Laparoscopy-assisted D2 radical distal subtotal gastrectomy
Clinical data
General data
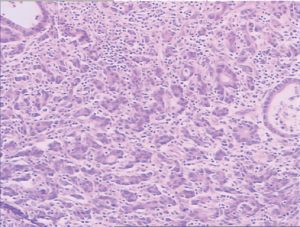
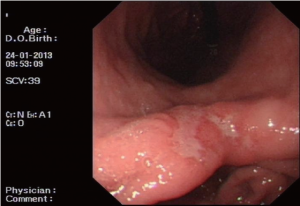
A 54-year-old male patient was admitted to our hospital due to repeated oppressive pain and discomfort in upper and middle abdomen for 3 months. The oppressive pain and discomfort in upper and middle abdomen occurred without significant causes 3 months prior to the admission, which manifested persistent, dull pain. Gastroscopy performed in our hospital 5 days ago showed ulcerative cancer in gastric angle (Figure 1); pathology: tubular adenocarcinoma grade II (Figure 2). He was then admitted as with “gastric cancer”.
Treatment
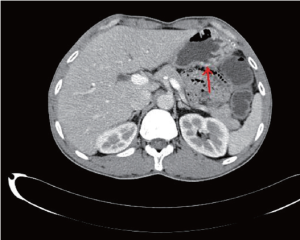
Abdominal CT after admission showed (Figure 3): (I) The stomach cavity was not fully distended with irregular gastric wall thickening in the gastric antrum, gastroscopy was then advised; (II) some small lymph nodes in the hepatogastric space were detected; (III) possibility of mild expansion of biliary system due to the muddy stones associated with inflammatory change in the lower part of common bile duct was considered; and (IV) bilateral renal multiple small cysts. CA199 12.0 U/mL, CA724 0.9 U/mL, CEA 5.67 ng/mL, AFP 1.8 ng/mL. Preoperative diagnosis was tubular adenocarcinoma in lesser curvature of gastric antrum with a pT1N0M0 stage. After relevant examinations were completed, D2 laparoscopy-assisted radical distal subtotal gastrectomy was performed on January 28, 2013, followed by anti-infection, acid resistance, and rehydration and nutrition support. The patient was discharged on postoperative day 7, with stitches removed.
Post-operative pathology
(I) Early grade II tubular adenocarcinoma (IIc) in lesser curvature of gastric antrum with mucosal muscle layer invaded; no cancer infiltration was found in the upper and lower incised margin of surgical specimen and the upper incised margin of the specimen additionally sent for examination. Cancer metastasis: 0/24 in the lymph nodes in lesser curvature, 0/5 at greater curvature, 1/2 at pylorus, and 0/12 under the pylorus; (II) (station 14): the specimen was a fat and vascular tissue. Immunohistochemistry: CK18 (+++) and Ki67 (+90%).
The approach of laparoscopy-assisted radical gastrectomy and lymph node dissection (Video 1)
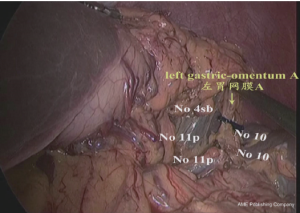
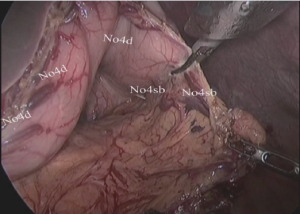
(I) Dissociation of the left and lower region of the greater gastric curvature: The greater gastric curvature was clamped by the assistant using intestinal clamp with the left hand to lift gastrocolic ligament to the head side to expose the surgical field. The greater omentum was separated along the colon from the central region of transverse colon to the splenic flexure of colon with an ultrasonic scalpel to separate the left gastro-omental vessels. The lymph node stations 4d and 4sb were dissected as shown in Figures 4,5.
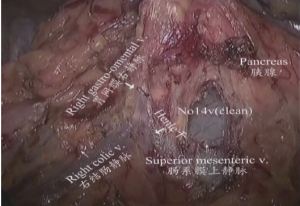
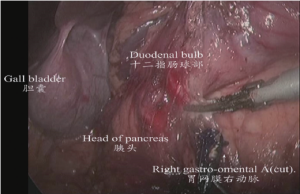
(II) Dissociation of the gastric antrum and the lower region of pylorus: The gastric antrum was clamped by an assistant using an intestinal clamp with the left hand to the head side. The transverse mesocolon was pulled to the caudal side by the operator with the left hand and separated along the middle colic vein to the inferior margin of pancreas using an ultrasonic scalpel with the right hand to expose the superior mesenteric vein. The lymph node station 14v was dissected from the inferior side to the superior side. The right colic vein and right gastro-omental vein were exposed by separating the deep surface of the anterior fascia of pacreaticodudenum. The lymph node station 6 was dissected after the right gastro-omental vein was transected at the point it joined the Hennel axis. After that, the right gastro-omental artery root was transected to separate the duodenal bulb as shown in Figures 6,7.
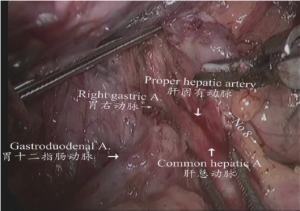
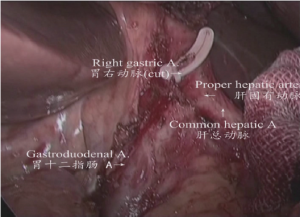
(III) The upper and left region of stomach: the gastric and pancreatic folds were clamped by the assistant using an intestinal clamp with the left hand to roll to the head side, pressing the pancreatic head with the right hand. The middle region of pancreas was gently pressed by the operator using an intestinal clamp with the left hand and the gastroduodenal artery; common hepatic artery, proper hepatic artery and right gastric artery were dissected and exposed using an ultrasonic scalpel closely along the margin of the gastric and pancreatic folds. Parts of the lymph node stations 8a, 5, and 12a were dissected as shown in Figure 8.
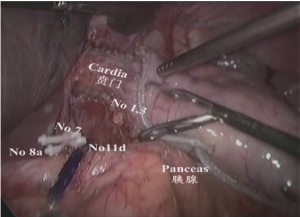
(IV) The region of the upper margin of pancreatic body: The gastric body was clamped by the assistant using an intestinal clamp with the left hand to roll to the head side, pressing the pancreatic body with the right hand. The pancreatic body was gently pressed by the operator using an intestinal clamp with the left hand and the upper margin of the pancreatic capsule was lifted. The common hepatic artery, splenic artery, celiac artery axis and left gastric blood vessels were separated using an ultrasonic scalpel along the anterior side and upper margin of the common hepatic artery from the lower side to the upper side and right to left. The lymph node stations 8a, 11d, 9, and 7 were dissected as shown in Figure 9.
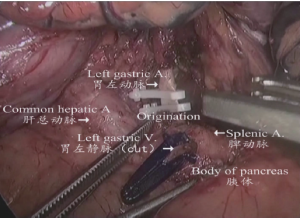
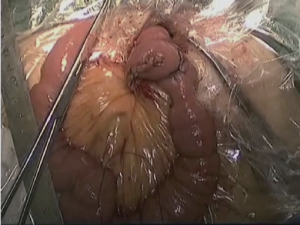
(V) Dissociation of the region of the gastric omentum: The liver was lifted by the assistant using an intestinal clamp with the left hand and the stomach was pulled to the lower abdomen using a clamp with the right hand. The operator tracted the hepatoduodenal ligament using an intestinal clamp with the left hand and transected the hepatogastric ligament along the liver to the cardia using an ultrasonic scalpel with the right hand. The lymph node station 12a was dissected from the upper side to the lower side along the anterior side of the proper hepatic artery. The right gastric artery was transected at the root segment to dissect the lymph node station 5. The lymphatic adipose tissue on the right side of the cardia was separated using an ultrasonic scalpel to the middle-upper 1/3 segment of lesser curvature to dissect the lymph node station 1 as shown in Figures 10,11.
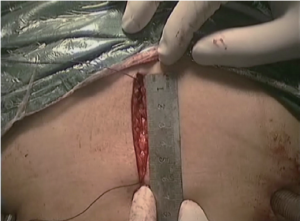
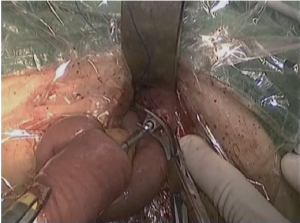
(VI) The duodenal stump, greater curvature and lesser curvature were transected and closed with Endo-GIA and the stomach was transected then for the local irrigation. The surgical field was inspected and the specimen was withdrawn through a 5 to 6 cm small abdominal incision under xiphoid. The digestive tract was reconstructed by RouX-en-Y gastrojejunostomy as shown in Figures 12-14.
Conclusions
The newly updated Japanese gastric cancer treatment guidelines has defined D2 radical treatment as the standard radical treatment for gastric cancer, with its indications including stage IB, stage II and some stage III cases. In 1991, Japanese surgeons for the first time reported the application of laparoscopy-assisted distal gastrectomy for early gastric cancer, which showed similar short- and long-term efficacies like the conventional open surgery (1-3). However, few studies with large number of cases have explored the role of D2 surgery in treating advanced gastric cancer. The key to a successful radical resection is the complete dissection of perigastric lymph nodes. Although this can be challenging, the relevant technques have became optimized, and many studies have demonstrated that D2 surgery can remarkably prolong the survival of patients with advanced gastric cancer. Some clinical trials have also confirmed that laparoscopy-assisted D2 radical resection can achieve the same efficacies as the open surgery in patients with early gastric cancer and part of patients with advanced gastric cancer (4-6). According to our clinical experiences, laparoscopy-assisted D2 radical resection, when we are performing dissociation and dissection in the separate regions, it is superior to the conventional open surgery in terms of surgical trauma, pain, recovery, and abdominal wall scar.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Yoo HM, Lee HH, Shim JH, et al. Long-term outcomes and survival after laparoscopy-assisted distal gastrectomy for gastric cancer: three-year survival analysis of a single-center experience in Korea. J Surg Oncol 2011;104:511-5. [PubMed]
- Bracale U, Rovani M, Bracale M, et al. Totally laparoscopic gastrectomy for gastric cancer: meta-analysis of short-term outcomes. Minim Invasive Ther Allied Technol 2012;21:150-60. [PubMed]
- Han JH, Lee HJ, Suh YS, et al. Laparoscopy-assisted distal gastrectomy compared to open distal gastrectomy in early gastric cancer. Dig Surg 2011;28:245-51. [PubMed]
- Hayashi H, Ochiai T, Shimada H, et al. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc 2005;19:1172-6. [PubMed]
- Bouras G, Lee SW, Nomura E, et al. Surgical outcomes from laparoscopic distal gastrectomy and Roux-en-Y reconstruction: evolution in a totally intracorporeal technique. Surg Laparosc Endosc Percutan Tech 2011;21:37-41. [PubMed]
- Kim MG, Yook JH, Kim KC, et al. Influence of obesity on early surgical outcomes of laparoscopic-assisted gastrectomy in gastric cancer. Surg Laparosc Endosc Percutan Tech 2011;21:151-4. [PubMed]