Laparoscopic radical resection for rectal cancer
Introduction
Compared with conventional open surgery for rectal cancer, laparoscopic radical resection requires a more profound understanding of the local anatomy. A successful approach relies on adequate resection scope and lymph node dissection. In laparoscopic radical resection for rectal cancer, the inferior mesenteric blood vessels are required to be ligated at the origin for dissection of the third station lymph nodes; the laparoscopic surgical plane is set so close to the lateral peritoneum that the lymph nodes in the sigmoid mesocolon, or the second station lymph nodes, can be dissected; and a complete, sharp separation is made along the pelvic fascia anterior to the sacrum along to ensure complete resection of the rectal tumor and mesorectum (first station juxtaintestinal lymph nodes). Given strict compliance with the principles of cancer surgery and a full understanding of the anatomical characteristics of the rectum and its surrounding structure, surgeons can use laparoscopic radical resection as a less invasive approach to the treatment of rectal cancer with reduced bleeding and comparable efficacy to laparotomy (Videos 1, 2, 3, 4, 5, 6, and 7).
Laparoscopic low anterior resection
Indications
(I) Tumors in the middle and upper parts of the rectum with a diameter of 5 cm or below;
(II) Tumors in the middle and lower parts without infiltration, where the anorectal ring and levator ani muscle are still intact after excision to 2 cm below the lower border of the lesions;
(III) Tumors 4-5 cm or farther from the anal margin on digital rectal examination.
Contraindications
(I) Lower rectal tumors with partial infiltration, particularly those that have invaded the anorectal ring;
(II) Middle to upper rectal tumors that have invaded the surrounding tissue with evidence of pelvic wall infiltration or metastasis;
(III) Poor general condition and/or other concurrent severe illnesses that contraindicate general anesthesia;
(IV) History of abdominal or pelvic surgery in which severe adhesions are expected.
Anesthesia
General anesthesia with endotracheal intubation.
Patient positioning and trocar placement
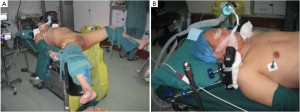
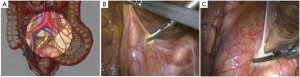
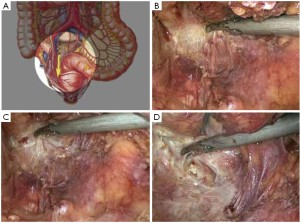
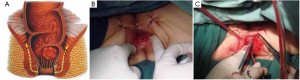
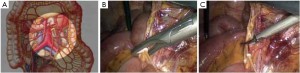
Modified lithotomy position, i.e. the right hip is straight and rotated externally at about 45º with the right knee straight, the right lower limb below the abdomen, the left hip slightly flexed at 30º and rotated externally at 45º, and the left knee flexed at 45º. The right upper limb is adducted and the left upper limb adducted or abducted as needed. After the start of surgery, the feet are raised 30º and tilted to the right 15º so that the head is lower than the rest of the body. The surgeon stands on the patient’s right side and to the right of the camera operator, while the assistants are on the opposite side (Figure 1).
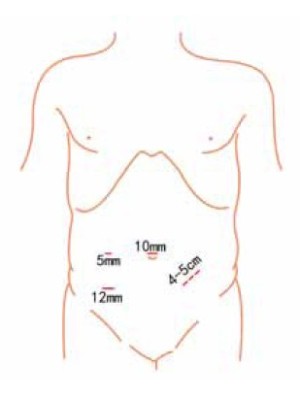
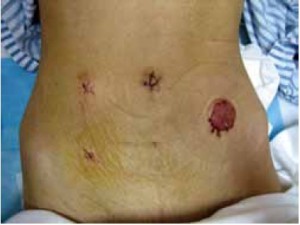
Using the open method of port insertion, a 10 mm trocar is placed in the upper umbilical edge as the observation port to introduce the laparoscope after inflation. A 12 mm working port is inserted in the right lower quadrant (in the midclavicular line at the level of the anterior superior iliac spines) and a 5 mm auxiliary port in the right midclavicular line parallel to umbilicus. A 5 mm or 5 mm secondary auxiliary working port is also inserted in the left midclavicular line parallel to umbilicus, which will be expanded to 4-5 cm for specimen extraction (Figure 2).
Relevant anatomy
Surgical procedures
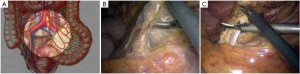
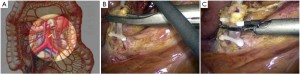
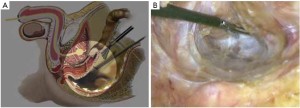
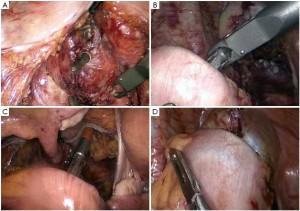
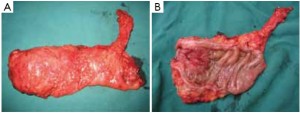
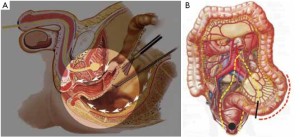
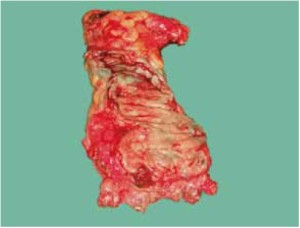
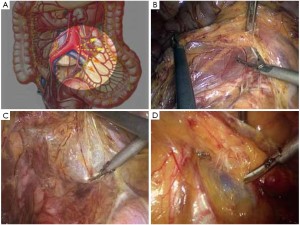
The peritoneum is opened at the junction of the sigmoid mesocolon and the minor pelvis, and dissection is continued from the bottom to the top along the root of the sigmoid mesocolon (Figure 5). The inferior mesenteric artery is separated at about 4 cm from the bifurcation of the right and left common iliac arteries (Figure 6), and the vessel is ligated with Hem-o-Lok clips or 7-0 suture and divided at the origin (Figures 7,8). The inferior mesenteric vein is litigated and transected at 1 cm lateral to the inferior mesenteric artery at the same plane (Figure 9). Dissection is continued into the Toldts space on the left side towards the lower left abdominal wall (Figure 10). The yellow-white boundary is separated between the sigmoid mesocolon and the left side of the abdominal wall for dissection into the retroperitoneal space along the Toldts fascia to join the Toldts space dissected from inside, so that the left psoas major muscle, the left common iliac artery and the ureter passing over it can be seen through the peritoneum (Figure 11). The inferior space along the surface of the abdominal aorta is dissected (Figure 12). The sacrorectal ligament is divided to expose the presacral space (Figure 13), and the loose tissues posterior to the rectum are dissected (Figure 14). The anterior structures are dissected along the spaces between the mesorectum and pelvic walls on both sides (Figure 15). The pelvic peritoneum at the rectovesical pouch or rectouterine pouch is separated so that the seminal vesicles and prostate (in men) (Figure 16) or the cervix and vaginal vault (in women) (Figure 17) are exposed. The rectal wall is denudated at 3-5 cm from the lower border of the tumor. This segment is then clamped with bowel forceps and the lower part of the rectum is rinsed with saline through the anus to minimize tumor cell contamination at the closed line. The rectum is then divided with a linear stapler (Figure 18). The working port in the left lower quadrant is extended to 4-5 cm (19Figure ). Incision protective films are placed while opening the abdominal cavity, and the resected rectum and its mesentery are then extracted (Figure 20). The sigmoid colon is transected at the level 10 cm above the upper edge of the lesion. Thus, the rectal tumor, the proximal part of the sigmoid colon and its mesentery lymphoid tissues are removed (Figure 21). The anvil head of a circular stapler (29-33 mm) is inserted in the stump of the sigmoid colon (Figure 22). The abdominal incision is closed and pneumoperitoneum is re-established. The handle of the stapler is inserted into the anus to connect with the intraperitoneal anvil head. After verifying that there is no evidence of intestinal torsion or tissues inside the convergence, the stapler is fired to create an anastomosis (Figure 23). Saline is injected into the pelvic cavity and the rectum is inflated to verify the anastomosis by evidence of bubbles. The surgical wounds are washed with plenty of distilled water and the effusion drained by suction. A drainage tube is then placed next to the presacral rectal anastomosis, protruding from the lower right trocar (Figure 24). The operation is completed after the trocar incision at the umbilicus is closed layer by layer. (Figures 25,26).
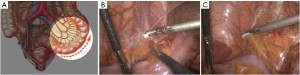

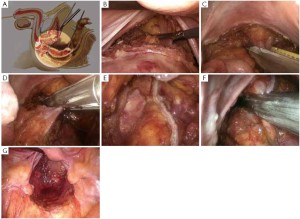
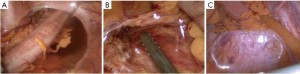
Key points
Proper separation of layers based on a clear awareness of the local anatomy
The dissection commences at the medial side of sigmoid mesentery at the junction with the minor pelvis and continues on the surface of the right common iliac artery. Taking advantage of the avascular zone at the root of the sigmoid mesocolon, separation can be done using ultrasound with minimal to no bleeding. The lateral side of the sigmoid mesentery is dissected along the yellow-white boundary on the left abdominal wall into the Toldts space and through to the inside. Dissection along the surface of the abdominal aorta is continued superiorly to the root of the inferior mesenteric artery and inferiorly to the pelvic cavity into the presacral space. The presacral space is naturally separated as the dissection reaches the bifurcation of the left and right common iliac arteries. A clearly magnified laparoscopic vision will contribute to the accurate identification of the loose clearance between the visceral and pleural layers of the pelvic fascia, and enable the operative field in the narrow pelvis from an alternative view. Complete sharp division of the mesorectum, including the visceral pelvic fascia, is then performed using ultrasonic scalpel. When dissecting on both sides of the rectum, the middle rectal artery can be divided at the root of the collateral rectal ligament using coagulation with ultrasonic scalpel, and vascular clips are rarely needed. When dissecting the rectal wall, caution should be given to identify its definite boundary with the seminal vesicle in men or the cervix in women.
Litigation of the inferior mesenteric artery with suture
In radical resection for rectal cancer, the inferior mesenteric artery must be litigated at its origin so that the third station lymph nodes in this region can be effectively dissected, as they are responsible for the lymphatic drainage from the tumor. Following litigation of the inferior mesenteric artery, blood supply to the sigmoid colon is provided by marginal arteries emerging from the middle colic artery. The origin of the inferior mesenteric artery is easily identifiable when dissecting superiorly along the surface of the abdominal aorta, which is about 1.5 cm from its accompanying vein. Direct ultrasonic dissection is possible as there is no small arteriovenous branch around. Caution should be given under two circumstances: (I) For patients with diabetes who have pathological variations of the vessels with limited length of blood supply from the marginal arteries, the left colic artery should be preserved as much as possible and litigation be made at the distal end of its starting point; (II) For those with congenital absence of the colonic artery (with an incidence of about 3% to 5%), the left colic artery should also be retained. Therefore, the inferior mesenteric artery should be occluded with a non-invasive clamp before litigation to verify whether there is arterial pulse of the sigmoid colon wall to be retained, so that a proper ligation decision can be made (vascular occlusion test).
Exposure and protection of the left ureter
After the inferior mesenteric vessels are ligated, continued dissection to the left will naturally lead to the Toldts fascia. The course of the left ureter can be safely exposed by dissecting on its surface over the retroperitoneum. An alternative approach is to free the lateral sigmoid colon along the left side of the yellow-white boundary to expose the ureter on the surface of the left external iliac artery. Ureteral peristalsis is clearly visible under laparoscope and can be gently induced with instrument for identification. To avoid injury to the ureter, caution should be given to preventing excessively deep dissection of the sigmoid lateral ligament that may potentially involve the ureter, and to identifying the reproductive vessels accompanying the ureter posterior to the left retroperitoneum (spermatic artery and vein in men and ovarian vessels in women), which are confusingly similar in diameter but easily distinguishable by evidence of peristalsis. Occasionally similar in diameter to the ureter, the left seminiferous duct is closer to the seminal vesicles and located at a relatively superficial level, so appropriate identification is needed during the operation.
Cautions
(I) In a modified lithotomy position, the right leg should be placed at a lower level (Figure 1B) so that it will not hinder the operation at the origin of the inferior mesenteric vessels.
(II) For lower rectal tumors, an additional 5 mm trocar can be inserted in the in the left lower quadrant or suprapubic site to help lift the upper rectal segment tied with gauze straps towards the abdominal cavity, so that the local field is enlarged in favor of the operation.
(III) In the case of difficulty identifying the rectal wall during lower rectal separation, the primary assistant may help identify the location of the rectal wall by digital rectal or vaginal examination to avoid injury to the rectal or vaginal wall.
Postoperative care
Anal dilatation: after the resection of rectal tumor, the anastomosis is so close to the anal sphincter that no buffer area is left in the lower segment. Hence, daily anal dilatation will be required from the second day after surgery until passage of gas to reduce the anastomotic pressure and prevent anastomotic fistula.
Complications and treatment
Postoperative complications: in view of the high risk of anastomotic fistula after resection of lower rectal cancer, the drainage traits and clinical signs should be monitored after surgery. Patients with anastomotic fistula that occur beyond three days after surgery, localized peritonitis in the lower abdomen and an otherwise good general condition can present noticeable improvement by conservative treatment and local drainage. However, surgical exploration is required for those with the condition accompanied by extensive abdominal tenderness and sustained high fever in three days after surgery to wash the abdominal cavity, place adequate drainage and perform proximal colostomy or terminal ileostomy. Exploratory laparoscopic cleaning and drainage, followed by proximal enterostomy, is possible if the condition is identified early and a stable general condition is confirmed.
Laparoscopic abdominoperineal resection for rectal cancer
With the continued development of laparoscopic techniques and improvement of the instrument, sphincter-sparing approaches have witnessed a growing application for surgical management for rectal cancer, and radical surgery that requires anal resection has been greatly reduced. In theory, sphincter-sparing surgery can be performed for radical resection of early rectal tumor, no matter how low it is. Due to the difficulty of defining the depth of tumor invasion under clinical settings, however, there is a wide variation in the understanding of the surgical efficacy (in terms of survival period, recurrence rate, ability to control stool, quality of life, etc.) Therefore, the surgical indications specified in traditional textbooks are strictly followed as a mainstream practice.
Indications
(I) Invasive, poorly differentiated rectal cancer less than 5 cm from the anal margin;
(II) Rectal cancer less than 3 cm from the anal margin;
(III) Anal canal and perianal cancer.
Contraindications
(I) Older age and frailty, poor general condition and/or other concurrent severe illnesses that contraindicate general anesthesia;
(II) Wide local infiltration.
Anesthesia
General anesthesia with endotracheal intubation.
Patient positioning and trocar placement
Modified lithotomy position, i.e. the right hip is straight and rotated externally at about 45º with the right knee straight, the right lower limb below the abdomen, the left hip slightly flexed at 30º and rotated externally at 45º, and the left knee flexed at 45º. The right upper limb is adducted and the left upper limb adducted or abducted as needed. After the start of surgery, the feet are raised 30º and tilted to the right 15º so that the head is lower than the rest of the body.
Using the open method of port insertion, a 10 mm trocar is placed in the upper umbilical edge as the observation port to introduce the laparoscope after inflation. A 12 mm working port is inserted in the right lower quadrant (in the midclavicular line at the level of the anterior superior iliac spines) and a 5 mm auxiliary port in the right midclavicular line parallel to umbilicus. A 5 or 10 mm secondary auxiliary working port is also inserted in the left midclavicular line parallel to umbilicus, which will be expanded to 3 cm for sigmoid colostomy.
Surgical procedures
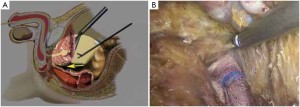
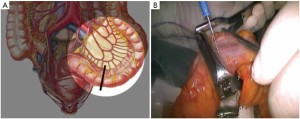
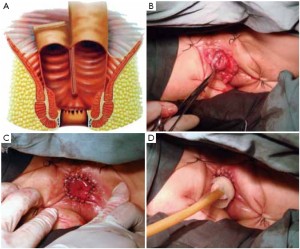
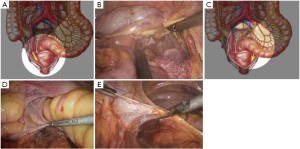
The initial steps are the same as those specified in laparoscopic low anterior resection. At a later stage, dissection of the rectum is continued posteriorly from the presacral space through the tip of the coccyx, anteriorly to the level below the prostate (in men) or the lower end of the rectovaginal subphrenic (in women), and to the pelvic floor bilaterally (Figure 27). The trocar in the left lower quadrant is expanded to a 3 cm port. The sigmoid colon is then extracted from the abdominal cavity via this port and transected. The lower rectal segment or anal tumor and part of the surrounding skin and subcutaneous tissues are resected using standard surgical techniques, and the distal sigmoid colon and the tumor are pulled out from the perineal port (Figure 28). The proximal intestine is extracted through the port in the left lower quadrant as an artificial anus (Figure 29).
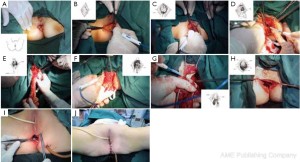
Key points
(I) Preoperative positioning is essential for laparoscopic Miles surgery as half the operation is conducted in the perineal area. Shoulder pads should be installed to prevent the patient from sliding.
(II) Dissection of the structure around the rectum should be ensured to the level as close to the pelvic floor muscles as possible to create the optimal environment for perineal operation. A suboptimal dissection can be undesirable in the perineal surgery after pneumoperitoneum is ended.
(III) It is recommended to create an extraperitoneal tunnel to accommodate the proximal sigmoid colon when extracting it through the left lower abdomen to form the artificial anus. This does not only eliminate the risk of internal hernia but also reduce the risk of a long-term complication, parastomal hernia.
(IV) Since a standard closure of the pelvic peritoneum is not possible during perineal operation, the pelvic floor muscles, subcutaneous tissues and skin have to be sutured layer by layer. The perineal drainage tube can extend out from the working port in the right lower quadrant via the pelvic cavity. Caution should be given to minimizing perineal wound infection and preventing wound dehiscence and intestinal hernia.
Postoperative care
(I) Injuries from the perineal surgery may have a significant impact on the bladder nerves. For example, the integrity of the ureter may be compromised due to obscure operative field. Therefore, it is recommended that urethral catheter stays for one week or above to maintain the patency of the urinary tract. The catheter is intermittently occluded from the seventh day after surgery to facilitate recovery of the bladder function, and is removed three days later.
(II) Gas and a small amount of intestinal discharge from the artificial anus is often observed one day after surgery. Hence, a small amount of water and liquid diet can be prescribed early after the surgery with gradual increment and shift to semi-liquid diet. This is conducive to the recovery of gastrointestinal functionality and nutritional support.
Complications and treatment
(I) Urethral injury: the urethral integrity should be protected during the perineal surgery. Upon definite identification of the ureter, if it is undesirably adjacent to the tumor, the surrounding tissues can be used to suture and wrap the thin urethral wall after removal of the tumor. In such cases, the urethral catheter should be maintained for one month or longer after surgery.
(II) Bleeding: having rich blood supply, the lower end of the prostate and pelvic diaphragm are prone to bleeding, which can be difficult to control due to poor exposure of the operative field. To secure hemostasis, the posterior structure and bilateral tissues of the anus can be divided to pull the tumor and its associated intestine out of the perineum, followed by division of tissues between the rectum, prostate and pelvic diaphragm.
(III) Infection of the perineal incision is relatively common. Proper sterile techniques are required to minimize contamination of the operative field. In the case of infection, open drainage should be prescribed with daily dressing change.
The remaining steps are the same as in laparoscopic low anterior resection.
Laparoscopic intersphincteric resection for low rectal cancer
Lower rectal tumor is a common type of rectal cancer in the Chinese population. Despite advanced surgical techniques, a permanent colostomy is still required in about 20% of these patients. In addition to radical resection, a successful rectal surgery is also expected to improve the quality of life for those patients. Introduced by Lyttle et al. in 1977, intersphincteric resection (ISR) is designed for complete colorectal resection of patients with inflammatory bowel disease by removing the internal anal sphincter while retaining the external anal sphincter and surrounding tissues. This is beneficial as it prevents prolonged healing of the perineal incision. Later, ISR is employed in the radical treatment for low rectal cancer, mostly lower (tumor <5 cm from the anus) lesions that have not invaded the external anal sphincter. It has also been applied to sphincter-sparing surgery for higher tumors in a particularly narrow pelvis. Since its clinical application, ISR has been associated with satisfactory therapeutic effect and a 5-year survival of up to 81% in foreign reports, but there is controversy over the indication assessment, outcome of radical treatment and postoperative anal function. In view of the development of preoperative examination means, we suggest that more clinical studies are required to develop a set of reliable preoperative evaluation criteria.
The combination of the laparoscopic techniques and ISR will serve as a new minimally invasive option for sphincter-preserving radical resection of low rectal cancer that improves the quality of life of patients. Laparoscopic techniques are associated with many advantages in the radical resection for low rectal cancer. It provides an in-depth view of the pelvis that reveals the local anatomical structure from multiple angles, enabling surgeons to dissect the lower rectal segment to a level as distal as possible until the puborectalisbased on an clearly enlarged vision. At present, few studies have been conducted to provide clinical data on laparoscopic TME + ISR. Further research will be needed to shed light on this area.
Indications
(I) Low (4 cm or less from the anal verge) rectal sessile villous adenoma that has become malignant;
(II) Low rectal cancer at the early stage that has not penetrated the muscular layer of the internal anal sphincter, those in a diameter of <3 cm, or those with an unfixed basal portion upon digital rectal examination;
(III) Early anal cancer that has not invaded the external anal sphincter, those in a diameter of <3 cm, or those with an unfixed basal portion upon digital rectal examination;
(IV) Low rectal stromal tumor in a diameter of <3 cm;
(V) Certain low rectal cancer (Dukes C) that has undergone radiotherapy and chemotherapy before the surgery.
Contraindications
(I) Rectal and anal canal cancer that has invaded the external sphincter, those in a diameter of >3 cm, or those with a fixed basal portion upon digital rectal examination;
(II) Concurrent cardiopulmonary disorders that contraindicate general anesthesia with endotracheal intubation;
(III) A history of complex abdominal surgery, where extensive abdominal and pelvic adhesions are expected.
Anesthesia
General anesthesia with endotracheal intubation.
Patient positioning and trocar placement
Modified lithotomy position, i.e. the right hip is straight and rotated externally at about 45º with the right knee straight, the right lower limb below the abdomen, the left hip slightly flexed at 30º and rotated externally at 45º, and the left knee flexed at 45º. The right upper limb is adducted and the left upper limb adducted or abducted as needed. After the start of surgery, the feet are raised 30º and tilted to the right 15º so that the head is lower than the rest of the body.
Using the open method of port insertion, a 10 mm trocar is placed in the upper umbilical edge as the observation port to introduce the laparoscope after inflation. A 12 mm working port is inserted in the right lower quadrant (in the midclavicular line at the level of the anterior superior iliac spines) and a 5 mm auxiliary port in the right midclavicular line parallel to umbilicus. A 10 mm secondary auxiliary working port is also inserted in the left midclavicular line parallel to umbilicus.
Surgical procedures
The initial steps are the same as those specified in laparoscopic low anterior resection. At a later stage, dissection of the rectum is continued posteriorly from the presacral space through the tip of the coccyx, anteriorly to the level below the prostate (in men) or the lower end of the rectovaginal subphrenic (in women), and to the pelvic floor bilaterally.
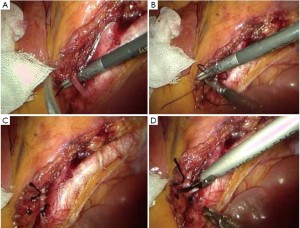
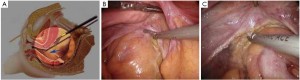
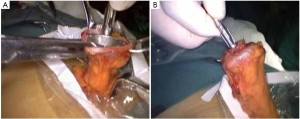
As the perineal surgery group takes over charge, a single layer of interrupted suture is made on the skin of the anal margin to lift the structure and fully expose the anal orifice. The skin and subcutaneous tissues are divided along the intermuscular groove of the anal sphincter to locate the intermuscular space of the internal sphincter (Figure 30). Sharp dissection is made along this space with an electrotome. For partial intersphincteric resection, the sphincter is perpendicularly cut through to the space between the internal and external sphincters, followed by sharp dissection to the proximal end (Figure 31). As proximal dissection reaches the dentate line, separation is continued superiorly between the levator ani muscle and the internal sphincter to join the separated internal pelvic surface. The division should reach the normal intestines and internal sphincter of which the distally separated boundary is 2 cm from the tumor. For patients who have undergone preoperative chemoradiotherapy, this criterion can be reduced to 1 cm as the distance of distal tumor infiltration is significantly shorter. In either case, intraoperative frozen section biopsy is required to confirm a tumor-free margin before the sphincter-preserving surgery.
The inferior mesenteric vessels are managed under laparoscope. The mesentery is cut from the inner side of the vascular arcade aside the colon to dissect sufficient portions of the descending colon and the sigmoid colon, while retaining enough marginal vascular supply (Figure 32) to the proximal colon. The tumor and the involved intestines are pulled out via the anus. The intestine is transected at the planned line and anastomosis between the colon and the anal canal is completed through the anus under direct vision. A full-thickness interrupted suture is applied with 3-0 absorbable suture or 1/0 thread. Alternatively, the distal colon can be used as a storage pouch and connected with the anal skin or distal residual internal sphincter (Figures 33,34). Pneumoperitoneum is recreated. The surgical wounds are rinsed with plenty of distilled water. A drainage tube is placed at the lowest position in the pelvis, protruding from the lower right port. The operation is completed after terminating pneumoperitoneum, removing all trocars and closing the umbilical port layer by layer.
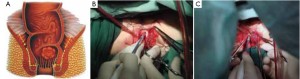
Key points
The key to successful resection using this technique lies in the division into the space between the internal and external sphincters. The internal sphincter is characterized by fine fibers, pale white color and “sliced chicken” appearance, while the external counterpart has more coarse fibers that look like red shredded beef. Both have muscular capsules and a naturally underlying space.
For the laparoscopic group, adequate colic dissection should be ensured without compromising sufficient blood supply to the distal colon, so that a tension-free anastomosis can be achieved with good blood circulation. The left colic artery can be preserved after denudation when necessary, while the inferior mesenteric artery is ligated at the distal end of its starting point.
The anal wall must be cut perpendicularly through the whole thickness of the internal sphincter into the muscular space between the internal and external sphincters. After that, dissection is continued into the pelvic cavity along this space.
Postoperative care
As stenosis is common at the anastomosis between the colon and the anal skin, daily anal dilatation should be prescribed from a half month after the surgery.
Complications and treatment
The most common intraoperative complication is bleeding due to vascular injury. When dissecting along the inferior mesenteric artery for preserving the left colonic artery, litigation of the mesenteric vessels may be required due to improper operation, leading to poor blood circulation of the distal colon. Meanwhile, a fairly long segment of the sigmoid mesocolon has to be separated, where injury can occur to the colic marginal vessels in the case of improper operation. On those occasions, the splenic flexure of the colon should be dissected to ensure a tension-free anastomosis and adequate blood supply.
Anastomotic fistula often occurs in 4-6 days after surgery, mostly as a result of anastomotic tension or compromised blood supply. A small fistula with little leakage can be effectively managed with conservative treatment such as fasting, parenteral nutrition support and adequate local drainage. Immediate laparotomy is warranted in the case of high fever and acute peritonitis, for thorough cleaning of the abdominal and pelvic cavity and placement of unobstructed drainage, followed by transverse colostomy or terminal ileostomy. In emergency situations when a large amount of contents are expected in the colon, the anastomosis can be removed and replaced with terminal colostomy. For peritonitis without severe symptoms, low-grade fever and a large pelvic drainage volume, a second exploratory laparoscopy can be considered to clean the cavities, place drainage and conduct terminal ileostomy or transverse colostomy under laparoscope, thus avoiding large wounds by open surgery.
Intractable diarrhea is the most common postoperative symptom, where up to 20-30 stools are passed each day. For these patients, water and electrolyte balance should be maintained in conjunction with prescription of antidiarrheal drugs such as berberine and loperamide. Diarrhea typically lasts several months to more than one year, but can be gradually reduced by conditioning diet, medication and bowel movement exercise.
Acknowledgements
Disclosure: The authors declare no conflict of interest.