Progranulin: a novel regulator of gastrointestinal cancer progression
Background
Progranulin (PGRN), also known as granulin-epithelin precursor, proepithelin, acrogranin and GP88/PC-cell derived growth factor, is a secreted glycoprotein conserved in most eukaryotes (1). It has been shown to mediate cell cycle progression, cell motility (2) and inflammatory processes (3). Structurally, it belongs to none of the well-established growth factor families, and inhibition of the known growth factor receptors such as IGF and EGF receptors fails to prevent the actions of PGRN (2).
PGRN has been implicated in a number of disease states. For example, a loss- of-function mutation in PGRN has been associated with the onset of frontotemporal lobar degeneration (4). Conversely, PGRN over-activity occurs in many types of cancer (2,5-8) and is thought to confer a more aggressive phenotype to the tumor cells (6). However, how the dysregulation of PGRN function leads to these pathogenic states is largely unknown.
Processing of PGRN to granulins
The human PGRN gene contains 12 protein-coding exons that result in 3 isoforms (9,10) and contains a signal sequence and a number of granulin-like domains. These domains are composed of highly conserved tandem repeats of a unique 12-cysteine sequence (11). The full-length PGRN protein is approximately 68.5 kDa, though it is heavily glycosylated and will therefore migrate as though it is much heavier (1). After secretion, the full-length protein can be proteolytically cleaved between the granulin-like domains by metalloproteinases such as matrix metalloproteinase-9 (MMP-9) (12), MMP-14 (13) and ADAMTS-7 (14). Complete cleavage of full length PGRN results in granulin peptides (GRN A-G and paragranulin) that are approximately 6 kDa in size, though intermediary cleavage products have also been identified (1). The full-length PGRN protein, the intermediaries and the GRN peptides all have biological activity, although it remains to be clarified if these are distinct or overlapping. Regardless, the proteolytic processing of full-length PGRN can be inhibited when secretory leukocyte protease inhibitor (SLPI) or high-density lipoprotein/apolipoprotein A-1 (HDL/ApoA-1) binds to full-length PGRN, preventing its cleavage (15). A schematic representation of the proteolytic cleavage of PGRN is described in Figure 1.
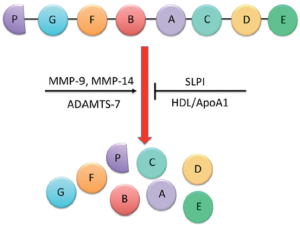
Modes of action of progranulin
To date, no unique receptor for PGRN has been identified. However, PGRN has been shown to bind to the membrane proteins sortilin and tumor necrosis factor receptors (TNFR) 1 and 2 (1). Sortilin was originally identified as a regulator of lysosomal enzyme trafficking (16), but has also been shown to bind and regulate neurotrophic factors such as neurotensin (17) and pro-nerve growth factor (18). It is thought that sortilin mediates the uptake of extracellular PGRN and regulates its internalization within the cell (19,20). The binding of PGRN to the TNFRs, on the other hand, is thought to antagonize TNF-α binding, thereby exerting anti-inflammatory effects (21).
Some of the downstream signaling pathways activated by PGRN include the extracellular signal-regulated kinase (ERK) signaling cascade via the activation of Src homologous and collagen (Shc) protein and p44/42 MAPK, as well as phosphatidylinositol-3 kinase (PI3K) via the activation of Akt (6-8). Activation of these two pathways results in increased expression of Cyclin D1 and Cyclin B and an enhanced proliferation rate (7,22). In addition, PGRN has been shown to activate focal adhesion kinase (FAK) (7,23) and promote the formation of the paxillin/FAK/ERK complex, which promotes cell migration and invasion (23).
Progranulin in cancer
Elevated PGRN expression has been demonstrated in a number of tumor types including ovarian, breast, prostate, renal, liver and esophageal cancers (2,5-8,23-27). In experimental systems, PGRN confers an aggressive phenotype on poorly tumorigenic epithelial cancer cells (2). The malignancy of highly tumorigenic PGRN-expressing cell lines depends on the expression level, since attenuating PGRN mRNA levels greatly inhibits tumor progression (2). Furthermore, PGRN expression has recently been identified as a potential prognostic biomarker for predicting the progression-free survival and overall survival of patients with epithelial ovarian cancer, as patients having significantly higher PGRN levels display poorer prognosis (5,28).
Hepatocellular carcinoma background
Hepatocellular carcinoma (HCC) is the most common type of liver cancer, arising from the malignant transformation of hepatocytes, occurring in most cases as a secondary consequence to hepatitis infections or liver cirrhosis. The incidence of HCC is highest in Asia and Africa where there is high prevalence of Hepatitis B and C, although prevalence in western countries is on the rise (29).
The median survival rate of HCC is approximately 6 months. The prognosis is poor due, in part, to the late presentation of symptoms, large tumors and lack of effective treatment options (29). However, the outcome of patients with HCC has been improved with the introduction of Sorafenib as a test chemotherapeutic agent. Sorafenib is a small inhibitor molecule that targets a number of receptor tyrosine kinases (e.g., VEGF and PCDGF) as well as the Raf kinases (30). In clinical trials, Sorafenib has improved median survival by approximately 3 months (31), although in other trials it proved ineffective as an adjunct therapy (32).
Progranulin in HCC
Using a cDNA microarray approach, PGRN was identified as being upregulated in HCC samples compared to adjacent non-tumor tissue (33,34). Strong PGRN expression was associated with large tumor size, venous infiltration and early intrahepatic recurrence (34). In vitro, the reduction of PGRN expression resulted in decreased cell proliferation, invasion and migration (34). Furthermore, blocking PGRN function in an HCC cell line, using a PGRN-specific neutralizing antibody, inhibited proliferation in vitro and tumor growth in vivo via a mechanism involving the inhibition of the p44/42 MAPK and Akt pathways (26). In addition, treatment with the PGRN-specific neutralizing antibody reduced tumor angiogenesis and vascular endothelial growth factor (VEGF) levels (25).
Cholangiocarcinoma background
Cholangiocarcinoma is a tumor that arises from the malignant transformation of the epithelial cells of the intrahepatic or extrahepatic bile ducts (35). This type of liver cancer has very poor prognosis and is extremely aggressive, with symptoms unobservable until there is bile duct blockage by the tumor (36). Treatment of cholangiocarcinoma by chemotherapy and radiation therapy is not very effective; surgical resection of the tumor is the only treatment option (36). Further study of the factors that lead to tumor initiation, promotion, and progression is necessary for designing alternative treatments for this devastating illness.
The incidence of intrahepatic and extrahepatic cholangiocarcinoma varies by geographic region, with the highest being in Asian countries. Intrahepatic cholangiocarcinoma mortality rates have continuously increased since 1970, conversely, deaths due to extrahepatic cholangiocarcinoma have been decreasing in most countries. Men are slightly more likely to develop cholangiocarcinoma, while incidence increases with age in both sexes (37). The geographic variation of cholangiocarcinoma incidence is partly due to the distribution of risk factors by region and ethnic groups (38). Regional risk factors share the involvement of chronic inflammation and biliary irritation (39). In Asian countries, prevalence of this disease is associated with infection by pathogens including liver flukes, Hepatitis B and Hepatitis C. Meanwhile, in Western countries, 90% of patients diagnosed with cholangiocarcinoma lack any of the reported risk factors (38). However, certain factors are associated with the remaining 10% of cases, including chronic inflammation, primary sclerosing cholangitis, obesity, hepatolithiasis, bacterial infection and/or bile stasis-related chronic cholangitis (40-42).
Progranulin in cholangiocarcinoma
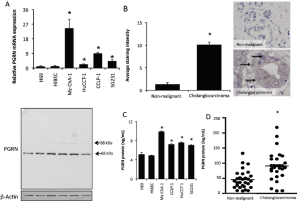
The expression and secretion of PGRN is increased in vitro in cholangiocarcinoma cell lines compared to their non-malignant counterparts (Figure 2) (25). Immunohistochemical analysis of human liver biopsy samples indicated that there is also increased PGRN immunoreactivity in cholangiocarcinoma samples compared to non-malignant controls (Figure 2) (24). In parallel, increased PGRN levels could be detected in the serum (but not bile) from patients with cholangiocarcinoma compared to non-malignant controls (Figure 2) (24).
Interleukin-6 (IL-6) is overexpressed in cholangiocarcinoma and shares a long-standing association with the neoplastic transformation of cholangiocytes to cholangiocarcinoma cells (44,45). Therefore, we hypothesized that IL-6 signaling might be driving the increase in PGRN expression seen in cholangiocarcinoma. Indeed, we demonstrated that increased PGRN expression in cholangiocarcinoma cells is driven by the IL-6-mediated activation of the ERK1/2/RSK1/C/EBP pathway (24).
Increased PGRN secretion was demonstrated to exert subsequent growth-promoting effects on cholangiocarcinoma cells via the activation of Akt and subsequent phosphorylation and nuclear extrusion of Forkhead box protein O1 (FOXO1) (24). A summary of our findings can be found in Figure 3 (24). These data suggest that the upregulation of PGRN may be a key feature associated with the progression of cholangiocarcinoma; inhibiting PGRN expression or function may be a viable target for the development of an effective adjunct therapy to treat this deadly disease.
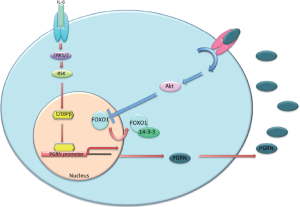
Interestingly, we have also demonstrated that PGRN expression and secretion is increased during hyperplastic cholangiocyte proliferation in a rodent model of extrahepatic biliary obstruction (43). Furthermore, PGRN exerted growth-promoting effects on cholangiocytes via the nuclear extrusion and inhibition of FOXO1, although the mechanism by which this occurred is different (43). Specifically, FOXO1 was extruded after phosphorylation via a mechanism involving the Akt signaling pathway in cholangiocarcinoma, whereas in hyperplastic cholangiocyte proliferation PGRN inhibited the expression of the deacetylase Sirt1 and subsequently increased acetylation of FOXO1, which also results in nuclear extrusion (43). One explanation for the difference in the mechanism by which FOXO1 is inhibited may be due to the increased reliance on Akt signaling by cholangiocarcinoma compared to their non-malignant counterparts (46-48).
Progranulin in other GI cancers
Esophageal squamous cell carcinoma
There are two types of esophageal cancer. Adenocarcinoma arises from glandular cells present at the junction of the esophagus and stomach, and squamous cell carcinoma arises from the cells that line the upper part of the esophagus. Research into the role of PGRN in esophageal cancer is sparse. However, Chen et al. (24) demonstrated that PGRN is upregulated in esophageal squamous cell carcinoma compared to normal mucosa, similar to other tumors. PGRN expression correlated with the depth of tumor invasion, lymph node metastasis and TNM classification, as well as microvessel density and VEGF expression (24). The precise mechanism by which PGRN expression is upregulated, and the downstream signaling pathways activated by PGRN in esophageal squamous cell carcinoma are unknown. No information exists concerning the role of PGRN in esophageal adenocarcinoma.
Colorectal cancer
Chemoresistant colorectal cancer cells (CRCs), selected by chronic exposure to high levels of chemotherapeutic drugs, secrete soluble factors that can stimulate growth in the parental chemonaïve cells and make the chemona?ve cells less sensitive to chemotherapy treatment (49). To identify the soluble factors released from the chemoresistant CRCs, proteomic analysis of the conditioned media was performed, demonstrating that PGRN levels (among other factors) were significantly higher in the media from chemoresistant CRCs than the chemona?ve cells (49). Furthermore, treatment of the parental cells with recombinant PGRN conferred a chemoresistant phenotype, suggesting that chemoresistant tumor cells may promote resistance through the release of PGRN and other soluble factors that mediate survival in otherwise chemosensitive tumor cells (49).
Conclusions
Regardless of the tumor type, it is evident that PGRN is upregulated in cancer cells and confers growth-promoting and chemoresistant effects. A role for PGRN in migration, invasion and angiogenesis is also evident. Specific targeting of progranulin may represent an alternative target for the development of therapeutic strategies to combat these gastrointestinal cancers.
Acknowledgements
Portions of these studies were supported by the following grants awarded to Dr. Sharon DeMorrow: American Cancer Society Research Scholar Award (RSC118760), and NIH K01 Award (DK078532) and NIH R03 Award (DK088012). This material is the result of work supported with resources and the use of facilities at the Central Texas Veterans Health Care System in Temple, TX.
The authors acknowledges Mr. Gabriel Frampton for assistance in proof reading this review.
Disclosure: The author declares no conflict of interest.
References
- De Muynck L, Van Damme P. Cellular effects of progranulin in health and disease. J Mol Neurosci 2011;45:549-60. [PubMed]
- He Z, Bateman A. Progranulin (granulin-epithelin precursor, pc-cell-derived growth factor, acrogranin) mediates tissue repair and tumorigenesis. J Mol Med 2003;81:600-12. [PubMed]
- Jian J, Konopka J, Liu C. Insights into the role of progranulin in immunity, infection, and inflammation. J Leukoc Biol 2013;93:199-208. [PubMed]
- Cruts M, Van Broeckhoven C. Loss of progranulin function in frontotemporal lobar degeneration. Trends Genet 2008;24:186-94. [PubMed]
- Han JJ, Yu M, Houston N, et al. Progranulin is a potential prognostic biomarker in advanced epithelial ovarian cancers. Gynecol Oncol 2011;120:5-10. [PubMed]
- He Z, Ismail A, Kriazhev L, et al. Progranulin (pc-cell-derived growth factor/acrogranin) regulates invasion and cell survival. Cancer Res 2002;62:5590-6. [PubMed]
- Lu R, Serrero G. Mediation of estrogen mitogenic effect in human breast cancer mcf-7 cells by pc-cell-derived growth factor (pcdgf/granulin precursor). Proc Natl Acad Sci U S A 2001;98:142-7. [PubMed]
- Zanocco-Marani T, Bateman A, Romano G, et al. Biological activities and signaling pathways of the granulin/epithelin precursor. Cancer Res 1999;59:5331-40. [PubMed]
- Bhandari V, Bateman A. Structure and chromosomal location of the human granulin gene. Biochem Biophys Res Commun 1992;188:57-63. [PubMed]
- Bhandari V, Palfree RG, Bateman A. Isolation and sequence of the granulin precursor cdna from human bone marrow reveals tandem cysteine-rich granulin domains. Proc Natl Acad Sci U S A 1992;89:1715-9. [PubMed]
- Shoyab M, McDonald VL, Byles C, et al. Epithelins 1 and 2: Isolation and characterization of two cysteine-rich growth-modulating proteins. Proc Natl Acad Sci U S A 1990;87:7912-6. [PubMed]
- Xu D, Suenaga N, Edelmann MJ, et al. Novel MMP-9 substrates in cancer cells revealed by a label-free quantitative proteomics approach. Mol Cell Proteomics 2008;7:2215-28. [PubMed]
- Butler GS, Dean RA, Tam EM, et al. Pharmacoproteomics of a metalloproteinase hydroxamate inhibitor in breast cancer cells: Dynamics of membrane type 1 matrix metalloproteinase-mediated membrane protein shedding. Mol Cell Biol 2008;28:4896-914. [PubMed]
- Bai XH, Wang DW, Kong L, et al. Adamts-7, a direct target of pthrp, adversely regulates endochondral bone growth by associating with and inactivating gep growth factor. Mol Cell Biol 2009;29:4201-19. [PubMed]
- Zhu J, Nathan C, Jin W, et al. Conversion of proepithelin to epithelins: Roles of slpi and elastase in host defense and wound repair. Cell 2002;111:867-78. [PubMed]
- Canuel M, Libin Y, Morales CR. The interactomics of sortilin: An ancient lysosomal receptor evolving new functions. Histol Histopathol 2009;24:481-92. [PubMed]
- Mazella J. Sortilin/neurotensin receptor-3: a new tool to investigate neurotensin signaling and cellular trafficking? Cell Signal 2001;13:1-6. [PubMed]
- Nykjaer A, Lee R, Teng KK, et al. Sortilin is essential for prongf-induced neuronal cell death. Nature 2004;427:843-8. [PubMed]
- Carrasquillo MM, Nicholson AM, Finch N, et al. Genome-wide screen identifies rs646776 near sortilin as a regulator of progranulin levels in human plasma. Am J Hum Genet 2010;87:890-7. [PubMed]
- Hu F, Padukkavidana T, Vaegter CB, et al. Sortilin-mediated endocytosis determines levels of the frontotemporal dementia protein, progranulin. Neuron 2010;68:654-67. [PubMed]
- Tang W, Lu Y, Tian QY, et al. The growth factor progranulin binds to tnf receptors and is therapeutic against inflammatory arthritis in mice. Science 2011;332:478-84. [PubMed]
- Lu R, Serrero G. Inhibition of pc cell-derived growth factor (pcdgf, epithelin/granulin precursor) expression by antisense pcdgf cdna transfection inhibits tumorigenicity of the human breast carcinoma cell line mda-mb-468. Proc Natl Acad Sci U S A 2000;97:3993-8. [PubMed]
- Monami G, Gonzalez EM, Hellman M, et al. Proepithelin promotes migration and invasion of 5637 bladder cancer cells through the activation of erk1/2 and the formation of a paxillin/fak/erk complex. Cancer Res 2006;66:7103-10. [PubMed]
- Chen XY, Li JS, Liang QP, et al. Expression of pc cell-derived growth factor and vascular endothelial growth factor in esophageal squamous cell carcinoma and their clinicopathologic significance. Chin Med J 2008;121:881-6. [PubMed]
- Frampton G, Invernizzi P, Bernuzzi F, et al. Interleukin-6-driven progranulin expression increases cholangiocarcinoma growth by an akt-dependent mechanism. Gut 2012;61:268-77. [PubMed]
- Ho JC, Ip YC, Cheung ST, et al. Granulin-epithelin precursor as a therapeutic target for hepatocellular carcinoma. Hepatology 2008;47:1524-32. [PubMed]
- Pan CX, Kinch MS, Kiener PA, et al. PC cell-derived growth factor expression in prostatic intraepithelial neoplasia and prostatic adenocarcinoma. Clin Cancer Res 2004;10:1333-7. [PubMed]
- Cuevas-Antonio R, Cancino C, Arechavaleta-Velasco F, et al. Expression of progranulin (acrogranin/pcdgf/granulin-epithelin precursor) in benign and malignant ovarian tumors and activation of mapk signaling in ovarian cancer cell line. Cancer Invest 2010;28:452-8. [PubMed]
- Blechacz B, Mishra L. Hepatocellular carcinoma biology. Recent Results Cancer Res 2013;190:1-20. [PubMed]
- Hotte SJ, Hirte HW. Bay 43-9006: Early clinical data in patients with advanced solid malignancies. Curr Pharm Des 2002;8:2249-53. [PubMed]
- Bai W, Wang YJ, Zhao Y, et al. Sorafenib in combination with transarterial chemoembolization improves survival of unresectable hepatocellular carcinoma: A propensity-score matching study. J Dig Dis 2013;14:181-90. [PubMed]
- Keating GM, Santoro A. Sorafenib: A review of its use in advanced hepatocellular carcinoma. Drugs 2009;69:223-40. [PubMed]
- Chen X, Cheung ST, So S, et al. Gene expression patterns in human liver cancers. Mol Biol Cell 2002;13:1929-39. [PubMed]
- Cheung ST, Wong SY, Leung KL, et al. Granulin-epithelin precursor overexpression promotes growth and invasion of hepatocellular carcinoma. Clin Cancer Res 2004;10:7629-36. [PubMed]
- Alpini A, Prall RT, LaRusso NF. The pathobiology of biliary epithelia. In: Arias IM, Boyer JL, Chisari FV, et al. eds. The Liver; Biology & Pathobiology, 4E. Philadelphia, PA: Lippincott Williams & Wilkins, 2001:421-35.
- Sirica AE. Cholangiocarcinoma: Molecular targeting strategies for chemoprevention and therapy. Hepatology 2005;41:5-15. [PubMed]
- Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer 2002;2:10. [PubMed]
- Ben-Menachem T. Risk factors for cholangiocarcinoma. Eur J Gastroenterol Hepatol 2007;19:615-7. [PubMed]
- Gores GJ. Cholangiocarcinoma: Current concepts and insights. Hepatology 2003;37:961-9. [PubMed]
- Catalano OA, Sahani DV, Forcione DG, et al. Biliary infections: Spectrum of imaging findings and management. Radiographics 2009;29:2059-80. [PubMed]
- Chen MF. Peripheral cholangiocarcinoma (cholangiocellular carcinoma): Clinical features, diagnosis and treatment. J Gastroenterol Hepatol 1999;14:1144-9. [PubMed]
- de Groen PC, Gores GJ, LaRusso NF, et al. Biliary tract cancers. N Engl J Med 1999;341:1368-78. [PubMed]
- Frampton G, Ueno Y, Quinn M, et al. The novel growth factor, progranulin, stimulates mouse cholangiocyte proliferation via sirtuin-1-mediated inactivation of FOXO1. Am J Physiol Gastrointest Liver Physiol 2012;303:G1202-11. [PubMed]
- Park J, Tadlock L, Gores GJ, et al. Inhibition of interleukin 6-mediated mitogen-activated protein kinase activation attenuates growth of a cholangiocarcinoma cell line. Hepatology 1999;30:1128-33. [PubMed]
- Yokomuro S, Tsuji H, Lunz JG 3rd, et al. Growth control of human biliary epithelial cells by interleukin 6, hepatocyte growth factor, transforming growth factor beta1, and activin a: Comparison of a cholangiocarcinoma cell line with primary cultures of non-neoplastic biliary epithelial cells. Hepatology 2000;32:26-35. [PubMed]
- Kobayashi S, Werneburg NW, Bronk SF, et al. Interleukin-6 contributes to mcl-1 up-regulation and trail resistance via an akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology 2005;128:2054-65. [PubMed]
- Leelawat K, Leelawat S, Narong S, et al. Roles of the MEK1/2 and AKT pathways in CXCL12/CXCR4 induced cholangiocarcinoma cell invasion. World J Gastroenterol 2007;13:1561-8. [PubMed]
- Xu X, Kobayashi S, Qiao W, et al. Induction of intrahepatic cholangiocellular carcinoma by liver-specific disruption of Smad4 and Pten in mice. J Clin Invest 2006;116:1843-52. [PubMed]
- Bose D, Zimmerman LJ, Pierobon M, et al. Chemoresistant colorectal cancer cells and cancer stem cells mediate growth and survival of bystander cells. Br J Cancer 2011;105:1759-67. [PubMed]


