Totally laparoscopic distal gastrectomy reconstructed by Roux-en-Y with D2 lymphadenectomy and needle catheter jejunostomy for gastric cancer
Patient information




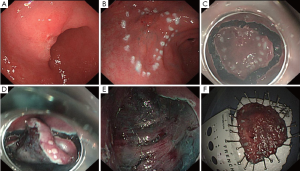
Plan of surgical strategy
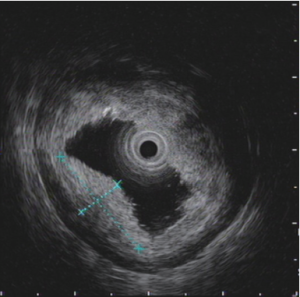
According to the pathologic result of ESD which showed tumor invasion to submucosa or perhaps more deep and suspected lymphatic metastasis from CT scan, laparoscopic distal gastrectomy with D2 lymphadenectomy was arranged. Intraoperative gastroscope was also planned to help locate the tumor position accurately and confirm the extent of resection because of relatively early T staging of tumor. Roux-en-Y reconstruction was demonstrated to be helpful for maintaining glucose homeostasis in diabetes (1), so it was chosen in this patient with Type 2 diabetes mellitus. NCJ was arranged for nutrition support therapy in early postoperative period and during expected chemotherapy after surgery according to suspected lymphatic metastasis from CT scan (2).
Operating procedure (Video 1)
The operation was performed in a regular way. After establishment of pneumoperitoneum and placement of laparoscopic instruments, adhesion between gastric antrum and gallbladder was revealed and no organic or peritoneal metastasis was seen during exploration. There was no gastric serosal involvement due to the tumor. Enlarged lymph nodes were detected along lesser curvature. ESD wound was located at the gastric angle by intraoperative gastroscope and distal gastrectomy was confirmed. Gastrocolic ligament was divided toward the splenic flexure of colon until cutting some of short gastric arteries and then toward the liver flexure until 3 cm distal to pylorus. Anterior lobe of transverse mesocolon and capsula pancreatic were removed until the superior margin of pancreas. The right gastroepiploic vessels were dissected and No.6 and No.14 lymph nodes were removed. The hepatoduodenal ligament was then dissected and No.12 Lymph nodes were cleaned. The right gastric artery was exposed and cut off and No.5 and No.8 lymph nodes were dissected. The left gastric vessels were exposed and cut off and No.7, No.9 and No.11 lymph nodes were removed. The soft tissues along lesser curvature and the right side of cardia including No.1 and No.3 lymph nodes were removed. The omentum along greater curvature including No.4 lymph nodes was divided. Gastroscope was used again to help determine the proximal cutting edge. Duodenum was cut off at 3 cm distal to pylorus by an endocutter. Jejunum was cut off at 40 cm distal to the ligament of Treitz. A precolonic anastomosis was made between the distal stump and posterior wall of stomach by an endocutter. The distal stomach with omentum was cut off at 6 cm proximal to the tumor. An anastomosis was made between the proximal stump of jejunum and the distal jejunum at 40 cm distal to the gastrojejunal anastomosis. NCJ was performed at 40 cm distal to the jejunojejunal anastomosis through the port on left upper quadrant. The whole resected specimen was got out of abdomen in a specimen bag through the prolonged 3 cm incision on right upper quadrant. The abdomen was irrigated with distilled water and no evidence of bleeding noted. A drainage tube was positioned adjacent to the gastrojejunal anastomosis through the incision on right upper quadrant. All the wounds were closed carefully.
Postoperative management
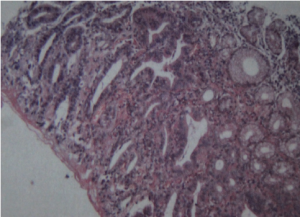
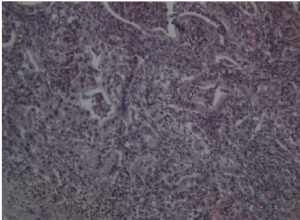
Postoperative treatment included fasting, fluid infusion and acid suppression. The blood pressure and sugar levels were monitored and controlled well. A small amount of enteral nutrition was given through NCJ tube on the first day after surgery. The volume of enteral nutrition was increased gradually and fluid infusion was reduced. The patient began to drink and eat on the fifth day after surgery and the drainage tube was removed. The patient recovered well and was discharged one week after surgery. The final pathologic result showed no residual cancer or lymph node metastasis. Inflammatory cells infiltration was seen in the ESD area. There was lymph node reactive hyperplasia in totally twenty-five resected lymph nodes (No.1 0/3, No.3 0/2, No.4 0/4, No.5 0/0, No.6 0/3, No.7 0/3, No.8 0/1, No.9 0/0, No.11 0/0, No.12 0/5, No.14 0/4). Immunohistochemical stain showed AE1/AE3(-), CD68(+), CEA(-) and Ki-67 index 15%. The final pathologic staging is pT1bN0M0 according to the pathologic result of ESD which showed tumor invasion to submucosa. Chemotherapy was not recommended and the patient followed up regularly in outpatient clinic.
Acknowledgements
This article was supported by the Teaching and Scientific Research Project of Peking Union Medical College Hospital (No. X102550), the Scientific Research Foundation for Middle-aged and Young Scientist of Peking Union Medical College Hospital (No. I102550), Beijing Municipal Natural Science Foundation (No. 7132209), the Teaching Reform Project of Peking Union Medical College (2010) and the Postgraduate Innovation Funding from Peking Union Medical College (No. 2011-1002-017).
Disclosure: The authors declare no conflict of interest.
References
- Patel RT, Shukla AP, Ahn SM, et al. Surgical control of obesity and diabetes: The role of intestinal vs gastric mechanisms in the regulation of body weight and glucose homeostasis. Obesity (Silver Spring) 2013. [Epub ahead of print].
- Wu Q, Yu JC, Kang WM, et al. Short-term effects of supplementary feeding with enteral nutrition via jejunostomy catheter on post-gastrectomy gastric cancer patients. Chin Med J (Engl) 2011;124:3297-301. [PubMed]