Radical surgery for cardia carcinoma: total gastrectomy, D2 + No.10 dissection, esophagojejunal Roux-en-Y anastomosis
Data of the patient
Medical history
The patient was a 71-year-old man, admitted for “upper abdominal discomfort for over 3 months, and gastric neoplasm found 2 months ago”. He had no history of surgery, trauma, hypertension, diabetes, and/or heart disease.
Admission examination
No palpable enlargement of the supraclavicular lymph nodes was found. The abdomen was flat and soft, without visible peristalsis, tenderness or rebound tenderness. There was no muscle guarding. The liver and spleen were not palpable under the ribs. Murphy’s sign was negative, and no percussion pain on the hepatic or renal regions was found. Bowel sounds were heard 4 times/min; and shifting dullness was negative. Digital rectal examination: a finger was inserted smoothly; the mucosa was smooth; no palpable or obvious mass was detected, and there was no blood on the glove after the examination.
Preoperative routine examination
Blood routine [2012-08-28]: WBC count: 4.73×109/L, RBC count: ↓3.93×1012/L, hemoglobin: ↓97 g/L, neutrophils: 66.8%, lymphocytes: 21.6%, platelets: ↑367×109/L; liver function [2012-08-28]: ALT: 7 U/L, AST: 16 U/L, ALP: 50 U/L, GGT: 14 U/L, TBA: ↑12 µmol/L; renal function [2012-08-28]: urea nitrogen: 3.8 mmol/L, creatinine: 65 mol/L, uric acid: 0.255 mmol/L; blood glucose [2012-08-28]: glucose: 5 mmol/L; clotting function [2012-08-28]: PT: 10.5, APTT: 23.9 seconds, FIB: ↑366.3 g/L; hepatic immune parameters [2012-08-28]: HBsAg: negative, HBs-Ab: 7.26, HBeAg: negative, HBe-Ab: negative, HBc-Ab: positive (+), HBcAb-IgM: negative, HCV-Ab: negative, anti-HAV IgM: negative; tumor markers [2012-08-28]: AFP: 4 µg/L, CEA: 2.22 µg/L, CA199: 17.05 U/mL.
Other tests before surgery
Preoperative endoscopy: neoplasm at the cardia; pathology: adenocarcinoma, moderately differentiated. Whole abdomen CT (performed in another hospital, no image data available): signifcantly thickened gastric wall at the cardia region, and gastric cancer was considered; chest X-ray: no obvious abnormalities.
Preoperative diagnosis
Cardia cancer (Siewert type III, preoperative staging: cT4N+M0).
Treatment protocol
Primary radical resection and postoperative adjuvant chemotherapy.
Surgery procedure (Video 1)
Exploration
An incision of about 20 cm was made in the middle of the upper abdomen and around the umbilicus, to gain access to abdominal cavity layer by layer. Abdominal exploration: a small amount of ascites was found. No obvious nodes were palpable in the pelvic cavity and the liver. No enlargement or nodes were detected in the small intestine, colon and their mesenteries. The tumor was an ulcerated mass of about 10 cm × 8 cm, located in the cardia, involving the body of the stomach. Enlarged lymph nodes were palpable at the lesser curvature side. A Kocher incision was made. After the duodenum and the head of the pancreas were isolated, enlargement of station 12b lymph nodes was found, though pathology of the frozen section pathology of the resected specimen showed “no metastasis”. No obvious enlargement of stations 3 and 16 was detected.
Isolation and dissection
The gastrocolic ligament was cut close to the transverse colon, and the anterior lobe of the transverse mesocolon was fully isolated to expose the lower edge of the pancreas. Station 14v lymph nodes were dissected along the surface of the SMV. As the gastrocolic trunk was exposed, the right gastroepiploic vein was cut and lymph nodes around the head of the pancreas were dissected. The right gastroepiploic artery was ligated and cut. Stations 4d and 6 were dissected. The separation was continued upwards until the pancreatic capsule was completely dissected to reach the upper edge of the pancreas. The duodenum was isolated to 3 cm below the pylorus. A 80 mm dividing stapler was triggered to closed and cut the duodenum. The hepatoduodenal ligament was cut open from the left edge of the common bile duct. The lymph nodes were dissected from left to right: the hepatogastric ligament was cut and lymph nodes around the proper hepatic artery (station 12a) were dissected until the hepatic portal was reached; the right gastric artery was ligated and cut (station 5), and lymph nodes to the left and posterior side of the portal vein were dissected (station 12p). The dissection was continued to the celiac trunk (station 9) along the common hepatic artery (station 8). The left gastric artery (station 7) and coronary vein were ligated at their roots. The small omentum was divided close to the liver, and stations 3 and 1 at the lesser curvature side were dissected. The diaphragm-esophageal fascia was open; both vagus nerves were cut, and lymph nodes (station 2) to the left of the cardia were dissected. An approximately 7 cm portion of abdominal esophagus was isolated, cut, and placed into the distal end of a 25 mm circular stapler (Weike). Pathology of the frozen section of the resected margin suggested “no tumor involvement”. The splenorenal, phrenicosplenic and splenocolic ligaments were separated. The spleen was flipped and raised; the dorsal tissues of the pancreas were divided, and the pancreatic tail and spleen were lifted. Lymph nodes along the upper edge and dorsal side of the pancreas were dissected along the splenic artery. The short gastric vessels (station 4sa) were cut, and the left gastroepiploic vessels (station 4sb) were divided at the roots. Lymph nodes of stations 11 and 10 were then dissected along the splenic artery, vein and their branches. The specimens were completely removed.
Reconstruction
The small intestine was cut 20 cm from the Treitz ligament. An end-to-side anastomosis was made between the distal small intestine and the esophagus using a 25 mm circular stapler (Johnson). A side-to-side anastomosis was made between the proximal intestine and the small intestine 50 cm below the previous esophageal anastomosis using a 25 mm circular stapler (Weike). The stumps were closed using a 80 mm linear dividing stapler (Weike). All stumps and anastomoses were consolidated with absorbable interrupted suture. Holes on the small bowel mesentery were closed using interrupted sutures
Postoperative pathology
(Gastric) ulcerative adenocarcinoma, Grade II, mostly mucinous adenocarcinoma, which invaded all of the layers. No involvement was found in the upper and lower surgical margins. One metastatic lymph node was found out of 22 lymph nodes at the lesser curvature. Multiple tumor nodules were observed. No metastasis was detected in the five lymph nodes from the greater curvature, one lymph node from station 13, and three lymph nodes from station 10 (1/31).
Postoperative chemotherapy regimen
DOF: docetaxel 100 mg/2 h ivgtt + oxaliplatin 200 mg/2 h ivgtt + calcium leucovorin 300 mg ivgtt + 5-FU 0.5 IV, 5-FU 4.5 g civ × 48 h q3w × 6 times.
Prognosis
The patient was followed up regularly three months after surgery. The patient was in a satisfactory general condition by eight months after surgery, without signs of recurrence or metastasis.
Instructions for key steps of the procedure
Kocher incision
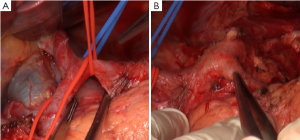
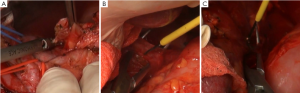
The Kocher incision was made at the external side of the duodenum to expose the duodenum and the head of the pancreas (Figure 1A). This does not only facilitate the exploration of lymph node stations number 12b, 13 and 16 for intraoperative staging and guiding treatment strategies, but also make it easier to completely remove the greater omentum from its rightmost attachment (Figure 1B). In addition, it is also helpful for reducing the anastomotic tension in Billroth I reconstruction following distal subtotal gastrectomy.
Lymph node stations 12b, 13, and 16 are explored
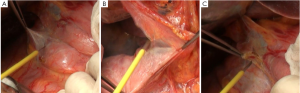
In the present case, enlarged lymph nodes were found in station 12b, lymph nodes around the common bile duct. According to the latest criteria in Japan, a metastatic lymph node in this location should be construed as a staging of M1, making the radical surgery unjustified. Hence, pathology of the frozen section was obtained, and the results suggested no tumor metastasis (Figure 1C). No significant enlargement was detected in stations 13, posterior to the pancreatic head, and 16, aside the aorta (Figure 2A,B).
Anatomical structures anterior to the pancreatic head and dissection of station 14v
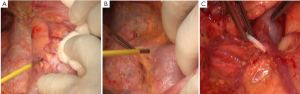
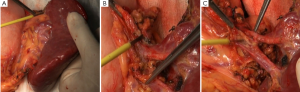
There are multiple variations of the veins anterior to the pancreatic head. In the present case, the right gastroepiploic vein entered the middle colic vein, rather than the Henle trunk. Therefore, caution should be paid when the subpyloric lymph nodes were dissecting, and the anterior superior pancreaticoduodenal vein should be preserved (Figure 2C). Dissection of station 14v has remained controversial. Despite the proximal location, the tumor was large and involving the gastric body, so this lymph node station was dissected as well (Figure 3A).
About dissection of lymph node station 12
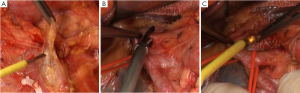
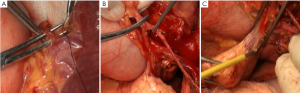
There were many lymph nodes in station 12a, and some were posterior to the PHA, making it difficult to dissect. Hence, CHA and PHA were individually isolated, and pulled with the color coded tubes to create a facilitating field under tension (Figure 3B,C). In addition, caution should be given to protect the common bile duct during the dissection. Enlarged lymph nodes were also found near the portal vein. To achieve R0 resection, PV was isolated and pulled in the same way for dissection of station 12p (Figure 4A).
About dissection of lymph node stations 4sa, 4sb, 10 and 11
Splenic hilum lymph node dissection was performed in this case of advanced proximal gastric cancer. This can be done using in vitro or in situ approaches. Based on our experience, we prefer cutting the splenorenal, phrenicosplenic and splenocolic ligaments, and separating the posterior pancreatic space (Figure 4B,C), so that the spleen and pancreatic head can be pulled out of the abdominal cavity for ease of dissection. This method is advantageous in that: (I) It allows safer and more thorough dissection under direct vision (Figure 5); (II) It is easier to identify the gastric and splenic branches of the splenic vessels, enabling preservation of the intact blood supply to the spleen when dissecting station 4sa (Figure 6A); (III) Compared with the in vivo and in situ methods, our approach enables better exposure of the root of the left gastroepiploic vessels, leading to thorough dissection of station 4sb (Figure 6B); (IV) The tail of the pancreas is better protected, avoiding pancreatic injury and postoperative pancreatic fistula (Figure 6C); (V) It provides good exposure in favor of the dissection of lymph nodes station 11 (Figure 7A,B); (VI) Dissection should be carefully performed to protect the vessels to the lowest splenic pole, as they are often thin and emerge early from the gastroepiploic artery. In situ dissection of station 4sb is associated with a high risk of lower pole ischemia because the vessels are often cut during the procedure. In the present case, these vessels were carefully isolated and protected (Figure 7C). The surgical field upon completion of dissection is shown in Figure 8A. After that, the spleen and the pancreas were put back in place without the need for fixation. A supine position for 24 hours would be sufficient as splenic torsion is very rare (Figure 8B).
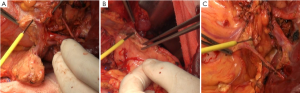
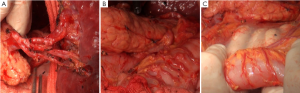
Surgical field after dissection (Figure 8C, Figures 9A,9B)
Acknowledgements
Disclosure: The authors declare no conflict of interest.