Video description
The patient was placed supine and in a split-legged position after general anesthesia. After routine disinfection, the surgical field was covered with a sterile surgical towel. An abdominal median incision of about 14 cm was made to gain access to abdominal cavity. After the exploration of the abdominal cavity, radical proximal subtotal gastrectomy was planned (
Video 1, Figures1-
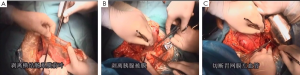
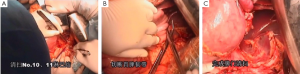
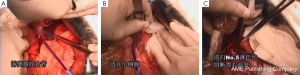
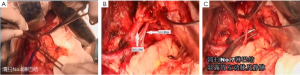
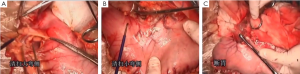
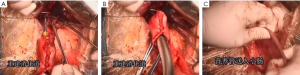
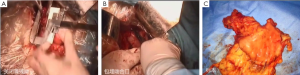
7). The adjacent tissues around the incision were covered by an incision protector. After the greater omentum was flipped and raised, the operator stretched the transverse colon, and separated the omentum along its attachment to the transverse colon with an electric knife. Meanwhile, the anterior lobe of the greater omentum was separated. Along the greater curvature of stomach, splenocolic ligament, gastrosplenic ligament, Huschke’s ligament and the left gastroepiploic artery, short gastric artery, and left gastric artery inside it were dissociated, double ligated, and cut. Furthermore, the anterior lobe of the transverse mesocolon was separated, and the adhesion between stomach and pancreatic capsule were dissociated, whereas the gastric branches of the right gastroepiploic vein and the right gastric artery were preserved. After the hepatogastric ligament was cut along the liver edge (up to the right side of the esophagus), both sides of the esophagus and its anterior and posterior walls (about 5 cm) were dissociated. The gastric body was pulled down. The gastric tube was inserted into the esophagus. Purse-string device was placed 3 cm above the cardia. After the esophagus was transected, the stapler was inserted and the purse string was tightened to cut away about three-fourths of the stomach. The body of stomach was transected at 6 cm to the lower border of the tumor. The residual stomach at the lesser curvature was closed with a surgical stapler. The stump was buried with purse, and the seromuscular layer was sutured. After the residual stomach was lifted up, the incision was opened to insert the stapler, which reached out the posterior wall of stomach. The staple (domestic 26# stapler) rod was attached to the staple body. The stapler and flip the switch was tightened to complete the anastomosis between stomach and esophagus. Efforts must be made to ensure the accuracy and patency of the anastomosis. The integrity of the anastomosis ring should be checked. After the placement of the feeding tube and the gastric tube, the greater curvature of stomach was closed using a stapler. The stump was similarly buried as in the seromuscular layer. The esophagogastric anastomosis was sutured in a continuous manner. The anastomosis tension was decreased. 5-fluorouracil was intraperitoneally injected, which would be released at a constant concentration in 500 hours. A drainage tube was placed at the left side of the esophagogastric anastomosis to check whether there was any active bleeding. After the gauzes and surgical instruments were carefully checked off, the peritoneum was closed, and the muscles and skins were sutured. The surgery was successful, during which the anesthesia was satisfactory. The intraoperative blood loss was about 180 mL. The patient was taken back to the ward after she had regained consciousness. After communication with the patient’s family, the specimens and lymph node stations were sent for pathological examination.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
Cite this article as: Zhang C, Miao W, Liu N, Ge R, Wang C. Radical proximal gastrectomy for gastric carcinoma. Transl Gastrointest Cancer 2013;2(S1):65-68. doi: 10.3978/j.issn.2224-4778.2013.05.21