Anatomy of laparoscopy-assisted distal D2 radical gastrectomy for gastric cancer
Laparoscopic gastrectomy has been widely accepted both in China and abroad. Due to the local complex anatomy of stomach and high demand for lymph node dissection, this approach is still hard to be widely performed in primary hospitals. We have begun the laparoscopic gastrectomy since 2009. So far we have completed 349 cases of laparoscopic distal gastric D2 radical surgery sand summarize the surgical anatomy ideas as follows.
Subjects and methods
General data
The study enrolled 349 patients with gastric cancer undergoing laparoscopy-assisted D2 radical distal gastrectomy from January 2009 to January 2012 in our department, including 180 men and 169 women, aged 29-86 years, with an average age of 57 years. All patients were confirmed as having cancer of the lower gastric body and the antrum by preoperative endoscopy and multi-detector enhanced CT scan. Based on the NCCN guidelines on the pathological staging of gastric cancer (second edition, 2011), there were 70 cases with stage IA, 30 cases with stage IB, 58 cases with stage II, 68 cases with stage IIIA, 73 cases with IIIB, and 50 cases with stage IV.
Methods
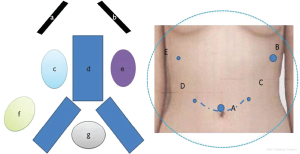
Laparoscopy-assisted D2 radical distal gastrectomy was performed on all patients. Under general anesthesia, each of the patients was placed in a supine position with the legs apart. The surgeon stood at the left side of the patient, the assistant at the right side of the patient, and the camera holder between his two legs. Trocar placement: the first port was created in the edge of the umbilical fossa for laparoscopic observation (port A); a 10 mm trocar was placed in the left anterior axillary line below the costal margin as the working port (port B); a 5 mm auxiliary port was created slightly above and 5 cm to the left of the umbilical fossa (port C); a 5 mm secondary auxiliary working port was inserted in the right midclavicular line parallel to umbilicus (port D); and the last 5 mm auxiliary port was created in the right anterior axillary line below the costal margin (port E) (Figure 1).
The surgery involved seven anatomical regions, focusing on proper exposure and dissection of layers of structures based on surface markers throughout the procedure.
Results
Procedures and experiences
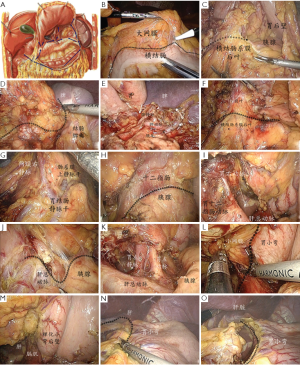
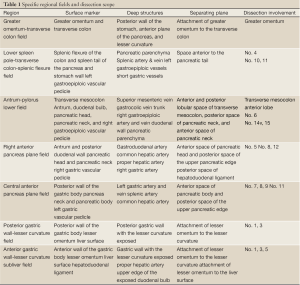
The surgery fields involved seven anatomical regions, including the greater omentum-transverse colon field, colon-spleen lower field, antrum-pylorus lower field, right anterior pancreas plane field, central upper pancreatic edge field, lesser curvature posterior wall field, and subhepatic lesser curvature anterior wall field. In each of the regions, the structures readily visible without surgical dissection were referred to as “surface markers”, in opposite to deep structures, which had to be exposed by proper separation of anatomical spaces. Proper exposure and dissection of various structural layers based on surface markers throughout the procedure was the key to successful lymph node dissection (Figure 2A, Table 1).
Full Table
Greater omentum-transverse colon field
The assistant lifted and extended the greater omentum to maintain mild tension at its attachment to the transverse colon. The operator stretched the transverse colon with forceps in the left hand, and separated the omentum along its attachment to the transverse colon with an ultrasonic scalpel or hook-type electrotome in the right hand. The operation started from the middle of the transverse colon, extending to the splenic flexure on the left and the hepatic flexure on the right (Figure 2B). The anterior and posterior lobular spacing of the transverse mesocolon was explored and identified. The goal in this region was to fully divide the gastrocolic ligament into the lesser sac and expose the deeper structures such as of the posterior wall of the stomach and pancreas (Figure 2C).
Colon-spleen lower field
The assistant pulled the omentum to the right side of the abdomen as far as possible, pushed the posterior gastric wall to the upper right with the left forceps to fully expose the pancreatic tail, and stretched part of the greater omentum with the right forceps. Using an ultrasonic scalpel, the surgeon divided along the anterior space of the pancreas between the capsule and the tail of the pancreas (Figure 2D) until the upper edge, exposing the splenic artery and vein around the splenic hilum. Lymph nodes No. 10 and No. 11 were dissected along the spleen vascular trunk to the splenic hilum, exposing the roots of the left gastroepiploic vessels. The gastroepiploic left blood vessels were clamped and cut. After some of the short gastric vessels were divided, the greater omentum was cut along the greater curvature to expose the greater curvature. Lymph nodes No. 4 were dissected (Figure 2E).
Antrum-pylorus lower field
The assistant lifted the gastric wall from the antrum and greater curvature side with the left forceps, and extended the remaining part of the greater omentum with the right forceps to fully expose the anterior and posterior lobular spacing of the transverse mesocolon. The surgeon then divided along this space along the mesocolon (Figure 2F) to gradually expose the middle colic vessels and right colic vein. Lymph nodes No. 15 were dissected. The trunks of the gastrocolic vein and right gastroepiploic vein were clamped at their intersection, and the latter was cut. The separation continued on the right side, involving the anterior mesocolon and omentum, until the posterior outer side of the duodenal bulb. As separation continued along the posterior pancreatic space under the pancreatic neck, the superior mesenteric vein was exposed and lymph nodes No. 14 were dissected. After the root of the right gastroepiploic artery was exposed during the course, the vessel was clamped and cut. The posterior medial wall of the duodenal bulb was exposed. Dissection of lymph nodes No. 6 was completed (Figure 2G).
Right anterior pancreas plane field
With the assistant holding the gastric body and the antrum up with two pairs of forceps, making the duodenal bulb straightened and slightly extended towards the right side, the operator separated the anterior pancreatic space between the capsule of the pancreatic head and neck and the pancreatic parenchyma towards the patient’s head (Figure 2H) to expose the gastroduodenal artery. The upper edge of the pancreas was then separated to divide the hepatopancreatic fold, entering the posterior pancreatic space and exposing the common hepatic artery. The separation continued towards the right side along the anterior space of the common hepatic vessels to the intersection with the gastroduodenal artery, entering the posterior space of the hepatoduodenal ligament on the head side to expose the trunks of the proper hepatic artery and the right gastric artery. The latter vessel was clamped and cut. The ligament was dissected along the surface of the proper hepatic artery. Some of the lymph nodes of No. 12, 5 and 8a were dissected in this region. (Figure 2I)
Central anterior pancreas plane field
The assistant lifted the gastropancreatic fold with forceps in the left hand to maintain a certain tension, flipped the greater curvature forward to make it under the liver, and held up the antrum or pressed down the pancreas with forceps in the right hand to fully expose the upper edge of the pancreas. The surgeon lifted the pancreatic capsule and separated the anterior pancreatic space to that upper edge (Figure 2J), divided the gastropancreatic fold along the edge, and entered the posterior pancreatic space to expose the trunks of the common hepatic artery the abdominal artery. The left gastric artery and vein, splenic artery, and part of the common hepatic artery were exposed. The left gastric artery and vein were clamped and cut. Lymph nodes No. 7, 8 and 9 were dissected. Group No. 11p was dissected along the anterior space of the splenic artery from the posterior pancreatic space towards the splenic hilum. The gastrophrenic ligament was partially separated to expose both crura of diaphragm (Figure 2K).
Lesser curvature posterior wall field
The assistant lifted the gastric wall on the greater curvature side with the left forceps, and extended the lesser omentum with the right forceps to expose its attachment to the lesser curvature. The operator divided individual layers of the lesser omentum from left to right along the lesser curvature to expose its gastric wall (Figure 2L,M).
Subhepatic lesser curvature anterior wall field
With the assistant holding up the liver, the surgeon dissected the lesser omentum along its attachment to the lesser curvature to expose the corresponding gastric wall, resected the lesser omentum along the hepatic surface, and removed the tissues on the surface of the hepatoduodenal ligament. The upper edge of the duodenum bulb was exposed and lymph nodes No. 3 and No. 5 were dissected (Figure 2N,O).
Surgical outcomes
The operative time ranged from 120 to 210 mins in the observed patients, with intraoperative bleeding of 50-200 mL. Transition to open surgery was required in five patients, mainly due to large BMI and the consequently unclear surgical field. No death due to the surgery was reported. Postoperative pathologic staging confirmed 60 cases with stage IA, 33 cases with stage IB, 62 cases with stage II, 60 cases with stage IIIA, 62 cases with stage IIIB, and 72 cases with stage IV. As for postoperative complications, there were five cases with duodenal stump leakage, two cases with gastroparesis, and three cases with small bowel obstruction. All of them were improved by conservative treatment.
One patient with intraperitoneal bleeding from small branches at the splenic hilum was improved via a second operation.
Discussion
The application of laparoscopic techniques in D2 radical distal gastrectomy for advanced gastric cancer has been gradually recognized (1). A full understanding of the complex anatomical structure around the stomach, as well as a refined procedure protocol, is the key to the success of a high quality operation (2). We have summed up some experience of laparoscopy-assisted D2 radical distal gastrectomy for advanced gastric cancer based on the anatomical characteristics of the stomach, in accordance with the principles of laparoscopic operation.
Subregional operations following the principles of laparoscopic operation
Compared with traditional open surgery, laparoscopic surgery enables larger view of a local field, and presents the anatomical structures more clearly. However, the vision under laparoscope is not sufficiently broad, and it is not as easy to switch across different fields as it would be in an open surgery. Therefore, sub-regional operations, in which the operating field is not switched before all possible operations have been done in a given region, become the inevitable choice for laparoscopic radical gastrectomy, because it is the only way to avoid the inconvenience of repeated switch between fields and improve the operation efficiency. This requires the operator take the following aspects into account: (I) what are the main tasks to be accomplished in each region; (II) what are the starting and ending points in each region; (III) what quality standard is achieved upon completion of each region; and (IV) what are the challenging points for each region. Qian et al. divided the entire field into five regions during laparoscopic gastric surgery (3), and Li et al. employed six subregions (4). These are innovative improvements for the confined field in laparoscopic surgery. Based on our surgical experience, we have developed a seven-subregion configuration for laparoscopy-assisted D2 radical distal gastrectomy, involving local separation and lymph node dissection in the clockwise order from the greater omentum-transverse colon field, to the colon-spleen lower field, the antrum-pylorus lower field, the right anterior pancreas plane field, the central anterior pancreas plane field, the lesser curvature posterior wall field, and the subhepatic lesser curvature anterior wall field.
Exploration of deep structures based on surface markers
Despite the lack of tactile feedback, laparoscopic operation is highly advantageous in the visualization ability compared with traditional open surgery.
We refer to the structures directly visible without dissecting in a fixed field as surface markers. Correct identification of these markers sets a good foundation for accurate surgery. Identification of anatomical structures under laparoscope is also different than the case with open surgery. The camera holder needs to be familiar with the correct distance, direction, and angle.
We use the greater omentum and transverse colon as the surface markers in the greater omentum-transverse colon field, where the operation begins from their attachment point. As soon as the gastrocolic ligament is separated to explore deeper structures, identification of the posterior gastric wall and surface of the pancreas or other structures in the lesser sac indicates the end of operation in this region.
In the colon-spleen lower field, the splenic area of the colon and the pancreatic tail are the surface markers. Although the lower pole of the spleen can be seen in certain patients, it is covered by the greater omentum due to adhesion, making operation in this particular region a challenge during the entire treatment. To deal with the difficulties, we suggest paying attention to the following technical aspects: (I) When the spleen pole is covered by the omentum, the operation should be carried out carefully while exposing it by dividing from shallow to deep individual layers of the greater omentum that is attached to the lateral abdominal wall and the splenic flexure. Clamping of excessive omental tissues with the ultrasonic scalpel should be avoided, so as to prevent splenic injuries by the scalpel tip in a non-visualized area; (II) The tail of the pancreas serves as the essential marker in this region. Separation is carried out from here to the upper edge of the pancreas and the splenic hilum to expose the splenic artery, left gastroepiploic vessels and other structures in depth; (III) After the separation, vessels supplying the spleen from the hilum should be protected and should not be clamped. Otherwise, it can result in focal necrosis of the spleen; (IV) Misidentification of the trunk or main branches of the splenic artery as the left gastroepiploic vessel should be avoided, or extensive necrosis of the spleen may be resulted. In the antrum-pylorus lower field, the surface markers include the antrum, the duodenal bulb, and the head and neck of the pancreas. This part is a continuation of the separation along the anterior and posterior lobular space of the transverse mesocolon from the previous field. The critical area involves this region to the lower edge of the pancreas. The separation is carried out at two levels—the posterior pancreatic space and the anterior pancreatic space—to expose the trunks of the superior mesenteric vein and gastrocolic vein so that lymph nodes No. 14 can be dissected, and to expose deep structures such as the right gastroepiploic artery, respectively.
In the right anterior pancreas plane field, the surface markers include the posterior wall of the duodenal bulb, posterior wall of the antrum, head and neck of the pancreas, and the hepatopancreatic fold. The deep structures to be exposed include the gastroduodenal artery, common hepatic artery, proper hepatic artery, and right gastric artery. The key to the operation in this region is to identify the two vascular converging points—one between the gastroduodenal artery and the common hepatic artery, and the other the right gastric artery and the proper hepatic artery, particularly the latter one. Caution should be paid to identify and avoid the proper hepatic gastric artery when the right gastric artery is clamped, thus preventing liver injury.
In the central anterior pancreas plane field, the surface markers include the posterior wall of the gastric body, the body of the pancreas, and the gastropancreatic fold. Deep structures to be exposed include the celiac trunk, left gastric vein, left gastric artery, and splenic artery. Identifying the left gastric vein is difficult in this region due to its variations. Typically, the left gastric vein is located in the head side of the common hepatic artery and will enter the portal vein trunk, while some will enter the portosplenic confluence or directly into the splenic vein (5). Those variations are located in the foot side of the common hepatic artery. When it is divided along the upper edge of the pancreas, it is easy to directly cut the left gastric vein without being able to locate the stumps. Hence, caution should be paid during this operation.
Operations in the lesser curvature posterior wall and the subhepatic lesser curvature anterior wall fields are carried out to expose the lesser curvature and dissect the lesser sac, aiming mainly to dissect lymph node groups 1, 3 and 5.
Anatomical spaces provide the proper operating pathway
With less control of bleeding compared with open surgery, and in view that bleeding may obscure the surgical field and make it difficult to proceed, laparoscopic surgery has a higher demand for blood-free operation, and separation along the correct anatomical spaces is the key to guaranteeing this. To sum up, laparoscopy-assisted D2 radical distal gastrectomy involves the following anatomical spaces: the anterior and posterior lobular space of transverse mesocolon, the posterior pancreatic space, the anterior pancreatic space, and the anterior vascular space of major branches of the celiac trunk (common hepatic artery and splenic artery). The pancreas serves as a central marker for identifying these spaces (2).
Subregional operation on a layer by layer basis facilitates understanding of the procedure and standardized dissection, which is essential for increasing the operational efficiency, shortening the learning curve, and improving the quality of surgery.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Viñuela EF, Gonen M, Brennan MF, et al. Laparoscopic versus open distal gastrectomy for gastric cancer: a meta-analysis of randomized controlled trials and high-quality nonrandomized studies. Ann Surg 2012;255:446-56. [PubMed]
- Li GX. Ideas of anatomy on laparoscopic D2 lymph node dissection for distal gastric cancer. Zhong Hua Wei Chang Wai Ke Za Zhi 2010;13:400-2.
- Qian F, Tang B, Yu PW, et al. Operation approaches for aparoscopic gastric surgery. Zhong Hua Xiao Hua Wai Ke Za Zhi 2010;9:299-302.
- Li GX, Zhang C, Yu J. laparoscopic-assisted gastrectomy with D2 lymph node dissection for distal gastric cancer: the art based on anatomy. Wai Ke Li Lun Yu Shi Jian 2007;12:533-9.
- Lan GH, Lin ZW, Liang XY, et al. The application of venous anatomy in laparoscopic D2 lymph node dissection for distal gastric cancer. Guang Zhou Yi Xue Yuan Xue Bao 2010;2:66-9.


