Intracorporeal laparoscopic esophagojejunostomy using endoscopic linear staplers: the experiences of 293 cases
Introduction
Due to its safety and efficacy, laparoscopic gastrectomy is becoming a widely used surgical method for treating early gastric cancer (EGC) (1-5). After its introduction in 2002, several types of totally laparoscopic gastrectomy using intracorporeal reconstruction have been made to improve early surgical outcome of laparoscopic gastrectomy (6-10). Recently, we also reported that early surgical outcomes of totally laparoscopic distal gastrectomy using an intracorporeal reconstruction (TLDG) are superior to those of laparoscopic assisted distal gastrectomy using an extracorporeal reconstruction (LADG) (11,12).
More recently, some investigators reported various types of totally laparoscopic total gastrectomy using an intracorporeal reconstruction (TLTG) (13-18). In practice, however, it is very hard to perform or try TLTG. Unlike TLDG method, TLTG demands a high level of surgical technique. Therefore, we introduce more practical TLTG method from our experiences.
Surgical techniques
Each patient was placed in the reverse Trendelenburg position. A carbon dioxide pneumoperitoneum was formed from the umbilical port, and pressure was maintained between 12 and 15 mmHg. Five trocars were placed in a U-shape. To retract the liver, the attachment site of the lesser omentum to the right diaphragmatic cruse was intracorporeally sutured, and then a thread pulled by a suture-passer was tied onto the skin in the xyphoid process area. If the operating field was not sufficient, an additional 5-mm trocar was inserted into the epigastric area to retract the liver.
Dissection was begun by dividing the greater omentum, from the mid-portion of the gastroepiploic arcade to the left gastroepiploic vessel. The lymph nodes around the left gastroepiploic, and short gastric vessels were dissected. After dissecting the lymph nodes around the short gastric area, the infrapyloric area was dissected. After lymph nodes around the suprapyloric area were dissected, the duodenum was transected just below the duodenal bulb using an endoscopic linear stapler (ECHELON FLEXTM 60) (Video 1). And then, lymph nodes around common hepatic, proximal or distal splenic, celiac, and left gastric arteries; and right paracardial and lesser curvature areas were dissected in that order.
After having cleared all lymph nodes, nearly two-thirds of the esophagus diameter was transected 2 cm above the gastroesophageal junction using the endoscopic linear stapler (ECHELON FLEXTM 60) and the first intracorporeal suture was placed at the end of the stapled line to retract the esophageal stump and this suture was cut 15 cm from the esophageal stump, which it was retracted by first assistant during reconstruction of esophagojejunostomy (EJ). The unstapled esophageal stump was then transected with laparoscopic scissors after grasping the remnant stomach with a laparoscopic intestinal clamp to avoid cancer cell spillage (Video 1). To make the lumen of esophagus easier to detect, a second round of intracorporeal suture was placed at the small esophagostomy of the esophageal stump and it was extracted outside the abdomen through the right lower trocar to retract it, which prevented slipping of the esophageal mucosa and submucosa during reconstruction of EJ. The specimen was subsequently removed through another suprapubic incision that was approximately 3-4 cm long. After removing the specimen, the suprapubic incision site was closed by continuous suture to reinstate the pneumoperitoneum. The proximal resection margin of the specimen was examined pathologically.
The jejunum was then divided 20 cm below the ligament of Treitz by using an endoscopic linear stapler (ECHELON FLEXTM 60), and an efferent loop was turned in a counter-clockwise direction to reconstruct the EJ. An enterostomy of jejunum was made in the antimesenteric side of the Roux-en-Y limb by using laparoscopic scissors, and an endoscopic linear stapler (ETS STRAIGHTTM 45) with closed staple height of 1.5 mm was inserted into esophagostomy and enterostomy of jejunum to form an EJ (Video 1).
Postoperative management
Gastrograffin studies were performed on postoperative day 3 to evaluate leakage after certain intraoperative events that occurred in nine patients during reconstruction of the esophagojejunostomy. A soft diet was commenced on the day when each patient felt comfortable enough to eat soft foods. The patients were discharged when they had no problems eating a soft diet and were generally comfortable, and inflammatory conditions, including leukocytosis, unstable vital signs, and abrupt onset of abdominal pain, were absent. The final decision about discharge was made by the patient.
Clinical analysis of early surgical outcomes of TLTG
The study sample included 185 men (63.1%) and 108 women (36.9%) with mean age 57.0 years (range, 22-84 years). The average body mass index was 24.8 kg/m2 (range, 16.6-32.4 kg/m2). Intracorporeal esophagojejunal anastomosis using an endoscopic linear stapler was successful in all patients. None of the patients required conversion to open surgery or other laparoscopic anastomosis techniques. All the operations were curative. The mean operation time was 141.8±43.9 min. The mean time to first flatus was 3.47±0.9 days and the mean post-operative day on which patients commenced a soft diet with no morbidity was 4.52±8.0 days. The mean length of hospital stay of patients with no morbidity was 7.80±3.6 days. Table 1 shows the postoperative complications and managements of the patients who underwent TLTG. The overall postoperative complication rate was 15.7%, the mild postoperative complication rate was 11.3%, and the severe postoperative complication rate was also 4.4%.
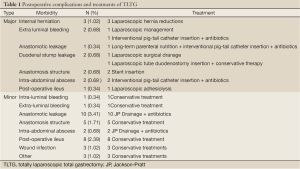
Full Table
Discussion
Recently, several types of totally laparoscopic gastrectomy using intracorporeal reconstruction were introduced. We also reported the benefits of totally laparoscopic distal gastrectomy with gastroduodenostomy using endoscopic linear staplers. Despite several articles about experiences for totally laparoscopic total gastrectomy using intracorporeal reconstructions reported, however there are few reports to evaluate early surgical outcomes of totally laparoscopic total gastrectomy. In practice, TLTG is rarely performed because of the complicated procedures. Therefore, we would like to introduce our method to perform TLTG safely and reduce the possibility of cancer cell spillage from our experiences.
In practical procedures, it is needed to prevent the slipping of esophageal stump during reconstruction of EJ because the resected esophageal stump moves easily into the thoracic cavity. To prevent the slipping of esophageal stump and perform the anastomosis in abdominal cavity during the reconstruction, we had devised improved techniques as follows. Two intracorporeal suturing using black silks were in the end of stapled line and opened esophagostomy of esophageal stump. To prevent slipping of esophageal slipping during the reconstruction, first assistant pulled first thread toward operator side in abdominal cavity and second assistant pulled second sutured thread outside the abdominal cavity through right lower troca. This retraction would have enabled operator to prevent falling of the anastomosis into thoracic cavity and confirm the safety of anastomosis. And, operator could insert without great difficulty an endoscopic linear stapler between opened hole of esophageal stump and jejunal stump to make common channel. As a result, we could minimize the size of remnant anterior hole of common channel. After completion of the EJ, we could confirm the safety of posterior and anterior side of the anastomosis.
In conclusion, we strongly believe that TLTG could be a best way to improve early surgical outcomes in gastric cancer patients. However, inexperienced surgeons for laparoscopic gastrectomy should be careful in performing TLTG because TLTG is made up of complex processes. Therefore, it is conceivable that our TLTG method from high volume center experiences can help surgeons decrease or overcome the learning period.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Adachi Y, Suematsu T, Shiraishi N, et al. Quality of life after laparoscopy-assisted Billroth I gastrectomy. Ann Surg 1999;229:49-54.
- Han JH, Lee HJ, Suh YS, et al. Laparoscopy-assisted distal gastrectomy compared to open distal gastrectomy in early gastric cancer. Dig Surg 2011;28:245-51.
- Hayashi H, Ochiai T, Shimada H, et al. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc 2005;19:1172-6.
- Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994;4:146-8.
- Yoo HM, Lee HH, Shim JH, et al. Long-term outcomes and survival after laparoscopy-assisted distal gastrectomy for gastric cancer: three-year survival analysis of a single-center experience in Korea. J Surg Oncol 2011;104:511-5.
- Kanaya S, Gomi T, Momoi H, et al. Delta-shaped anastomosis in totally laparoscopic Billroth I gastrectomy: new technique of intraabdominal gastroduodenostomy. J Am Coll Surg 2002;195:284-7.
- Oki E, Sakaguchi Y, Ohgaki K, et al. Surgical complications and the risk factors of totally laparoscopic distal gastrectomy. Surg Laparosc Endosc Percutan Tech 2011;21:146-50.
- Bracale U, Rovani M, Bracale M, et al. Totally laparoscopic gastrectomy for gastric cancer: meta-analysis of short-term outcomes. Minim Invasive Ther Allied Technol 2012;21:150-60.
- Bouras G, Lee SW, Nomura E, et al. Surgical outcomes from laparoscopic distal gastrectomy and Roux-en-Y reconstruction: evolution in a totally intracorporeal technique. Surg Laparosc Endosc Percutan Tech 2011;21:37-41.
- Sakaguchi Y, Ikeda O, Ohgaki K, et al. Totally Laparoscopic Gastrectomy for Gastric Cancer Associated with Recklinghausen’s Disease. Diagn Ther Endosc 2010;2010:682401.
- Kim MG, Kim KC, Kim BS, et al. A totally laparoscopic distal gastrectomy can be an effective way of performing laparoscopic gastrectomy in obese patients (body mass index≥30). World J Surg 2011;35:1327-32.
- Kim MG, Kawada H, Kim BS, et al. A totally laparoscopic distal gastrectomy with gastroduodenostomy (TLDG) for improvement of the early surgical outcomes in high BMI patients. Surg Endosc 2011;25:1076-82.
- Bracale U, Marzano E, Nastro P, et al. Side-to-side esophagojejunostomy during totally laparoscopic total gastrectomy for malignant disease: a multicenter study. Surg Endosc 2010;24:2475-9.
- Hirahara N, Monma H, Shimojo Y, et al. Reconstruction of the esophagojejunostomy by double stapling method using EEA™ OrVil™ in laparoscopic total gastrectomy and proximal gastrectomy. World J Surg Oncol 2011;9:55.
- Jeong O, Park YK. Intracorporeal circular stapling esophagojejunostomy using the transorally inserted anvil (OrVil) after laparoscopic total gastrectomy. Surg Endosc 2009;23:2624-30.
- Ziqiang W, ZhiMin C, Jun C, et al. A modified method of laparoscopic side-to-side esophagojejunal anastomosis: report of 14 cases. Surg Endosc 2008;22:2091-4.
- Wang ZQ, Yu PW, Qian F, et al. A modified method of laparoscopic side-to-side esophagojejunal anastomosis after laparoscopic total gastrectomy: a report of 12 cases. Zhonghua Wei Chang Wai Ke Za Zhi 2007;10:323-5.
- Kim MG, Yook JH, Kim KC, et al. Influence of obesity on early surgical outcomes of laparoscopic-assisted gastrectomy in gastric cancer. Surg Laparosc Endosc Percutan Tech 2011;21:151-4.


