalin buffer solution is used; the specimen should be placed in solution for more than 6 hours and less than 48 hours before embedding.
Paraffin embedding: after removal of the filter paper, the tissue should be embedded vertically.
Standard of HE slides: prepare the wax blocks, cut successively 6-8 tissue layers and put all on one glass slide, which is then mounted after routine HE staining.
Pathological diagnostic criteria
Low-grade intraepithelial neoplasia: mild atypia of the glandular structure and cellular morphology within the mucosa can be observed. Compared with surrounding normal glands, the glands are densely arranged, glandular cells were pseudostratified with no or little mucus, the nucleus dyed obviously, and mitosis is present.
High-grade intraepithelial neoplasia: severe atypia is found in the mucosal glandular structure and cellular morphology (epithelial carcinoma in situ). Compared with surrounding normal glands, the glands density is high, cell polarity disorder is obvious. On the basis of low-grade intraepithelial neoplasia, shared cell walls or even cribriform-shaped structures exist with absence of mucus secretion and high mitotic activity. Focal necrosis is observed without interstitial infiltration.
Intramucosal cancer: also known as intramucosal invasive carcinoma. Irregular nests of epithelial cell clusters or isolated epithelial cells invade the lamina propria stroma, the muscularis mucosa.
Submucosal cancer: intramucosal invasive carcinoma penetrate the muscularis mucosa to the submucosa without invasion of the muscularis propria of the stomach.
Early gastric cancer (T1N0/1M0): this includes intramucosal and submucosal invasive gastric cancer, regardless of evidence of regional lymph node metastasis.
Pathological assessment
Standard tissue fixation
(I) Fixative: Use 10-13% neutral formalin buffer solution and avoid any fixative containing heavy metals;
(II) Fixative volume: more than 10 times of the specimen; Fixation temperature: Normal room temperature.
(III) Fixation time: greater than 6 hours and less than 48 hours for specimens of endoscopic mucosal resection or biopsy.
(IV) Gastrectomy specimens: expand the specimen along the greater curvature of the stomach and fix for more than 12 hours and less than 48 hours.
Sampling requirements.
(I) Biopsy specimens: Verify the number of biopsy specimens sent for test--all of them must be sampled. Make sure each wax block contains at most five biopsy specimens; and Wrap the specimens in gauze or soft permeable paper to prevent loss.
(II) Specimens of endoscopic mucosal resection: Each submitted specimen is flattened, fixed and marked with orientation by the surgeon. The size of the tumor and distance to the cutting edges are recorded. The specimen is then cut perpendicularly to the stomach wall with an interval of 0.3 cm and divided into blocks of suitable sizes. Sampling along the same embedding direction is recommended. The orientation of each block is recorded.
(III) Gastrectomy specimens (gross examination is described in Annex 1):
(i)Tumor and resection margin: >=4 blocks are collected depending on tumor size, depth of invasion, textures, colors and other regional variation, including 1-2 blocks of the whole layer tissue at the site of the deepest invasion in order to determine the invasion depth; 1-2 blocks of tissues adjacent to the tumor for visual examination of the relationship between the tumor and the surrounding normal mucosa. At least one block of the distal and proximal surgical margin should be taken separately. Principles of sampling for early-stage cancer: All surgical dissected specimens should be photographed showing the sampling location, in case of further consultation.
(ii) Lymph nodes: lymph nodes dissection by group according to local anatomy and findings amid operation is recommended for localization of the lymph drainage area. In the absence of medical advice or markings, a pathologist should examine the lymph nodes of a specimen following the principles: all lymph nodes should be sampled; the number should be ≥15 if patients do not receive any treatment before surgery. All gross negative lymph nodes should be intact before pathological examination. For gross positive ones, the integrity can be compromised.
(iii)Recommended block volume: less than 2 cm × 1.5 cm × 0.3 cm.
(IV) Disposal of specimens and reserving period after sampling:
(i) Preservation of the remaining specimens The remaining tissues shall be preserved in a standard fixative. The volume and concentrat ion of the formaldehyde fixative must be maintained to prevent the specimens from drying up or decaying as a result of insufficient volume or decreased concentration, so that gross review or additional sampling may be possible whenever required for diagnosis based on microscopic findings, or clinical feedback is received on the last pathological report;
(ii) Time limit for handling residue: The remaining specimens can be disposed depending on the hospital if no clinical feedback is submitted or revision is demanded because of different opinion from another hospital one month after issue of the pathological report.
Pathological types
Gross type of early gastric cancer
(I) Protruded type
(i) Superficial (elevated subtype);
(ii) Superficial (flat subtype);
(iii) Superficial (depressed subtype)
(iv) Excavated type
Gross type of advanced gastric cancer
(I) Elevated type: tumor body protrudes into the intestine;
(II) Ulcerative type: tumor invades as deep as or through the muscle complicated with ulcers;
(III) Infiltrative type: tumor infiltrates into intestinal layers resulting in local wall thickening but often without obvious ulcer or surface uplift.
Histological type.
(I) WHO classification: the most commonly used method of histological classification of gastric cancer (Annex 2).
(II) Lauren classification: intestinal, diffuse and mixed type.
Pathology report
The pathology report of a biopsy specimen should include the following:
(I) Describe the basic information of the patient and the specimen;
(II) If intraepithelial neoplasia (dysplasia), describe the classification;
(III) Suspected infiltration: biopsy should be repeated and immunohistochemical staining if necessary;
(IV)Early invasive carcinoma: indicates the depth of invasion.
Clinicians should be aware the limitation of biopsy depth may affect the results ,leaving the depth of invasion undetermined.
The pathology report of a specimen of endoscopic mucosal resection should include the following:
(I) Describe the basic information on the patient and the specimen;
(II) Tumor size;
(III)The classification of intraepithelial neoplasia (dysplasia);
(IV) In the case of invasive cancer, the histological type, grade, depth of invasion, vascular invasion and margins should be reported.
For pT1 ,poorly differentiated carcinoma with vascular invasion and positive surgical margin, additional extended surgical resection should be performed. For other cases, complete endoscopic resection is adequate though regular follow-up is still needed.
Histological factors that indicate poor outcomes include: poor differentiation, invasion of blood vessels and lymph nodes, and positive surgical margin.
Positive margin is defined as a distance of less than 1 mm from the tumor to the resection margin or visible cancer cells at the cauterized margin.
The pathology report of a surgically resected specimen should include the following:
(I) Describe the basic information of the patient and the specimen;
(II) General information: tumor location, size, gross type, macroscopic depth of invasion, and distances of the tumor to the upper and lower margins;
(III) Degree of tumor differentiation (tumor type and grade);
(IV) Depth of tumor invasion (T stage, and T stage or pT is determined by the morphological evidence of tumor cells. A cell-free mucus pool in a specimen after neoadjuvant treatment is not considered residual tumor) (see Annex 3 for TNM staging criteria);
(V) The numbers of detected lymph nodes and positive lymph nodes (N stage);
(VI) Conditions of the proximal and distal margins. Any tumor close to the margin should be measured under the microscope and the distance reported. Positive margin is marked in case the distance is less than 1 mm;
(VII) Vascular and neural invasion;
(VIII) Any special examinations useful in differential diagnosis and for clinical treatment, including immunohistochemistry and molecular pathology testing such as HER-2.
Clinicians should complete a detailed pathological request form, which describes surgical findings and auxiliary examination results with lymph node status explicitly marked.
Laboratory testing
Blood tests: complete blood cell (CBC), chemistry profile, serum tumor markers and so on.
Urine, stool routine, fecal occult blood tests.
Imaging examination
Computed tomography (CT): CT scan with or without contrast is important in the evaluation of gastric lesions, lymph node metastasis and distant metastasis, and should be routinely performed for preoperative staging of gastric cancer. CT scan with contrast, if no contraindication, is recommended for a well prepared stomach. Scan should include the primary lesion as well as any site of potential metastases.
Magnetic resonance imaging (MRI): MRI examination is also an important imaging tool, which is recommended when a patient is allergic to the CT contrast agent or metastasis is suspected by other imaging method. MRI has an advantage over others in case of peritoneal metastasis.
Upper gastrointestinal imaging: this may help to determine the scope of the primary lesion and the functionality. In particular, air-barium double contrast is commonly used for diagnosis of gastric cancer. Water-soluble contrast agents are recommended for patients suspected of having pyloric obstruction.
Chest X-ray: this should include the anterior-lateral view, which can be used to evaluate the presence of lung metastasis or other major pulmonary lesions. The lateral view is helpful in identifying lesions posterior to the heart shadow.
Ultrasound: with certain value for evaluation of regional lymph node metastasis of gastric cancer and superficial metastases, it can be used as a preliminary staging tool preoperatively. Abdominal ultrasound is useful in assessing abdominal and pelvic metastases. In particular, ultrasound imaging is helpful in identifying the nature of lesions.
PET-CT: this is not recommended for routine exam, though it may be considered when there is any metastatic lesion not definable by conventional image methods.
Bone scan: this is not recommended for routine exam, though it may be considered when bone metastasis is suspected.
Differential diagnosis
Benign diseases
Since gastric cancer is not characterized by any specific symptoms and signs, it has to be differentiated from benign lesions such as gastric ulcer, gastric polyp (adenoma or adenomatous polyps), large gastric folds, hypertrophic gastritis, verrucous gastritis, gastric mucosal prolapse, gastric varices and granuloma.
Other gastric malignancies
Other gastric malignancies mainly include malignant gastric lymphoma, gastric stromal tumors, and neuroendocrine tumors. Liver metastases should be differentiated from primary liver cancer.
Treatment
Treatment principles
A comprehensive treatment plan should be developed, i.e. to achieve radical cure or maximize the control of tumor and improve the cure rate, improve the patient's quality of life and prolong survival by a well-planned multidisciplinary treatment model (MDT) combining surgery, chemotherapy, radiotherapy and targeted biological therapy based on patients' conditions, tumor cytology, pathological type, and clinical stage. For early gastric cancer without evidence of lymph node metastasis, endoscopic therapy or surgery may be considered according to the depth of tumor invasion. Adjuvant radiotherapy or chemotherapy is not necessary after the operation.
For locally advanced gastric cancer or early gastric cancer with lymph node metastasis, surgery-based MDT should be adopted. Radical surgery could be performed immediately or following neoadjuvant chemotherapy depending on the depth of tumor invasion and the presence of lymph node metastasis. After successful radical surgery, adjuvant therapy protocols for local advanced gastric cancer (adjuvant chemotherapy, or adjuvant chemoradiotherapy if necessary) are based on postoperative pathology staging.
For recurrent/metastatic gastric cancer, medication-based MDT should play the major part, associated with palliative surgery, radiation therapy, interventional therapy, radiofrequency ablation and other local therapies appropriately, as well as optimal supportive care such as pain relief, stent implant and nutritional support.
Surgical treatment
Principles
Surgical resection is the mainstream treatment of gastric cancer and the only way to achieve clinical cure. Common approaches include radical surgery and palliative surgery. Radical resection is always the ultimate goal. Radical procedures include EMR, ESD, D0 and D1 resections for early gastric cancer and D2 and enlargement surgery (D2+) for advanced gastric cancer. Palliative options include palliative gastric resection, gastrojejunostomy, jejunal feeding tube implantation and so on.
The primary lesion should be completely removed and regional lymph nodes thoroughly dissected. The interval between tumors and surgical margins should be at least 3 cm for local lesions, or 5 cm for invasive lesions. Complete removal should be performed of gastric cancer adjacent to the esophagus and duodenum. Intraoperative frozen biopsy might be needed to ensure elimination of residual cancer along the margins. D (dissection) is still used to indicate the scope of lymph node dissection. For example, in D1 dissection, all of the group 1 lymph nodes are removed, while both groups 1 and 2 are dissected in D2 surgery. D0 is used when the dissection does not involve all the group 1 lymph nodes.
Laparoscopy, a rapidly developing minimally invasive technique, is appropriate in stage I patients.
Techniques and indications
Reduction surgery: This category involves radical operations with a lesser resection range than standard radical procedure.
(I) Endoscopic mucosa resection (EMR) and endoscopic submucosa dissection (ESD) are indicated for well-differentiated or moderately differentiated intramucosal cancer with no ulcer or lymph node metastases and a diameter less than 2 cm;
(II) D1 gastric resection is indicated for intramucosal cancer which is more than 2 cm in diameter and invades the gastric submucosa. D2 dissection should be implemented as long as lymph node metastasis is present.
Standard surgery: D2 radical resection is a standard procedure for gastric cancer if the tumor invasion is deeper than the submucosa (muscularis or beyond) or is accompanied by lymph node metastasis without invasion to adjacent organs. Standard surgery combined with organ resection: indicated when tumor invasion of adjacent organs is present (Table 1).
| Distal gastrectomy | Proximal gastrectomy | Total gastrectomy | |
| D1 | 1,3,4sb,4d,5,6,7 | 1,2,3,4sa,4sb,7 | 1-7 |
| D2 | D1+8a,9,11p,12a | D1+8a,9,10,11 | D1+8a,9,10,11,12a |
Palliative surgery: only indicated for patients with distant metastasis or tumor invasion of a vital organ, which is irresectable, and complicated with hemorrhage, perforation, obstruction, etc. Palliative surgery is to relieve symptoms and improve quality of life.
Contraindications
(I) Surgery ineligibility or intolerability due to systemic conditions;
(II) Complete removal is impossible due to extensive local invasion;
(III) Definite evidence of distant metastases, including distant lymph node metastasis, peritoneal dissemination, or more than three liver metastatic lesions; and so on;
(IV) Significant malfunction of the heart, lung, liver, kidney and other vital organs, severe hypoproteinemia, anemia, malnutrition, resulting in intolerability.
Classification and grouping of lymph nodes in gastric cancer
See Annex 4.
Radiation therapy
Indications
Radiotherapy or radiochemotherapy is conducted as preoperative or postoperative adjuvant therapy and palliative care, to improve quality of life. Postoperative radiotherapy and chemotherapy are mainly indicated for T3-4 or N+ (lymph node positive) of gastric cancer, preoperative radiochemotherapy for irresectable local advanced or advanced gastric cancer, and palliative radiotherapy for local recurrence and/or distant metastasis.
(I) Concurrent radiochemotherapy is recommended postoperatively for patients with a pathological stage of T3-4 or node-positive (T3-4N+M0) after curative resection (R0) who did not receive standard D2 operation or preoperative radiochemotherapy;
(II) Concurrent radiochemotherapy may be considered preoperatively for those with irresectable local advanced gastric cancer (T4NxM0), followed by reassessment to evaluate the chance of radical resection;
(III) Concurrent radiochemotherapy is recommended postoperatively for patients with residual tumor (R1 or R2 resection) after non-radical surgery;
(IV) Radiotherapy or chemotherapy is recommended for patients with local/regional recurrence of gastric cancer;
(V) Palliative radiotherapy of tumor metastases or primary lesions may be considered for patients suffering pain of bone, brain or other metastases with relatively localized lesion.
Radiation therapy
Techniques: Different radiotherapy techniques may be used based on facilities, such as conventional radiotherapy, threedimensional conformal radiotherapy, intensity modulated radiotherapy, and image-guided radiotherapy. Three-dimensional conformal radiotherapy or intensity modulated radiation therapy is recommended as they protect better the surrounding normal tissues e.g the liver, spinal cord, kidney and intestine, reducing the dose and tissue toxicity, resulting in higher radiation tolerance.
(I) Simulated positioning: CT simulation is recommended. If not available, a conventional simulator must be used. Patients have to take a fixed, supine position during the procedure. Food intake should be minimized within 3 hours before positioning. Oral or intravenous contrast agent may help to position and profile the target lesion;
(II) Multi-field irradiation is recommended (3 fields or above);
(III) Before intensitymodulated radiotherapy, the protocol must be verified in prior;
(IV) Intraoperative radiotherapy or external irradiation may be performed with a local additional dose;
(V) Radioactive seed implantation is not recommended for routine application.
Target definition: The target should be radiated after radical gastrectomy, which includes the primary tumor site with high risk of recurrence and high-risk regional lymph nodes.
(I) Primary tumor site with high risk of recurrence: includes anastomosis site and adjacent invaded organs or sites;
(II) Highrisk regional lymph node area: depend on the primary tumor site, tumor invasion depth and lymph node metastasis;
(III)
Adjacent organs: the pancreas or part of the pancreas areas.
Dose limitation for normal tissues: Dose limitation for normal tissues: 60% liver <30 Gy, 2/3 single kidney <20 Gy, spinal cord <45 Gy, 1/3 heart <50 Gy, and minimize the radiation dose of intestinal and duodenum.
Radiation dose: The volume-dose definition equation should be used for three-dimensional conformal radiation and intensitymodulated radiotherapy, and the isocenter definition mode for conventional irradiation.
(I) DT45-50.4 Gy dispersed 25-28 courses with 1.8 Gy each is recommended for the primary tumor site with high risk of recurrence and regional lymph drainage area after radical resection;
(II) Wide field radiation followed by reduced field radiation with an addition dose is used for patients with tumor and/or residues, at DT 5-10 Gy.
Regimens of concurrent chemoradiotherapy
A program of concurrent chemoradiation based on 5-fluorouracil (5-FU) or capecitabine should be appropriate.
Chemotherapy
Chemotherapy consists of palliative chemotherapy, adjuvant chemotherapy and neoadjuvant chemotherapy, which should be administered within rigorous indications and under the guidance of oncologists. The stages of disease, physical condition, adverse reactions, quality of life and patient's wishes should be taken into account in order to avoid over-treatment or inadequate treatment. Assessment of the chemotherapy efficacy should be conducted timely along with close monitoring and control of adverse reactions, so that drugs and/or doses could be adjusted correspondingly. Efficacy is assessed following the WHO Response Evaluation Criteria in Solid Tumors or Annex 5. Adverse reactions are assessed following the NCI-CTC criteria.
Palliative chemotherapy
The therapy aims to alleviate the symptoms caused by cancer, improve quality of life and prolong survival. It is indicated for patients with unresectable or recurrent lesions or after palliative resection with good general conditions and normal main organ functions.
Commonly used systematic chemotherapy drugs include: 5-fluorouracil (5-FU), capecitabine, gimeracil and oteracil porassium, cisplatin, epirubicin, docetaxel, paclitaxel, oxaliplatin and irinotecan.
Two-drug or three-drug combination regimens are available. The former may include 5-FU/LV+cisplatin (FP), capecitabine and cisplatin, gimeracil and cisplatin, capecitabine and XELOX, FOLFOX, capecitabine and paclitaxel, or FOLFIRI. The threedrug program is suitable for advanced gastric cancer patients with a good physical condition. Commonly used combinations include ECF and its derivative regimes (EOX, ECX, EOF), DCF and its modified regime, and so on. For elderly patients and those with poor physical states, single-agent chemotherapy of oral fluoropyrimidine or taxane may be considered. The molecular targeted agent trastuzumab may be considered on the basis of chemotherapy for HER-2 positive (immunohistochemical staining +++ or immunohistochemical staining ++ with FISHpositive) patients with advanced gastric cancer.
Adjuvant chemotherapy
Eligible candidates include patients with confirmed stage Ib lesions by postoperative pathology and lymph node metastasis, or confirmed stage II or above. Postoperative adjuvant chemotherapy begins when physical conditions return to normal, usually 3-4 weeks after surgery, and is completed within 6 months. Singleagent chemotherapy should not exceed one year. Fluorouracil plus platinum is recommended as a two-drug adjuvant chemotherapy regimen. Single oral fluoropyrimidine chemotherapy may be considered in patients with Ib lesions, poor physical condition, older age, and intolerability to combination therapy.
Neoadjuvant chemotherapy
This is recommended for patients with local advanced gastric cancer and no evidence of distant metastasis (T3/4, N+). Two- or three-drug regimes should be used instead of single drug administration. ECF and its modified plans are the recommended neoadjuvant chemotherapy regimen. Generally, neoadjuvant chemotherapy should not last more than 3 months. Timely assessment of the efficacy and monitoring of adverse reactions are needed to avoid surgical complications.
Postoperative adjuvant therapy regimens base on the preoperative staging and efficacy of neoadjuvant chemotherapy; maintenance if effective, or adjustment, depending on the tolerance. The regimen may also be replaced if ineffective.
Supportive care given to relieve symptoms, ease pain and improve quality of life, supportive care should be considered when decision is made on the choice of treatment options and efficacy assessment, and should involve ameliorating anemia, improving nutrition and appetite, relieving obstruction and pain, and psychological treatment. Specific measures include stent placement, enteral nutritional support, control of ascites and treatment with Chinese traditional medicine.
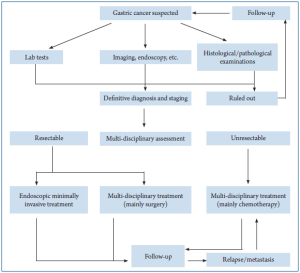
Process of gastric cancer diagnosis and treatment
Follow-up
Patients with gastric cancer should be monitored for symptoms, signs and laboratory examinations with regular followup. Follow-up is aimed at monitoring disease recurrence or treatment-related adverse reactions, assessing and improving the nutritional status of patients, which should include hematology, imaging, endoscopy and other tests.
The follow-up is performed every 3 to 6 months in the first 3 years after treatment, every 6 months from 3 to 5 years, and every one year from the fifth year. Endoscopy should be performed once a year. Vitamin B12 and folic acid should be added if largecell anemia appears after total gastrectomy.
| Routine gross examination of gastric and cardia cancer specimens |
| Description |
| (Total gastrectomy, gastrectomy or gastric remnant) resection specimens: the greater curvature length ___ cm, lesser curvature ___ cm; with the lower part of the pyloric ring/duodenum/lower esophagus, length ___ cm; at (cardia/pylorus/stomach body/sinus; greater/lesser curvature) side there is a ___ type (early or advanced) tumor (including the appearance): ___ cm away from the upper margin and ___ cm from the lower margin, size ___×___×___ cm. Section properties ___; depth of invasion ___; involving/not involving the pyloric ring/lower esophagus. Findings of surrounding mucosa adjacent to/within the muscle wall (erosion/rough/granular/depression/plaque//negative findings if necessary). Lymph nodes found in the greater curvature (number/many/dozen/few), ___ to ___ cm in diameter; lymph nodes found in the lesser curvature (number/many/dozen/few), ___ to ___ cm in diameter. Omentum size × ___×___cm, with or without tumor and lymph nodes. |
| Gastric cancer pathology report |
| 1. Tumor |
| (1) histological type |
| (2) histological grade |
| (3) depth of invasion |
| (4) esophageal or duodenal invasion (if cut ) |
| (5) vascular invasion |
| (6) perineural infiltration |
| 2. Margin |
| (1) proximal |
| (2) distal |
| 3. Other pathological findings |
| (1) chronic gastritis |
| (2) intestinal metaplasia |
| (3) dysplasia |
| (4) atrophy |
| (5) adenoma |
| (6) polyps |
| (7) Helicobacter pylori |
| (8) Other |
| 4. Regional lymph nodes (including the lesser curvature, greater curvature, greater omentum and lymph nodes submitted separately) |
| (1) total |
| (2) number of affected nodes |
| 5. Distant metastasis |
| 6. Other tissue/organ |
| 7. Special test results (staining, immunohistochemical staining, etc.) |
| Undetermined pathology should be submitted to a higher level hospital for consultation (providing the original pathology report to verify the correct slice so as to reduce inspection errors, as well as adequate lesion biopsy or wax blocks, and intraoperative findings, etc.). |
| Epithelial tumours | |
| Intraepithelial neoplasia - Adenoma | 8140/0 |
| Carcinoma | |
| Adenocarcinoma | 8140/3 |
| Intestinal type | 8144/3 |
| Diffuse type | 8145/3 |
| Papillary adenocarcinoma | 8260/3 |
| Tubular adenocarcinoma | 8211/3 |
| Mucinous adenocarcinoma | 8480/3 |
| Signet-ring cell carcinoma | 8490/3 |
| Adenosquamous carcinoma | 8560/3 |
| Squamous cell carcinoma | 8070/3 |
| Small cell carcinoma | 8041/3 |
| Undifferentiated carcinoma | 8020/3 |
| Others | |
| Carcinoid (well differentiated endocrine neoplasm) | 8240/3 |
| Non-epithelial tumours | |
| Leiomyoma | 8890/0 |
| Schwannoma | 9560/0 |
| Granular cell tumour | 9580/0 |
| Glomus tumour | 8711/0 |
| Leiomyosarcoma | 8890/3 |
| GI stromal tumour | 8936/1 |
| Benign | 8936/0 |
| Uncertain malignant potential | 8936/1 |
| Malignant | 8936/3 |
| Kaposi sarcoma | 9140/3 |
| Others | |
| Malignant lymphomas | |
| Marginal zone B-cell lymphoma of MALT-type | 9699/3 |
| Mantle cell lymphoma | 9673/0 |
| Diffuse large B-cell lymphoma | 9680/3 |
| Others | |
| Secondary tumours |
| Primary tumor (T) |
| TX: Primary tumor cannot be assessed |
| T0: No evidence of primary tumour |
| Tis: Carcinoma in situ: intraepithelial tumor without invasion of the lamina propria |
| T1a: Tumour invades lamina propria or muscularis mucosae |
| T1b: Tumour invades submucosa |
| T2: Tumour invades muscularis propria |
| T3: Tumour penetrates subserosa connective tissues without invasion of visceral peritoneum or adjacent structures |
| T4a: Tumor invades serous membrane (visceral peritoneum) |
| T4b: Tumor invades adjacent structures |
| Regional lymph nodes (N) |
| NX: Regional lymph nodes cannot be assessed |
| N0: No regional lymph node metastasis |
| N1: Metastasis in 1 to 2 regional lymph nodes |
| N2: Metastasis in 3 to 6 regional lymph nodes |
| N3: Metastasis in 7 or more regional lymph nodes |
| N3a: Metastasis in 7 to 15 regional lymph nodes |
| N3b: Metastasis in more than 15 regional lymph nodes |
| Distant metastasis (M) |
| M0: No distant metastasis |
| M1: Distant metastasis |
| Stage 0 |
| Stage IA |
| Stage IB T1N1M0 or T2N0M0 |
| Stage IIA T1N2M0, T2N1M0, or T3N0M0 |
| Stage IIB T1N3M0, T2N2M0, T3N1M0, or T4aN0M0 |
| Stage IIIA |
| Stage IIIB T3N3M0, T4aN2M0, T4bN0M0, or T4bN1M0 |
| Stage IIIC T4aN3M0, T4bN2M0, or T4bN3M0 |
| Stage IV |
| The stomach is anatomically divided into three equal portions from the greater curvature to the lesser curvature:the upper (U), middle (M), and lower (L) parts. Tumor extension into the esophagus or the duodenum is recorded as E or D, respectively. If more than one portion is involved, each portion should be described in order of severity. | |
| 1. Grouping of lymph nodes | |
| No. 1 | Right paracardial LN |
| No. 2 | Left paracardial LN |
| No. 3 | LN along the lesser curvature |
| No. 4sa | LN along the short gastric vessels |
| No. 4sb | LN along the left gastroepiploic vessels |
| No. 4d | LN along the right gastroepiploic vessels |
| No. 5 | Suprapyloric LN |
| No. 6 | Infrapyloric LN |
| No. 7 | LN along the left gastric artery |
| No. 8a | LN along the common hepatic artery (Anterosuperior group) |
| No. 8p | LN along the common hepatic artery(Posterior group) |
| No. 9 | LN around the celiac artery |
| No. 10 | LN at the splenic hilum |
| No. 11p | LN along the proximal splenic artery |
| No. 11d | LN along the distal splenic artery |
| No. 12a | LN in the hepatoduodenal ligament (along the hepatic artery) |
| No. 12b | LN in the hepatoduodenal ligament (along the bile duct) |
| No. 12p | LN in the hepatoduodenal ligament (behind the portal vein) |
| No. 13 | LN on the posterior surface of the pancreatic head |
| No. 14v | LN along the superior mesenteric vein |
| No. 14a | LN along the superior mesenteric artery |
| No. 15 | LN along the middle colic vessels |
| No. 16a1 | LN in the aortic hiatus |
| No. 16a2 | LN around the abdominal aorta (from the upper margin of the celiac trunk to the lower margin of the left renal vein) |
| No. 16b1 | LN around the abdominal aorta (from the lower margin of the left renal vein to the upper margin of the inferior mesenteric artery) |
| No. 16b2 | LN around the abdominal aorta (from the upper margin of the inferior mesenteric artery to the aortic bifurcation) |
| No. 17 | LN on the anterior surface of the pancreatic head |
| No. 18 | LN along the inferior margin of the pancreas |
| No. 19 | Infradiaphragmatic LN |
| No. 20 | LN in the esophageal hiatus of the diaphragm |
| No. 110 | Paraesophageal LN in the lower thorax |
| No. 111 | Supradiaphragmatic LN |
| No. 112 | Posterior mediastinal LN |
| ** Lymph node station | * Location | |||||
| LMU MUL MLU UML |
LD L |
LM M ML |
MU UM |
U | U | |
| No.1 | 1 | 2 | 1 | 1 | 1 | |
| No.2 | 1 | *** M | 3 | 1 | 1 | |
| No.3 | 1 | 1 | 1 | 1 | 1 | |
| No.4sa | 1 | M | 3 | 1 | 1 | |
| No.4sb | 1 | 3 | 1 | 1 | 1 | |
| No.4d | 1 | 1 | 1 | 1 | 2 | |
| No.5 | 1 | 1 | 1 | 1 | 3 | |
| No.6 | 1 | 1 | 1 | 1 | 3 | |
| No.7 | 2 | 2 | 2 | 2 | 2 | |
| No.8a | 2 | 2 | 2 | 2 | 2 | |
| No.8b | 3 | 3 | 3 | 3 | 3 | |
| No.9 | 2 | 2 | 2 | 2 | 2 | |
| No.10 | 2 | M | 3 | 2 | 2 | |
| No.11p | 2 | 2 | 2 | 2 | 2 | |
| No.11d | 2 | M | 3 | 2 | 2 | |
| No.12a | 2 | 2 | 2 | 2 | 3 | |
| No.12b | 3 | 3 | 3 | 3 | 3 | |
| No.12p | 3 | 3 | 3 | 3 | 3 | |
| No.13 | 3 | 3 | 3 | M | M | |
| No.14v | 2 | 2 | 3 | 3 | M | |
| No.14a | M | M | M | M | M | |
| No.15 | M | M | M | M | M | |
| No.16a1 | M | M | M | M | M | |
| No.16a2 | 3 | 3 | 3 | 3 | 3 | |
| No.16b1 | 3 | 3 | 3 | 3 | 3 | |
| No.16b2 | M | M | M | M | M | |
| No.17 | M | M | M | M | M | |
| No.18 | M | M | M | M | M | |
| No.19 | 3 | M | M | 3 | 3 | 2 |
| No.20 | 3 | M | M | 3 | 3 | 1 |
| No.110 | M | M | M | M | M | 3 |
| No.111 | M | M | M | M | M | 3 |
| No.112 | M | M | M | M | M | 3 |
| 1. WHO Response Evaluation Criteria in Solid Tumors |
| Complete remission (CR): tumor disappears for more than 1 month. |
| Partial response (PR): the product of maximum tumor diameter and maximum vertical diameter decreases up to 50% and none of the other lesions increase for more than 1 month. |
| Stable disease (SD): product of two diameters reduces less than 50% or increases less than 25% for more than 1 month. |
| Disease progression (PD): product of two diameters increases more than 25%. |
| 2. Response Evaluation Criteria In Solid Tumors (RECIST) |
| Evaluation of target lesions: |
| (I). Complete response (CR): All the target lesions disappear. |
| (II). Partial response (PR): At least a 30% decrease in the sum of the LD of target lesions, taking as reference the baseline sum. |
| (III). Progressive Disease (PD): At least a 20% increase in the sum of the LD of target lesions, taking as reference the smallest sum LD recorded since the treatment started ,or the appearance of one or more new lesions. |
| (IV). Stable Disease (SD): Neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD, taking as reference the smallest sum diameters while on study. |
| Evaluation of non-target lesions: |
| (I). Complete response (CR): Disappearance of all non-target lesions and normalization of tumor marker level. |
| (II). Incomplete Response/ Stable Disease (IR/SD): Persistence of one or more non-target lesion(s) or/and maintenance of tumor marker level above the normal limits. |
| (III). Progressive Disease (PD): Appearance of one or more new lesions and/or unequivocal progression of existing non-target lesions. |
| Evaluation of best overall response: |
| The best overall response is the best response recorded from the start of the treatment until disease progression/recurrence. In general, the patient's best response assignment will depend on the achievement of both measurement and confirmation criteria. |