Malignant epithelioid hemangioendothelioma of liver
Introduction
Hepatic epithelioid hemangioendothelioma (HEH) is a rare neoplasm arising from the vascular endothelial cells of liver. It commonly affects middle-aged females and entails a low to intermediate malignant potential between hepatic hemangioma and hepatic angiosarcoma. All the current treatment recommendations are based on evidence from limited retrospective clinical series. Because of the rarity of this tumour and unpredictable natural history, a direct comparison between various therapeutic modalities has not been possible. Herein, clinical presentation of HEH in a woman is described. The pertinent pathological and radiological details along with the management strategy are discussed.
Case report
A 65-year-old woman presented with a 9-month history of pain and heaviness in upper abdomen. She had history of anorexia and weight-loss. She did not have any history of usage of oral contraceptive pills or alcohol. She did not have history of exposure to vinyl chloride or asbestos. Besides, she did not have history of abdominal trauma or viral hepatitis. Ultrasonography of abdomen showed a cystic lesion arising from left lobe of liver. Magnetic resonance imaging (MRI) of abdomen was performed which revealed a well defined circumscribed lesion measuring 7 cm (AP) × 6 cm (TR) × 6.5 cm (SI) in left lobe of liver (Figure 1). It showed heterogeneous iso- to hyperintense signal with peripheral hyperintense rim and moderate to marked hypointense signal on T1- and T2-weighted images, respectively. The lesion showed heterogeneous enhancement along inferior aspect on post-contrast images. Her hydatid serology i.e., Anti Echinococcus IgG was positive [26.53 U/mL (normal <8.0 U/mL)]. Her liver function tests were within normal limits. In addition, serum CA19.9 level was normal [11.91 U/mL (normal <37.0 U/mL)] and viral markers i.e., HBsAg and Anti HCV were non-reactive. Considering the diagnosis of hydatid cyst, exploratory laparotomy was performed. During the operative period, a mass lesion arising from inferior surface of left lobe of liver infiltrating the anterior parieties was visualized. In addition, a lesion measuring 2 cm × 2 cm in greater omentum was visualized. The frozen biopsy of the omental lesion revealed features of metastatic adenocarcinoma. Hence, in view of the aforementioned findings, no further operative intervention was carried out after biopsy. The histopathological examination of the biopsy specimen demonstrated features consistent with hemangioendothelioma.

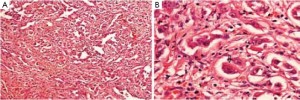
Positron emission tomography (PET) scan was performed for further evaluation. It showed intensely hypermetabolic uptake in a soft tissue lesion arising in liver and anterior abdominal wall in the epigastric region. There was no evidence of any distant metastasis. Her serum alfa-feto protein (AFP) level was within normal limits [3.0 ng/mL (normal <7.0)]. Subsequently, she underwent exploratory laparotomy with hepatic lobectomy (segment 2, 3, and 4), sleeve resection of stomach, anterior abdominal wall resection, and mesh application. The histopathological examination of the resected specimen showed a well encapsulated variegated tumor mass with a rim of hepatic parenchyma. The mass measured 7 cm in maximum dimension and showed areas of necrosis and haemorrhage. It was seen infiltrating the capsule. The adjacent liver parenchyma showed micro- and macrovesicular steatosis with portal triaditis. The microscopic features of the tumor were consistent with epithelioid hemangioendothelioma (Figure 2). On immunohistochemistry, the tumor cells showed positivity for the endothelial markers viz., CD31, CD34, and vimentin (Figure 3). The cells were negative for cytokeratin. An anterior abdominal wall tumor deposit measuring 4.5 cm × 3 cm × 2 cm was identified, the sections of which showed a tumor morphologically similar to the hepatic tumor. The sections examined from the lesser curvature of stomach showed a tumor morphologically similar to the hepatic tumor. In addition, a resected peripancreatic lymph node revealed involvement by the tumor with perinodal soft tissue infiltration.

A contrast enhanced computed tomography (CECT) of abdomen was performed after the surgery. It showed post-operative changes with no residual disease. There was no evidence of any significant uptake on post-operative PET scan. Considering the extensive nature of disease, she was planned for adjuvant radiotherapy. A dose of 45 Gy in 25 fractions over 5 weeks was delivered to the tumor bed and regional nodal regions with three-dimensional conformal radiation therapy (3-DCRT) technique using 6 MV X-rays. One month later, she presented with complaints of abdominal pain and constipation. The CECT of abdomen showed a hypodense cystic lesion in right lobe of liver and adherent bowel loops. She died of hepatic encephalopathy after 2 months.
Discussion
The course of natural history for HEH is variable ranging from a favourable disease with prolonged survival to a rapidly progressive disease with a grave outcome. Peripheral confluent mass with capsular retraction is the hallmark feature that should suggest a diagnosis of HEH (1). The management options for HEH include liver resection, liver transplantation, chemotherapy, radiotherapy, immunotherapy, or surveillance alone. Mehrabi et al. (2) reviewed the therapeutic modalities used in 402 patients with HEH treated from 1984 to 2005. The mean age of patients with HEH was 41.7 years. The male-to-female ratio recorded was 2:3. The most common clinical manifestations included right upper quadrant pain (48.6%), hepatomegaly (20.4%), and weight loss (15.6%). The remaining manifestations were weakness, anorexia, epigastric mass, ascites, nausea/emesis, jaundice, and fatigue. Extrahepatic involvement was observed in 36.6% of patients. The extrahepatic sites included lungs (8.5%), regional lymph nodes (7.7%), peritoneum (6.1%), bone (4.9%), spleen (3.2%), and diaphragm (1.6%). The MRI features observed in those patients revealed low signal intensity on T1-weighted images in 89% of patients. Whereas on T2-weighted images, high signal intensity was seen in 48.5% of patients, mixed signal intensity with a peripheral dark rim in 29%, high signal intensity with peripheral dark rim in 16%, and central low signal intensity with a peripheral high signal rim in 6.5% of cases. Of the 16 patients who had undergone gadolinium-enhanced MRI, high enhancement was seen in 37.5%, peripheral and delayed central enhancement in 37.5%, concentric layers of variable intensity in 19%, and no enhancement in 6%. Liver resection produced a favorable outcome with 1- and 5-year survival rates of 100% and 75% respectively. The results with liver transplantation were also encouraging with 1- and 5-year survival rates of 96% and 54.5%. In a study of 59 patients managed with liver transplantation, the 5- and 10-year survival rates were 83% and 74% respectively (3). A recent report using data from the United Network for Organ Sharing registry for patients undergoing liver transplantation for HEH during an approximately 20-year period reported a 5-year survival of 64% (4). Enhanced aggression of any undetectable residual tumor following resection has been reported. This phenomenon has been postulated to be due to tumour cell reactivity to increased hepatotropic growth factors (5).
A retrospective review of 30 patients with HEH treated at Mayo Clinic (6) between 1984 and 2007 identified three factors associated with prolonged disease-free survival (DFS). These were size of the tumour ≤10 cm, number of nodules ≤10, and extent of hepatic involvement ≤4 segments. Evidently, extrahepatic metastasis at the time of diagnosis was not associated with a worse overall survival. Two of the three patients with extrahepatic metastasis treated with resection were alive at 3 and 10 years, respectively. The difference in overall 5-year survival after liver resection and orthotopic liver transplantation (OLT) was not statistically significant (86% and 64%). Furthermore, there was no statistical difference for 1- and 5-year DFS after liver resection (78% and 62%) and OLT (64% and 46%). Thus, liver resection is a surgical option for many patients with HEH regardless of bilobar distribution provided the hepatic disease can be resected. The authors considered orthotopic liver transplantation to be a more appropriate treatment for patients with >10 nodules or >4 involved hepatic segments. Most patients in this review with extrahepatic disease underwent adjunctive chemotherapy. However, chemotherapy was not associated with improved survival (P=0.42). The chemotherapeutic agents that were used included: Doxorubicin, Ifosfamide, Gemcitabine, Carboplatin, Docetaxel, and Paclitaxel. Interferon-alpha and Bevacizumab were used in some patients.
Makhlouf et al. (7) reviewed 137 cases of HEH. The tumor was diagnosed incidentally in 42% of cases. The follow-up data was available for 60 patients. The median survival noted was of 51 months, ranging from 4 to 336 months. Two of the 60 patients had received adjuvant radiotherapy in addition to chemotherapy. At the time of last follow-up, 1 patient had residual tumor while the other one had widespread metstases at 38 months and 120 months, respectively. In the series by Cardinal et al. (8), there was a statistically significant difference in the mean survival of patients who had no extrahepatic disease (202 months; 95% CI: 157-248 months) compared to those with extrahepatic disease (59 months; 95% CI: 37-80 months) [log-rank test - 6.19; P=0.01]. Their series failed to identify angiolymphatic invasion or positive lymph node involvement as poor prognostic factors. However, the presence of extrahepatic disease beyond regional portal nodes was a negative predictor of outcome.
Treatment with Interferon alfa-2a has been used for patients with HEH, however the benefit in outcome is unclear (9,10). Recent data suggests that anti-VEGF therapy is an option that should be explored in patients with this disease as a means of reducing tumour volume, treating unresectable lesions, or as an adjuvant after OLT (11). The drugs that have been investigated include anti-VEGF monoclonal antibodies i.e., Bevacizumab and Ranibizumab; Anti-VEGF aptamer i.e., Pegaptanib; and inhibitor of VEGF receptor i.e., Sunitinib (12). Sangro et al. treated a patient with metastatic HEH with Sorafenib. Sorafenib is an inhibitor of tyrosine-kinase associated with VEGFR-2, VEGFR-3, and PDGF-β. The patient had stable disease for duration of 2 years (13). Recent studies have also shown that thalidomide can control the progression of the metastatic disease and can even provide symptomatic relief in some cases of HEH (14,15). A case of metastatic hepatic epithelioid hemangioendothelioma treated with thalidomide was reported by Salech et al. (16) The patient had symptomatic relief with stable disease evident upto 9 years of follow-up period.
The patient concerning this case study was treated with resection of the disease. She had extrahepatic involvement evident in anterior abdominal wall, lesser curvature of stomach, and peripancreatic lymph node. With the intent of decreasing the chances of loco-regional recurrence, she was given adjuvant radiation therapy. However, she presented with recurrence in the uninvolved part of liver and succumbed to the disease. Her survival period from the time of diagnosis was of 8 months.
The mode of hepatic involvement and the presence or absence of extrahepatic disease determines the treatment modality for HEH. The clinical presentation and the rate of disease progression influence the decision pertaining to various therapeutic modalities. Liver resection is the primary modality of treatment in resectable cases. In the presence of extrahepatic involvement, adjuvant therapy may be considered. The use of immunotherapy and anti-VEGF therapy needs to be explored in the adjuvant setting. In patients who have massive involvement of the liver, a total hepatectomy with liver transplantation is the therapeutic option.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Earnest F 4th, Johnson CD. Case 96: Hepatic epithelioid hemangioendothelioma. Radiology 2006;240:295-8.
- Mehrabi A, Kashfi A, Fonouni H, et al. Primary malignant hepatic epithelioid hemangioendothelioma: a comprehensive review of the literature with emphasis on the surgical therapy. Cancer 2006;107:2108-21.
- Lerut JP, Orlando G, Adam R, et al. The place of liver transplantation in the treatment of hepatic epitheloid hemangioendothelioma: report of the European liver transplant registry. Ann Surg 2007;246:949-57; discussion 957.
- Rodriguez JA, Becker NS, O’Mahony CA, et al. Long-term outcomes following liver transplantation for hepatic hemangioendothelioma: the UNOS experience from 1987 to 2005. J Gastrointest Surg 2008;12:110-6.
- Ben-Haim M, Roayaie S, Ye MQ, et al. Hepatic epithelioid hemangioendothelioma: resection or transplantation, which and when? Liver Transpl Surg 1999;5:526-31.
- Grotz TE, Nagorney D, Donohue J, et al. Hepatic epithelioid haemangioendothelioma: is transplantation the only treatment option? HPB (Oxford) 2010;12:546-53.
- Makhlouf HR, Ishak KG, Goodman ZD. Epithelioid hemangioendothelioma of the liver: a clinicopathologic study of 137 cases. Cancer 1999;85:562-82.
- Cardinal J, de Vera ME, Marsh JW, et al. Treatment of hepatic epithelioid hemangioendothelioma: a single-institution experience with 25 cases. Arch Surg 2009;144:1035-9.
- d’Annibale M, Piovanello P, Carlini P, et al. Epithelioid hemangioendothelioma of the liver: case report and review of the literature. Transplant Proc 2002;34:1248-51.
- Läuffer JM, Zimmermann A, Krähenbühl L, et al. Epithelioid hemangioendothelioma of the liver. A rare hepatic tumor. Cancer 1996;78:2318-27.
- Miller MA, Sandler AD. Elevated plasma vascular endothelial growth factor levels in 2 patients with hemangioendothelioma. J Pediatr Surg 2005;40:e17-9.
- Breen EC. VEGF in biological control. J Cell Biochem 2007;102:1358-67.
- Sangro B, Iñarrairaegui M, Fernández-Ros N. Malignant epithelioid hemangioendothelioma of the liver successfully treated with Sorafenib. Rare Tumors 2012;4:e34.
- Mascarenhas RC, Sanghvi AN, Friedlander L, et al. Thalidomide inhibits the growth and progression of hepatic epithelioid hemangioendothelioma. Oncology 2004;67:471-5.
- Singhal S, Mehta J. Thalidomide in cancer. Biomed Pharmacother 2002;56:4-12.
- Salech F, Valderrama S, Nervi B, et al. Thalidomide for the treatment of metastatic hepatic epithelioid hemangioendothelioma: a case report with a long term follow-up. Ann Hepatol 2011;10:99-102.


