Prevention of obesity-associated colon cancer by (-)-epigallocatechin-3 gallate and curcumin
Abstract: Obesity is now recognised as a major global health problem. It accounts for a large proportion of the population and is increasing in both developed and developing countries. Epidemiological evidence and studies in animal models showed that obesity increased the incidence of colon cancer. As obesity is difficult to prevent and treat, it is important to find effective approaches to prevent obesity-associated colon cancer. The prevention strategy should be different from that used for the treatment as clinically used drugs are not suitable for the prevention due to side-effects and cost. Phytochemicals are ideal for the prevention. This review summarises the effect of green tea component (-)-epigallocatechin-3 gallate and turmeric component curcumin in the prevention of obesity-associated colon cancer and the mechanisms for their preventive effects. Both agents have been demonstrated to reduce obesity increased polyp formation in animal models and inhibit PI3K/Akt and MAPK signal pathways.
Key words: Obesity; colon cancer; (-)-epigallocatechin-3 gallate; curcumin; phosphoinositide 3-kinase/protein kinase B (PI3K/Akt); mitogen activated protein kinase (MAPK)
Introduction
Obesity is now recognised as a major global health problem. It accounts for a large proportion of the population and is increasing in both developed and developing countries. Obesity can cause many co-morbidities including cancer, heart disease, osteoarthritis and diabetes (1-8). Among them, obesity-associated colon cancer has been studied extensively. Several epidemiological studies showed that obesity increased the incidence of colon cancer (9,10). This is further demonstrated in animal models. Two common animal models have been employed in colon cancer study: genetic defect model - Apcmin/- and chemical azoxymethane (AOM)-induced colon polyp model (11,12). Obesity has been demonstrated to increase colon polyp formation in both models (13,14).
As it is difficult to prevent or treat obesity at present, prevention of obesity-associated colon is an important issue. To be able to prevent obesity-associated colon cancer, it would be great helpful to understand the mechanisms of the disease. Indeed, the mechanisms for obesity-associated colon cancer are now partially elucidated. It is known that multiple cancer risk factors that are increased in obesity are responsible for increased incidence of colon cancer cancer (1,15,16). The main increased risk factors include elevated levels of insulin, insulin-like growth factor-1 (IGF-1), leptin, interleukin (IL)-6, IL-17, tumor necrosis factor (TNF)-alpha and decreased levels of adiponection (14,17-24). These factors in turn cause activation of multiple signal pathways which play key roles in obesity-associated colon cancer such as, phosphoinositide 3-kinase/protein kinase B (PI3K/Akt), mitogen activated protein kinase (MAPK) and signal transducer and activator of transcription 3 (STAT3) (25,26). Thus, inhibition of these factors and associated pathways can be used to prevent or treat obesity-associated colon cancer (27,28). Although many approaches can be used to inhibit these pathways, phytochemicals may be the best to be used for the prevention due to low side-effects and low cost. This review focuses on the effects of green tea component (-)-epigallocatechin-3 gallate (EGCG) and turmeric component curcumin in the prevention of obesity-associated colon cancer and the mechanisms for such effects.
Signalling pathways in the initiation of obesity-associated colon cancer and prevention implications
Several studies showed that the PI3K/Akt pathway play a key role in obesity-associated colon cancer (25). A most recent study demonstrated the importance of the PI3K/Akt and MAPK in the initiation of obesity -associated colon cancer in A/J mice (29). High-fat diet was shown to increase AOM-induced colon carcinogenesis. In these mice, the activity of the PI3K/Akt pathway is increased as detected by phosphorylated Akt. Activation of MAPK pathway is indicated by p-ErK1/2. In addition, anti-apoptotic protein bcl-xl and cell cycle regulator cyclin D1 are increased. This study thus demonstrated that both pathways are important in the initiation of obesity-associated colon cancer and provide the basis for the prevention of obesity-associated colon cancer.
Various approaches have been used to inhibit these signalling pathways to prevent obesity-associated cancer. In energy restrict model, it has been shown that 30% decrease in energy could decrease AOM-induced polyp numbers markedly (30). Calorie restrict reduced IGF-1 and associated signal pathways (31). An angiotensin-converting enzyme (ACE) inhibitor captopril and angiotensin-II type 1 receptor blocker (ARB) telmisartan can also decrease AOM-induced colon cancer in obese mouse model (32). Pitvastatin and branch amino acids are effective in the prevention of colon cancer in db/db obese model (33,34). However, clinic drugs may be not practical to be used for the prevestion of obesity-associated colon cancer as they are expensive and have side-effects. Alternatively, phytochemicals may be ideal for prevention.
Effects of phytochemicals on obesity-associated colon cancer
Phytochemicals have been studied extensively for the prevention of cancer. It has been estimated that phytochemicals can reduce cancer risk as much as 20% via inhibition of cell cycle and increase of apoptosis as well as increase of host immune responses (35). Several phytochemicals have been shown to be effective on the prevention of obesity-associated colon cancer. Eskin et al. demonstrated that mustard mucilage was shown to inhibit obesity-associated colon cancer (36). A meta-analysis showed that coffee consumption decreased the incidence of colon cancer (37,38). Flavonoids (chrysin, quercetin and nobiletin) is also effective in AOM/db/db model (39). The effects of EGCG and curcumin are decribed in details in below sections.
EGCG
Green tea, made from the leaves of the plant Camellia sinensis, is a common beverage in China for thousands of years and now is popular worldwide. Many health beneficial properties of green tea have been revealed such as anti-inflammatry, anti-hypertensive, hypocholesterolemic, hypoglycemic, antidiabetic and anti-carcinogenic effects (40-43). The components of green tea include polyphenolic compounds (catechins): EGCG, (-)-epigallocatechin (EGC), (-)-epicatechin-3-gallate (ECG) and (-)-epicatechin (EC), and flavonols: quercetin, kaempferol and myricitin (44). EGCG is a main component of green tea and has been studied extensively in the prevention of cancer (45).
The preventive effect of green tea and EGCG in colon cancer carcinogenesis has been demonstrated in both Apcmin/- and AOM animal models. Green tea extract alone or in combination with sulindac reduced intestinal tumour formation via down-regulation of beta-catenin in Apcmin/- mouse model (12,46). Ju et al. showed that EGCG can effectively prevent colon cancer carcinogenesis in this model (47). EGCG was also demonstrated to reduce polyp formation in AOM model of colon cancer (48,49).
Several studies have extended the study of EGCG to prevent obesity-associated colon cancer. Shimiz et al.v showed that EGCG at concentrations 0.01% or 0.1% in drinking water significantly decreased the number of total aberrant crypt foci induced by AOM in obese C57BL/KsJ-db/db mice, via reduction of beta-catenin (50). High-fat diet feeding is a common model for obesity-associated cancer which has been shown to increase colon cancer incidence. Ju et al. showed that 0.6% of EGCG in drinking water reduced AOM-induced abbarrant crypt foci formation in high-fat diet induced obese mice (51).
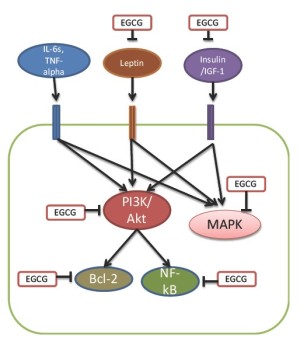
The mechanisms for the preventive effect of EGCG on carcinogenesis have been elucidated to be its properties to inhibit multiple signal pathways (52). EGCG can inhibit both PI3K/Akt and MAPK pathways (47) (Figure 1), which are important in obesity-associated colon cancer. EGCG also interacts directly with the PI3K downsteam target bcl-2, an anti-apoptotic protein (53,54). EGCG can inhibit NF-κB, a transcriptional factor, also a downstream target of the PI3K/Akt pathway (55). Therefore, EGCG can cause cell cycle arrest (56). The effects of EGCG on cancer risk factors increased in obesity include increasing serum level of IGFBP-3, decreasing the serum levels of IGF-I, insulin and leptin. Therefore, EGCG can inhibit activated signal pathways of obesity-associated colon cancer at multiple points (50).
Curcumin
Tumeric, derived from the plant Curcuma longa L of the Zingiberaceae family, has been used as a medicinal agent for thousands of years in Asian countries (57,58). Curcumin is a major component of the spice turmeric which has similar chemical structure of aspirin, a well known chemical that can prevent carcinogenesis (59).
In vitro studies, curcumin has been shown to decrease proliferation and increase apoptosis in several colon cancer cell lines. Addition of curcumin at the concentration of 50 µM to cultured colon cancer cell line HT29 induced apoptosis (60-62). Curcumin was also shown to effectively inhibit the growth of HCT116 cells that survived through fluorouracil and oxaliplatin treatment (63,64). Wei et al. tested the effect of curcumin on 5 cell lines SW480, HCT116, LoVo, SW48, HCT15 and demonstrated it caused apoptosis in all these cells (65). The preventive effect of curcumin on colon cancer has been confirmed in animal models of colon cancer (66).
It has been demonstrated that curcumin can inhibit tumour initiation in obese animal models. Pettan-Brewer et al. showed that diet containing 35% of fat increase polyp number by 23% and 0.5% curcumin reversed the high-fat accelerated polyp formation in an Apcmin/- mouse model (67). Curcumin increased apoptosis and DNA repair. It also reduced high-fat induced weight gain in the model. The anti-obesity property of curcumin (57,68) could also mediate its preventive effect on obesity-associated colon cancer. Kubota investigated the effect of curcumin in C57BL/KsJ-db/db obese mice in AOM model and found that 0.2% and 2% curcumin feeding reduced polyp formation markedly (69-71).
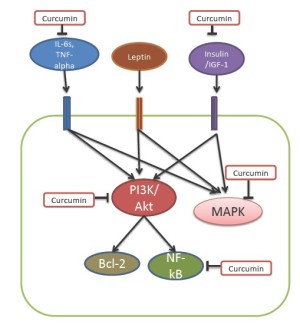
The mechanisms for the protective effect of curcumin on carcinogensis in obesity-associated colon cancer are multiple. Curcumin can inhibit MAPK and Akt pathways (63) (Figure 2). It has been shown to reduce TNF-alpha-induced NF-κB. Curcumin reduced levels of TNF-alpha, IL-6, IGF-1 receptor and cyclooxygenase-2 (COX2) mRNA (63). Cox-2 was considered to be a good target for the prevention of colon cancer (72). Indeed, increased Cox-2 is also involved in obesity-associated colon cancer (73). In addition, curcumin reduces inflammation in obesity (74).
Curcumin is not well dissolved in water and this limits its bioavailability. Nanotechnology has been used to increase its bioavailability and a polymeric nanocarrier-curcumin (PNCC) has been used for colon cancer prevention in AOM-induced tumours in rats (75). PNCC markedly reduced tumour number and size with decreased cell proliferation and increased apoptosis. The study showed that beta-catennin and Bcl-2 proteins were decreased and Bax protein was increased. Several curcumin analogues have also been developed to increase its effectiveness (76-79).
Combinatorial implications of EGCG and curcumin
As both EGCG and curcumin have preventive effects on obesity-associated colon cancer, it is interesting to investigate whether combination of both agents has synergistic effect. Indeed, two studies have revealed the synergistic effect of EGCG and curcumin. Manikandan showed that EGCG and curcumin produced highest levels of inhibitory effect on HCT15 and HCT116 cells in combination although EGCG and curcumin can cause apoptosis individually (38). Kondo et al. also showed that EGCG significantly lowed the dose needed for curcumin to inhibit pAkt/mTOR pathway (80). Further studies are needed to characterise the effect of combinatorial use of EGCG and curcumin on obesity-associated colon cancer and on the key signalling molecules.
Conclusions
Both EGCG and curcumin have been demonstrated to have preventive effects on obesity-associated colon cancer. The mechanisms are their properties to inhibit multiple signalling pathway components especially that in PI3K/Akt and MAPK pathways. These components are activated in obesity and responsible for increased incidence of colon cancer. Combinational use of EGCG and curcumin produced synergistic effect on colon cancer cells. Further studies are warrant for such as effect in the obesity-associated colon cancer model and to characterise the inhibition of signalling molecules.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Drew JE. Molecular mechanisms linking adipokines to obesity-related colon cancer: focus on leptin. Proc Nutr Soc 2012;71:175-80.
- Percik R, Stumvoll M. Obesity and cancer. Exp Clin Endocrinol Diabetes 2009;117:563-6.
- Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist 2010;15:556-65.
- Ning Y, Wang L, Giovannucci EL. A quantitative analysis of body mass index and colorectal cancer: findings from 56 observational studies. Obes Rev 2010;11:19-30.
- Vance V, Mourtzakis M, McCargar L, et al. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obes Rev 2011;12:282-94.
- Ali AS, Ali S, Ahmad A, et al. Expression of microRNAs: potential molecular link between obesity, diabetes and cancer. Obes Rev 2011;12:1050-62.
- Gill RS, Al-Adra DP, Shi X, et al. The benefits of bariatric surgery in obese patients with hip and knee osteoarthritis: a systematic review. Obes Rev 2011;12:1083-9.
- Runhaar J, Koes BW, Clockaerts S, et al. A systematic review on changed biomechanics of lower extremities in obese individuals: a possible role in development of osteoarthritis. Obes Rev 2011;12:1071-82.
- Ben Q, An W, Jiang Y, et al. Body mass index increases risk for colorectal adenomas based on meta-analysis. Gastroenterology 2012;142:762-72.
- Pischon T, Lahmann PH, Boeing H, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). Natl Cancer Inst 2006;98:920-31.
- Chen J, Huang XF. The signal pathways in azoxymethane-induced colon cancer and preventive implications. Cancer Biol Ther 2009;8:1313-7.
- Orner GA, Dashwood WM, Blum CA, et al. Suppression of tumorigenesis in the Apc(min) mouse: down-regulation of beta-catenin signaling by a combination of tea plus sulindac. Carcinogenesis 2003;24:263-7.
- Teraoka N, Mutoh M, Takasu S, et al. High susceptibility to azoxymethane-induced colorectal carcinogenesis in obese KK-Ay mice. Int J Cancer 2011;129:528-35.
- Flores MB, Rocha GZ, Damas-Souza DM, et al. Obesity-Induced Increase in Tumor Necrosis Factor-α Leads to Development of Colon Cancer in Mice. Gastroenterology 2012;143:741-53.e4.
- Huang XF, Chen JZ. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes Rev 2009;10:610-6.
- Sikalidis AK, Varamini B. Roles of hormones and signaling molecules in describing the relationship between obesity and colon cancer. Pathol Oncol Res 2011;17:785-90.
- Gislette T, Chen J. The possible role of IL-17 in obesity-associated cancer. Scientific World Journal 2010;10:2265-71.
- Renehan AG, Zwahlen M, Minder C, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet 2004;363:1346-53.
- Chen J, Katsifis A, Hu C, et al. Insulin decreases therapeutic efficacy in colon cancer cell line HT29 via the activation of the PI3K/Akt pathway. Curr Drug Discov Technol 2011;8:119-25.
- Paz-Filho G, Lim EL, Wong ML, et al. Associations between adipokines and obesity-related cancer. Front Biosci 2011;16:1634-50.
- Renehan AG, Dive C. Obesity, insulin and chemoresistance in colon cancer. J Gastrointest Oncol 2011;2:8-10.
- Chen J, Huang XF, Qiao L, et al. Insulin caused drug resistance to oxaliplatin in colon cancer cell line HT29. J Gastrointest Oncol 2011;2:27-33.
- Howard JM, Pidgeon GP, Reynolds JV. Leptin and gastro-intestinal malignancies. Obes Rev 2010;11:863-74.
- Johnson C, Han Y, Hughart N, et al. Interleukin-6 and its receptor, key players in hepatobiliary inflammation and cancer. Transl Gastrointest Cancer 2012;1:58-70.
- Chen J. Multiple signal pathways in obesity-associated cancer. Obes Rev 2011;12:1063-70.
- Huang XF, Chen JZ. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes Rev 2009;10:610-6.
- Chen JZ. Targeted therapy of obesity-associated colon cancer. Transl Gastrointest Cancer 2012;1:44-57.
- Chen J, Wang B. The roles of miRNA-143 in colon cancer and therapeutic implications. Transl Gastrointest Cancer 2012;1:169-74.
- Park SY, Kim JS, Seo YR, et al. Effects of diet-induced obesity on colitis-associated colon tumor formation in A/J mice. Int J Obes (Lond) 2012;36:273-80.
- Moore T, Beltran L, Carbajal S, et al. Dietary energy balance modulates signaling through the Akt/mammalian target of rapamycin pathways in multiple epithelial tissues. Cancer Prev Res (Phila) 2008;1:65-76.
- Harvey AE, Lashinger LM, Otto G, et al. Decreased systemic IGF-1 in response to calorie restriction modulates murine tumor cell growth, nuclear factor-κB activation, and inflammation-related gene expression. Mol Carcinog 2012. [Epub ahead of print].
- Kubota M, Shimizu M, Sakai H, et al. Renin-angiotensin system inhibitors suppress azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Biochem Biophys Res Commun 2011;410:108-13.
- Yasuda Y, Shimizu M, Shirakami Y, et al. Pitavastatin inhibits azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Cancer Sci 2010;101:1701-7.
- Shimizu M, Shirakami Y, Iwasa J, et al. Supplementation with branched-chain amino acids inhibits azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Clin Cancer Res 2009;15:3068-75.
- Bradford PG, Awad AB. Phytosterols as anticancer compounds. Mol Nutr Food Res 2007;51:161-70.
- Eskin NA, Raju J, Bird RP. Novel mucilage fraction of Sinapis alba L. (mustard) reduces azoxymethane-induced colonic aberrant crypt foci formation in F344 and Zucker obese rats. Phytomedicine 2007;14:479-85.
- Je Y, Liu W, Giovannucci E. Coffee consumption and risk of colorectal cancer: a systematic review and meta-analysis of prospective cohort studies. Int J Cancer 2009;124:1662-8.
- Manikandan R, Beulaja M, Arulvasu C, et al. Synergistic anticancer activity of curcumin and catechin: an in vitro study using human cancer cell lines. Microsc Res Tech 2012;75:112-6.
- Miyamoto S, Yasui Y, Ohigashi H, et al. Dietary flavonoids suppress azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Chem Biol Interact 2010;183:276-83.
- Benelli R, Venè R, Bisacchi D, et al. Anti-invasive effects of green tea polyphenol epigallocatechin-3-gallate (EGCG), a natural inhibitor of metallo and serine proteases. Biol Chem 2002;383:101-5.
- Butt MS, Sultan MT. Green tea: nature’s defense against malignancies. Crit Rev Food Sci Nutr 2009;49:463-73.
- Henning SM, Wang P, Heber D. Chemopreventive effects of tea in prostate cancer: green tea versus black tea. Mol Nutr Food Res 2011;55:905-20.
- Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol 2011;82:1807-21.
- Yang CS, Lambert JD, Ju J, et al. Tea and cancer prevention: molecular mechanisms and human relevance. Toxicol Appl Pharmacol 2007;224:265-73.
- Jankun J, Selman SH, Swiercz R, et al. Why drinking green tea could prevent cancer. Nature 1997;387:561.
- Suganuma M, Ohkura Y, Okabe S, et al. Combination cancer chemoprevention with green tea extract and sulindac shown in intestinal tumor formation in Min mice. J Cancer Res Clin Oncol 2001;127:69-72.
- Ju J, Hong J, Zhou JN, et al. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (-)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res 2005;65:10623-31.
- Ohishi T, Kishimoto Y, Miura N, et al. Synergistic effects of (-)-epigallocatechin gallate with sulindac against colon carcinogenesis of rats treated with azoxymethane. Cancer Lett 2002;177:49-56.
- Shirakami Y, Shimizu M, Tsurumi H, et al. EGCG and Polyphenon E attenuate inflammation-related mouse colon carcinogenesis induced by AOM plus DDS. Mol Med Report 2008;1:355-61.
- Shimizu M, Shirakami Y, Sakai H, et al. (-)-Epigallocatechin gallate suppresses azoxymethane-induced colonic premalignant lesions in male C57BL/KsJ-db/db mice. Cancer Prev Res (Phila) 2008;1:298-304.
- Ju J, Liu Y, Hong J, et al. Effects of green tea and high-fat diet on arachidonic acid metabolism and aberrant crypt foci formation in an azoxymethane-induced colon carcinogenesis mouse model. Nutr Cancer 2003;46:172-8.
- Yang CS, Wang H. Mechanistic issues concerning cancer prevention by tea catechins. Mol Nutr Food Res 2011;55:819-31.
- Leone M, Zhai D, Sareth S, et al. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res 2003;63:8118-21.
- Ermakova S, Choi BY, Choi HS, et al. The intermediate filament protein vimentin is a new target for epigallocatechin gallate. J Biol Chem 2005;280:16882-90.
- Ahmad N, Gupta S, Mukhtar H. Green tea polyphenol epigallocatechin-3-gallate differentially modulates nuclear factor kappaB in cancer cells versus normal cells. Arch Biochem Biophys 2000;376:338-46.
- Ahmad N, Cheng P, Mukhtar H. Cell cycle dysregulation by green tea polyphenol epigallocatechin-3-gallate. Biochem Biophys Res Commun 2000;275:328-34.
- Aggarwal BB. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Annu Rev Nutr 2010;30:173-99.
- Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol 2009;41:40-59.
- Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol 2012;9:259-67.
- Lee YK, Park SY, Kim YM, et al. Regulatory effect of the AMPK-COX-2 signaling pathway in curcumin-induced apoptosis in HT-29 colon cancer cells. Ann N Y Acad Sci 2009;1171:489-94.
- Goel A, Boland CR, Chauhan DP. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett 2001;172:111-8.
- Singh N, Shrivastav A, Sharma RK. Curcumin induces caspase and calpain-dependent apoptosis in HT29 human colon cancer cells. Mol Med Report 2009;2:627-31.
- Patel BB, Gupta D, Elliott AA, et al. Curcumin targets FOLFOX-surviving colon cancer cells via inhibition of EGFRs and IGF-1R. Anticancer Res 2010;30:319-25.
- Watson JL, Hill R, Yaffe PB, et al. Curcumin causes superoxide anion production and p53-independent apoptosis in human colon cancer cells. Cancer Lett 2010;297:1-8.
- Wei SC, Lin YS, Tsao PN, et al. Comparison of the anti-proliferation and apoptosis-induction activities of sulindac, celecoxib, curcumin, and nifedipine in mismatch repair-deficient cell lines. J Formos Med Assoc 2004;103:599-606.
- Villegas I, Sánchez-Fidalgo S, de la Lastra CA. Chemopreventive effect of dietary curcumin on inflammation-induced colorectal carcinogenesis in mice. Mol Nutr Food Res 2011;55:259-67.
- Pettan-Brewer C, Morton J, Mangalindan R, et al. Curcumin suppresses intestinal polyps in APC Min mice fed a high fat diet. Pathobiol Aging Age Relat Dis 2011;1.
- Ejaz A, Wu D, Kwan P, et al. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J Nutr 2009;139:919-25.
- Kubota M, Shimizu M, Sakai H, et al. Preventive effects of curcumin on the development of azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db obese mice. Nutr Cancer 2012;64:72-9.
- Kawamori T, Lubet R, Steele VE, et al. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res 1999;59:597-601.
- Kwon Y, Magnuson BA. Effect of azoxymethane and curcumin on transcriptional levels of cyclooxygenase-1 and -2 during initiation of colon carcinogenesis. Scand J Gastroenterol 2007;42:72-80.
- Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer 2001;1:11-21.
- Delage B, Rullier A, Capdepont M, et al. The effect of body weight on altered expression of nuclear receptors and cyclooxygenase-2 in human colorectal cancers. Nutr J 2007;6:20.
- Weisberg SP, Leibel R, Tortoriello DV. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology 2008;149:3549-58.
- Alizadeh AM, Khaniki M, Azizian S, et al. Chemoprevention of azoxymethane-initiated colon cancer in rat by using a novel polymeric nanocarrier-curcumin. Eur J Pharmacol 2012;689:226-32.
- Chen C, Liu Y, Chen Y, et al. C086, a novel analog of curcumin, induces growth inhibition and down-regulation of NFκB in colon cancer cells and xenograft tumors. Cancer Biol Ther 2011;12:797-807.
- Kanwar SS, Yu Y, Nautiyal J, et al. Difluorinated-curcumin (CDF): a novel curcumin analog is a potent inhibitor of colon cancer stem-like cells. Pharm Res 2011;28:827-38.
- Lai CS, Wu JC, Yu SF, et al. Tetrahydrocurcumin is more effective than curcumin in preventing azoxymethane-induced colon carcinogenesis. Mol Nutr Food Res 2011;55:1819-28.
- Lin L, Liu Y, Li H, et al. Targeting colon cancer stem cells using a new curcumin analogue, GO-Y030. Br J Cancer 2011;105:212-20.
- Kondo A, Takeda T, Li B, et al. Epigallocatechin-3-gallate potentiates curcumin›s ability to suppress uterine leiomyosarcoma cell growth and induce apoptosis. Int J Clin Oncol 2012. [Epub ahead of print].


