Chronic administration of omeprazole decreases biliary proliferation of cholestatic rats by increased gastrin serum levels
Introduction
Cholangiocytes, which line the intrahepatic biliary epithelium (1,2), modify bile originally secreted at the bile canaliculus (3) before it reaches the duodenum (4). The modification of ductal bile is regulated by the secretion/reabsorption of water and electrolytes that is regulated by a number of gastrointestinal hormones, peptides and neurotransmitters (1,4-9). Regarding gastrointestinal hormones, secretin stimulates the secretion of water and HCO3– ions by interaction with basolateral SR (1,5-7), an interaction that leads to increased cyclic adenosine 3',5'-monophosphate (cAMP) levels, phosphorylation of protein kinase A (PKA), opening of the cystic fibrosis transmembrane regulator (CFTR) leading to activation of the chloride bicarbonate anion exchanger 2 (Cl–/HCO3– AE2) (10) with subsequent secretion of HCO3– into bile (1,4-9).
In addition to regulate ductal bile secretion, cholangiocytes are the target cells in chronic liver diseases such as primary biliary cirrhosis (PBC) and primary sclerosing cholangitis (PSC), diseases that are characterized by dysregulation between biliary growth/apoptosis (11,12). Animal models of cholestasis such as extrahepatic bile duct ligation (BDL) (4,8,13), acute treatment with carbon tetrachloride (CCl4) (14) and chronic administration of the toxin, a-naphthylisothiocyanate (15) or gamma-aminobutyric acid (GABA) (16) induces the proliferation and/or loss of bile ducts (4,8,13-15). We have shown that in pathological conditions associated with biliary hyperplasia (e.g., following BDL) (4,8) there is enhanced expression of SR and increased secretin-stimulated cAMP levels and bile and bicarbonate secretion (4,6-8,13), parameters which have been suggested to be functional indices of cholangiocyte proliferation (4,6-8,13). In models associated with biliary damage [e.g., after administration of carbon tetrachloride (CCl4) or GABA] (14,15) there is reduced expression of SR and decreased responsiveness to secretin (14,15).
There is growing information regarding the mechanisms of biliary proliferation (17-22). For example, recent studies have shown that cytokines/growth factors such as interleukins (e.g., IL6), serotonin, melatonin, angiogenic factors [e.g., vascular endothelial growth factor (VEGF)-A/C] and nerve growth factor (NGF) regulate the proliferation of bile ducts by autocrine mechanisms (17-22). Serotonin inhibits biliary hyperplasia in cholestatic rats by activation of serotonin receptor 1A and 1B (17). While vascular endothelial growth factor-A/C (VEGF-A/C) and NGF stimulate cholangiocyte hyperplasia by interacting with VEGFR-2/3, melatonin has been shown to inhibit biliary growth by down-regulation of cAMP signaling (17,19,20,22). Both autocrine and paracrine VEGF signaling stimulates the growth of liver cysts in Pkd2KO mice (23). In polycystic liver diseases, VEGF and angiopoietin-1 stimulate the growth of biliary cysts and their vascular supply (24). Also, gastrin inhibits both hyperplastic and neoplastic biliary growth by interaction with CCK-B receptors through translocation of Ca2+-dependent PKC isoforms (8,25,26). We have also shown that gastrin inhibits secretin-stimulated ductal secretion in cholestatic rats (27). Since omeprazole has been shown to increase gastrin levels in rodents and humans (28-30), we performed studies aimed to demonstrate that chronic administration of omeprazole inhibits biliary growth and secretin-stimulated ductal secretion by enhanced release of gastrin serum levels.
Materials and methods
Materials
Reagents were purchased from Sigma Chemical Co. (St. Louis, MO, USA) unless otherwise stated. The antibody against proliferating cell nuclear antigen (PCNA) was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). The mouse anti-cytokeratin-19 (CK-19) antibody was purchased from Caltag Laboratories Inc. (Burlingame, CA, USA). The substrate for γ-glutamyl transpeptidase (γ-GT), N- (γ-L-glutamyl)-4-methoxy-2-naphthylamide, was purchased from Polysciences (Warrington, PA).
Animal models
All animal procedures were performed according to protocols approved by the Scott and White and Texas A&M Health Science Center IACUC. Normal, BDL or bile duct incannulated (BDIN) (immediately after surgery) (4) rats were treated with omeprazole (40 mg/kg BW by daily gavage administration in 500 mmol/L of sodium bicarbonate, pH 9.0 containing 0.75% methylcellulose) (31) or vehicle for 1 week. While BDL rats were used for the collection of serum, liver tissue and cholangiocytes, BDIN were used for the in vivo studies of biliary physiology. In all animals used, we measured liver and body weight and liver to body weight ratio, an index of liver cell growth including cholangiocytes (4).
Freshly isolated cholangiocytes
Virtually pure cholangiocytes (by histochemistry for γ-glutamyl transpeptidase) (32) were isolated by immunoaffinity separation by a monoclonal antibody (a rat IgG2a, a gift from Dr. R. Faris, Brown University, Providence, RI) against an unidentified antigen expressed by all mouse cholangiocytes (2).
Evaluation of serum chemistry, intrahepatic bile duct mass (IBDM) in liver sections and H3 histone mRNA and PCNA protein expression in purified cholangiocytes
The serum levels of the transaminases, glutamate pyruvate transaminases (SGPT) and glutamic oxaloacetic transaminase (SGOT), and total bilirubin were measured by a Dimension RxL Max Integrated Chemistry system (Dade Behring Inc., Deerfield IL) by the Chemistry Department, Scott & White. We determined in paraffin-embedded liver sections (4-5 mm thick) IBDM NGF (2) of cholangiocytes; the IBDM was evaluated as area occupied by CK-19 positive-bile duct/total area ×100. Sections were examined in a coded fashion by BX-51 light microscopy (Olympus, Tokyo, Japan) equipped with a camera.
Gene expression for H3 histone (an index of cell replication) (33,34) was measured by the lysate RNase protection assay (Direct Protect™ kit, Ambion Inc., Austin, TX) using lysate samples from organs (100 mg) or cholangiocytes (5.0×106) as described (33). For each cell sample, we used 45 µL of lysate containing 4.5×105 cells. Antisense riboprobes were transcribed with either T7 or SP6 RNA polymerase using [α-32P] UTP (800 Ci/mmol; Amersham, GE Health Care, Piscataway, NJ) by the Maxiscript kit (Ambion). The 32P-labeled antisense riboprobes were as follows: a 204-bp riboprobe encoding for the H3 histone gene was obtained from Dr. S. Gupta (Albert Einstein Hospital, Bronx, NY); and a 316-bp riboprobe encoding a complementary sequence for rat glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA was purchased from cDNA purchased from Ambion. We used the following controls: rat spleen (positive) and yeast transfer RNA (negative) for the H3 histone genes; and rat kidney and yeast transfer RNA were the positive and negative controls, respectively, for GAPDH the housekeeping gene (34).
We measured cholangiocyte proliferation by immunoblots (8,16) for proliferating cellular nuclear antigen (PCNA) in protein (10 µg) obtained from whole cell lysate from purified cholangiocytes from BDL rats treated with omeprazole or saline for 1 week. Immunoblots were normalized by β-actin (16). The intensity of the bands was determined by scanning video densitometry using the phospho-imager, Storm 860, (GE Health Care) and the ImageQuant TL software version 2003.02 (GE Health Care, Little Chalfont, Buckinghamshire, England).
Measurement of secretin receptor gene expression, and secretin-stimulated cAMP levels and bile and bicarbonate secretion
We next measured SR mRNA expression, and secretin-stimulated cAMP levels and bile and bicarbonate secretion, as functional indices of enhanced biliary proliferation (4,6-8,13,35). The expression of SR mRNA was measured by the lysate RNase protection assay (Direct Protect™ kit, Ambion Inc., Austin, TX) (see above) (33). The 32P-labeled 318 bp riboprobe was generated from the rat SR cDNA clone (a gift of Dr. N. LaRusso, Mayo Clinic, Rochester, MN). We used rat heart (positive) and yeast transfer RNA (negative) for the SR gene.
Basal and secretin-stimulated intracellular cAMP levels were measured in purified cholangiocytes from BDL rats treated with vehicle or omeprazole for 1 week by commercially available RIA kits (Amersham, GE Health Care). Briefly, after isolation cholangiocytes were incubated for 1 hour at 37 °C to restore membrane proteins damaged during the isolation procedure by proteolytic enzymes (8,36,37) and subsequently treated with 0.2% BSA (basal) or secretin (100 nmol/L) (8,37) in the presence of 0.2% BSA for 5 minutes.
The in vivo studies aimed to evaluate the effect of secretin on bile and bicarbonate secretion were performed in BDIN rats, which were prepared for bile collection as described by us (4). When steady-state bile flow was reached (approximately 60 minutes after infusion of Krebs-Henseleit bicarbonate solution, KRH, via a jugular vein), secretin (10-7 M) administered for 30 min (by a jugular vein) followed by a final infusion of KRH for 60 min. Bicarbonate concentration (measured as total CO2) was determined by a Natelson microgasometer apparatus (Scientific Industries, Bohemia, NY).
Measurement of serum levels of gastrin
Since omeprazole has been shown to increase gastrin serum levels in rodents and humans (28-30), we evaluated the levels of this gastrointestinal hormone in serum from the selected groups of animals. The serum levels of gastrin were measured by radioimmunoassay using commercially available kits obtained respectively from Diagnostic Products Corporation (DPC, Los Angeles, CA) according to the instructions supplied by the vendor.
Proliferation assay
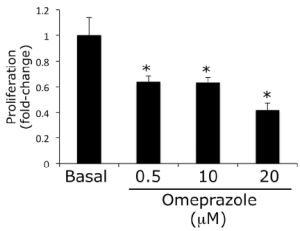
Proliferation assay was performed in our normal rat intrahepatic cholangiocyte cell line (NRIC) following in vitro treatment with omeprazole (0.5, 10 and 20 µM) for 48 h. Cell proliferation was measured as previously described (16,38). NRICs were seeded (7,000 cells/well) onto 96-well plates in complete medium and allowed to adhere overnight at 37 °C. Cells were then serum-starved in medium containing 0.5% FBS for 24 h, washed twice with 1x phosphate buffered saline (PBS). Cells were incubated for 48 h with the respective treatments, at which time 10 µL of Cell Titer 96 (Promega, Madison, WI) was added to each well (16,38). Absorbance at 490 nm was measure with a microplate reader (Microplate Spectrophotometer, Spectra Max 3,400 pc, Molecular Devices, Sunnyvale, CA). Absorbance is directly correlated to the number of viable cells and data is presented as fold-change compared to basal (16,38).
Statistical analysis
Data are expressed as mean ± SEM. Differences between groups were analyzed by the Student’s unpaired t-test when two groups were analyzed, and by ANOVA when more than two groups were analyzed, followed by an appropriate post hoc test.
Results
Evaluation of serum chemistry, IBDM in liver sections and cell proliferation in purified cholangiocytes
There was no significant difference in body weight between normal and BDL treated with omeprazole compared to control rats (Table 1). In agreement with other studies (4), the body weight of BDL rats decreased compared to normal rats (Table 1). As expected (4), the liver to body weight ratio increased in BDL compared to normal rats (Table 1). There was decreased liver to body weight ratio in BDL rats treated with omeprazole compared to BDL rats treated with saline for 1 week (Table 1).

Full Table
In BDL rats, the serum levels of transaminases and bilirubin were significantly higher than those of normal rats (Table 1). There were no significant differences in the serum levels of transaminases and bilirubin in normal and BDL rats treated with omeprazole compared to the corresponding rats treated with saline for 1 week (Table 1).
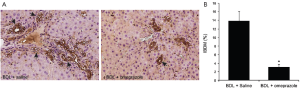
There was no difference in IBDM between normal rats treated with saline or omeprazole for 1 week (data not shown). In agreement with previous studies (4,8), there was increased IBDM in BDL compared to normal rats (data not shown). Prolonged administration of omeprazole to BDL rats decreased IBDM compared to BDL rats treated with saline (Figure 1A,B). In purified cholangiocytes from BDL rats treated with omeprazole there was reduced mRNA H3 histone (Figure 2A,B) and PCNA protein (Figure 2C) expression compared to the corresponding value from BDL rats treated with saline. The levels of GAPDH and β-actin were similar in cholangiocytes from BDL rats treated with saline or omeprazole (Figure 2A,B).

Measurement of secretin receptor gene expression, and secretin-stimulated cAMP levels and bile and bicarbonate secretion
There was reduced SR mRNA expression in purified cholangiocytes from BDL rats treated with omeprazole compared to cholangiocytes from BDL rats treated with saline (Figure 2A). The levels of GAPH were similar in cholangiocytes from the two experimental groups (Figure 2A).
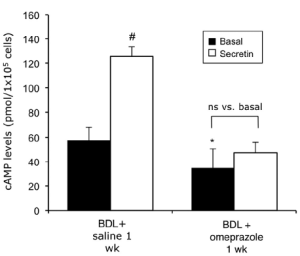
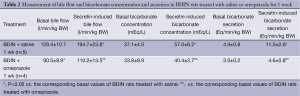
At the functional level, prolonged treatment with omeprazole decreased basal cAMP levels in cholangiocytes of BDL rats compared to cAMP levels in cholangiocytes from BDL rats treated with saline (Figure 3). Furthermore, omeprazole inhibited secretin-stimulated cAMP synthesis that is enhanced by secretin in BDL rats (Figure 3). As expected, in BDIN rats secretin increased bile flow, and both bicarbonate concentration and secretion (Table 2). Prolonged omeprazole treatment blocked the stimulatory effect of secretin on bile flow, and both bicarbonate concentration and secretion (Table 2).
Full Table
Measurement of serum levels of gastrin
To determine if omeprazole effects are mediated by changes in serum gastrin levels, we measured the levels of this gastrointestinal hormone in both normal and BDL rats treated with saline or omeprazole. Consistent with our previous findings (33), we have shown that gastrin serum levels are higher in BDL compared to normal rats (Table 1). Prolonged administration of omeprazole to normal or BDL rats increased gastrin serum levels compared to their corresponding values of normal and BDL rats treated with saline for 1 week (Table 1).
Proliferation assay
To determine if omeprazole has a dose-dependent effect on cholangiocyte proliferation, NRIC were stimulated with omeprazole for 48 h. We found that omeprazole induces a significant dose-dependent reduction in proliferation (Figure 4).
Discussion
The study relates to the in vivo inhibitory effect of omeprazole on biliary proliferation, secretin receptor gene expression and basal and secretin-stimulated cAMP levels and bile and bicarbonate secretion. Specifically, we have treated normal and BDL rats with omeprazole or saline for 1 week and have demonstrated that omeprazole decreases IBDM in liver sections and biliary proliferation in purified cholangiocytes from BDL rats. Also, omeprazole decreased SR gene expression and both basal and secretin-stimulated cAMP levels in purified cholangiocytes from BDL rats, and secretin-induced bile and bicarbonate secretion in BDIN rats. Conflicting data exists regarding the role of omeprazole on liver cell growth. While a study has shown that omeprazole stimulates liver regeneration after partial hepatectomy by increased gastrin serum levels (39), another study has demonstrated an inhibitory effect of omeprazole on hepatic regeneration (40). Also, while a study has demonstrated liver tumor promoting effects of omeprazole in rodents (41), several other studies have provided evidence for anti-carcinogenic properties of omeprazole against the growth of human colon cancer cells both in vivo and in vitro (42-44). The inhibitory effects of omeprazole were mediated by increase serum levels of gastrin that we have previously shown to inhibit both hyperplastic and neoplastic biliary proliferation (8,25,26). The study has strong clinical implications since the diffuse use of proton-pump inhibitors may not only reduce gastric stomach secretion (45,46), but may ameliorate biliary disorders in chronic liver diseases.
A number of studies have increased our knowledge on the mechanisms of biliary hyperplasia in the cholestatic BDL model that is characterized by growth/damage of cholangiocytes (4,6-8,13,14,18-20,26,27,33). Using this model, several studies have shown that a number of gastrointestinal hormones and growth factors regulate the balance between cholangiocyte proliferation/damage that is key for maintaining the homeostasis of the biliary epithelium (4,6-8,13,14,18-20,26,27,33). We used the BDL model of cholestasis since it is characterized by enhanced gastrin serum levels (33); this would allow us to relate the antiproliferative effects of omeprazole on biliary growth on changes of gastrin serum levels. The increase in serum gastrin levels observed in BDL compared to normal rats (33) may be due to a compensatory mechanism aimed to decrease the enhanced biliary mass.
Supporting this view, the inhibitory effect of omeprazole on biliary mass was associated with enhanced gastrin serum levels. Although we did not observe any changes in serum chemistry in BDL rats treated with omeprazole compared to BDL rats treated with vehicle, rare cases of liver damage by proton pump inhibitors (e.g., pantoprazole) and omeprazole have been described in clinical setting (47-49). For example, acute liver failure (massive centrolobular necrosis without fibrosis) attributed to omeprazole was observed in a 62-year old man with gastroesophageal reflux (48). Controlled trial of two doses of omeprazole in 32 patients with duodenal ulcer described higher SGPT serum levels within the first 1-2 weeks, resolving rapidly on withdrawal of the drug (47). One female patient receiving pantoprazole during a corticosteroid therapy for encephalomyelitis disseminate developed sudden idiosyncratic hepatocellular damage (49). On the contrary, other studies in patients showed no alterations in SGPT serum levels, jaundice or hepatitis after omeprazole administration (50-52).
No information exists regarding the role of omeprazole in the regulation of biliary growth and liver damage. Thus, our studies due to the diffuse use of omeprazole in the clinical setting open the new possibility that proton pump inhibitors may be beneficial during the progression of biliary disorders. Since we demonstrated that omeprazole treatment decrease biliary mass in vivo and cell proliferation in purified cholangiocytes, we performed studies aimed to demonstrate that the inhibitory effects of omeprazole on biliary hyperplasia are due to increased serum levels of gastrin that we have recently demonstrate to inhibit hyperplastic and neoplastic biliary growth (8,25-27) and secretin-stimulated choleresis, a functional marker of biliary proliferation (4,8,13,14,35). At the functional level, the decrease in intrahepatic bile ductal mass was also supported by reduced choleretic response of large bile ducts to secretin (4,8,14,27,35). We also found that omeprazole can inhibit cholangiocyte proliferation in a cholangiocyte cell line, which suggests omeprazole may directly influence biliary proliferation. Recently, omeprazole has been identified as an aryl hydrocarbon receptor agonist, which may mediate its antiproliferative effects in several cancer cell types (53,54). This concept will be addressed in future studies hyperplastic and neoplastic cholangiocytes.
Contrasting information exists regarding the trophic or antiproliferative effects of gastrin on epithelia cell growth. For example, gastrin has been shown to stimulate the proliferation of enterochromaffin-like cells of the stomach and colon epithelial cells, (55,56) but it does not stimulate cell replication of the oxyntic mucosal D cells (57) and rat liver (58). Activation of CCK-B/gastrin receptor inhibits the growth of the pancreatic cell lines, MiaPaca-2 and Panc-1 (59), in contrast to the mitotic stimulatory effects observed in AR4-2J cells (60).
In conclusion, we have demonstrated that the proton pump inhibitor, omeprazole, inhibits biliary hyperplasia and liver damage concomitant with reduced cholangiocyte proliferation and secretin receptor gene expression and decreased choleretic response to secretin. The study raises the novel concept that proton pump inhibitors may also ameliorate biliary hyperplasia and liver damage.
Acknowledgements
This work was supported by an NIH RO1 grant, DK-081442, to Dr. Glaser.
Disclosure: The authors declare no conflict of interest.
References
- Alpini G, Roberts S, Kuntz SM, et al. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology 1996;110:1636-43. [PubMed]
- Glaser SS, Gaudio E, Rao A, et al. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest 2009;89:456-69. [PubMed]
- Nathanson MH, Boyer JL. Mechanisms and regulation of bile secretion. Hepatology 1991;14:551-66. [PubMed]
- Alpini G, Lenzi R, Sarkozi L, et al. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest 1988;81:569-78. [PubMed]
- Alpini G, Glaser S, Robertson W, et al. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol 1997;272:G1064-74. [PubMed]
- Alpini G, Ulrich C, Roberts S, et al. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol 1997;272:G289-97. [PubMed]
- Alpini G, Ulrich CD 2nd, Phillips JO, et al. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. Am J Physiol 1994;266:G922-8. [PubMed]
- Glaser S, Benedetti A, Marucci L, et al. Gastrin inhibits cholangiocyte growth in bile duct-ligated rats by interaction with cholecystokinin-B/Gastrin receptors via D-myo-inositol 1,4,5-triphosphate-, Ca(2+)-, and protein kinase C alpha-dependent mechanisms. Hepatology 2000;32:17-25. [PubMed]
- Kanno N, LeSage G, Glaser S, et al. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol 2001;281:G612-25. [PubMed]
- Banales JM, Arenas F, Rodríguez-Ortigosa CM, et al. Bicarbonate-rich choleresis induced by secretin in normal rat is taurocholate-dependent and involves AE2 anion exchanger. Hepatology 2006;43:266-75. [PubMed]
- Alpini G, Prall RT, LaRusso NF. The pathobiology of biliary epithelia. In: Arias I, Boyer JL, Chrisari F, et al. eds. The Liver: Biology & Pathobiology. 4th ed. Philadephia, PA: Lippincott Williams & Wilkins, 2001:421-35.
- Lazaridis KN, Strazzabosco M, Larusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology 2004;127:1565-77. [PubMed]
- Alpini G, Glaser SS, Ueno Y, et al. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol 1998;274:G767-75. [PubMed]
- LeSage GD, Glaser SS, Marucci L, et al. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol 1999;276:G1289-301. [PubMed]
- Lesage G, Glaser S, Ueno Y, et al. Regression of cholangiocyte proliferation after cessation of ANIT feeding is coupled with increased apoptosis. Am J Physiol Gastrointest Liver Physiol 2001;281:G182-90. [PubMed]
- Mancinelli R, Franchitto A, Gaudio E, et al. After damage of large bile ducts by gamma-aminobutyric acid, small ducts replenish the biliary tree by amplification of calcium-dependent signaling and de novo acquisition of large cholangiocyte phenotypes. Am J Pathol 2010;176:1790-800. [PubMed]
- Marzioni M, Glaser S, Francis H, et al. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology 2005;128:121-37. [PubMed]
- Alvaro D, Mancino MG, Glaser S, et al. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology 2007;132:415-31. [PubMed]
- Gaudio E, Barbaro B, Alvaro D, et al. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology 2006;130:1270-82. [PubMed]
- Gigliozzi A, Alpini G, Baroni GS, et al. Nerve growth factor modulates the proliferative capacity of the intrahepatic biliary epithelium in experimental cholestasis. Gastroenterology 2004;127:1198-209. [PubMed]
- Johnson C, Han Y, Hughart N, et al. Interleukin-6 and its receptor, key players in hepatobiliary inflammation and cancer. Transl Gastrointest Cancer 2012;1:58-70. [PubMed]
- Renzi A, Glaser S, Demorrow S, et al. Melatonin inhibits cholangiocyte hyperplasia in cholestatic rats by interaction with MT1 but not MT2 melatonin receptors. Am J Physiol Gastrointest Liver Physiol 2011;301:G634-43. [PubMed]
- Spirli C, Okolicsanyi S, Fiorotto R, et al. Mammalian target of rapamycin regulates vascular endothelial growth factor-dependent liver cyst growth in polycystin-2-defective mice. Hepatology 2010;51:1778-88. [PubMed]
- Fabris L, Cadamuro M, Fiorotto R, et al. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology 2006;43:1001-12. [PubMed]
- Kanno N, Glaser S, Chowdhury U, et al. Gastrin inhibits cholangiocarcinoma growth through increased apoptosis by activation of Ca2+-dependent protein kinase C-alpha. J Hepatol 2001;34:284-91. [PubMed]
- Glaser S, Alvaro D, Ueno Y, et al. Gastrin reverses established cholangiocyte proliferation and enhanced secretin-stimulated ductal secretion of BDL rats by activation of apoptosis through increased expression of Ca2+- dependent PKC isoforms. Liver Int 2003;23:78-88. [PubMed]
- Glaser SS, Rodgers RE, Phinizy JL, et al. Gastrin inhibits secretin-induced ductal secretion by interaction with specific receptors on rat cholangiocytes. Am J Physiol 1997;273:G1061-70. [PubMed]
- Koop H, Klein M, Arnold R. Serum gastrin levels during long-term omeprazole treatment. Aliment Pharmacol Ther 1990;4:131-8. [PubMed]
- Takeuchi Y, Kitano S, Bandoh T, et al. Acceleration of gastric ulcer healing by omeprazole in portal hypertensive rats. Is its action mediated by gastrin release and the stimulation of epithelial proliferation? Eur Surg Res 2003;35:75-80. [PubMed]
- Klingensmith ME, Neville LJ, Delpire E, et al. Gastrin-mediated effects of omeprazole on rat colon mucosa. Surgery 1999;126:272-8. [PubMed]
- Kashfi K, McDougall CJ, Dannenberg AJ. Comparative effects of omeprazole on xenobiotic metabolizing enzymes in the rat and human. Clin Pharmacol Ther 1995;58:625-30. [PubMed]
- Rutenburg AM, Kim H, Fischbein JW, et al. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem 1969;17:517-26. [PubMed]
- LeSagE G, Alvaro D, Benedetti A, et al. Cholinergic system modulates growth, apoptosis, and secretion of cholangiocytes from bile duct-ligated rats. Gastroenterology 1999;117:191-9. [PubMed]
- Alpini G, Glaser S, Robertson W, et al. Bile acids stimulate proliferative and secretory events in large but not small cholangiocytes. Am J Physiol 1997;273:G518-29. [PubMed]
- Lesage G, Glaser SS, Gubba S, et al. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal secretion. Gastroenterology 1996;111:1633-44. [PubMed]
- Kato A, Gores GJ, LaRusso NF. Secretin stimulates exocytosis in isolated bile duct epithelial cells by a cyclic AMP-mediated mechanism. J Biol Chem 1992;267:15523-9. [PubMed]
- Francis H, Glaser S, Ueno Y, et al. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol 2004;41:528-37. [PubMed]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55-63. [PubMed]
- Ohtake M, Aono T, Sakaguchi T, et al. Liver regeneration is enhanced by omeprazole in rats following partial hepatectomy. Br J Surg 1994;81:1179-80. [PubMed]
- Kucuk HF, Akyol H, Kaptanoglu L, et al. Effect of proton pump inhibitors on hepatic regeneration. Eur Surg Res 2006;38:322-8. [PubMed]
- Hayashi H, Shimamoto K, Taniai E, et al. Liver tumor promoting effect of omeprazole in rats and its possible mechanism of action. J Toxicol Sci 2012;37:491-501. [PubMed]
- Penman ID, el-Omar E, McGregor JR, et al. Omeprazole inhibits colorectal carcinogenesis induced by azoxymethane in rats. Gut 1993;34:1559-65. [PubMed]
- Tobi M, Chintalapani S, Goo R, et al. Omeprazole inhibits growth of cancer cell line of colonic origin. Dig Dis Sci 1995;40:1526-30. [PubMed]
- Patlolla JM, Zhang Y, Li Q, et al. Anti-carcinogenic properties of omeprazole against human colon cancer cells and azoxymethane-induced colonic aberrant crypt foci formation in rats. Int J Oncol 2012;40:170-5. [PubMed]
- Kittang E, Aadland E, Schjønsby H. Effect of omeprazole on the secretion of intrinsic factor, gastric acid and pepsin in man. Gut 1985;26:594-8. [PubMed]
- Olbe L, Cederberg C, Lind T, et al. Effect of omeprazole on gastric acid secretion and plasma gastrin in man. Scand J Gastroenterol Suppl 1989;166:27-32; discussion 41-2. [PubMed]
- Gustavsson S, Adami HO, Lööf L, et al. Rapid healing of duodenal ulcers with omeprazole: double-blind dose-comparative trial. Lancet 1983;2:124-5. [PubMed]
- Jochem V, Kirkpatrick R, Greenson J, et al. Fulminant hepatic failure related to omeprazole. Am J Gastroenterol 1992;87:523-5. [PubMed]
- Sandig C, Flechtenmacher C, Stremmel W, et al. Pantoprazole induces severe acute hepatitis. Z Gastroenterol 2011;49:207-10. [PubMed]
- Lööf L, Adami HO, Gustavsson S, et al. Omeprazole: no evidence for frequent hepatic reactions. Lancet 1984;1:1347-8. [PubMed]
- Joelson S, Joelson IB, Lundborg P, et al. Safety experience from long-term treatment with omeprazole. Digestion 1992;51 Suppl 1:93-101. [PubMed]
- Klinkenberg-Knol EC, Festen HP, Jansen JB, et al. Long-term treatment with omeprazole for refractory reflux esophagitis: efficacy and safety. Ann Intern Med 1994;121:161-7. [PubMed]
- Jin UH, Lee SO, Safe S. Aryl hydrocarbon receptor (AHR)-active pharmaceuticals are selective AHR modulators in MDA-MB-468 and BT474 breast cancer cells. J Pharmacol Exp Ther 2012;343:333-41. [PubMed]
- Ishiguro T, Ishiguro R, Ishiguro M, et al. Co-treatment of dichloroacetate, omeprazole and tamoxifen exhibited synergistically antiproliferative effect on malignant tumors: in vivo experiments and a case report. Hepatogastroenterology 2012;59:994-6. [PubMed]
- Yassin RR, Clearfield HR, Little KM. Gastrin’s trophic effect in the colon: identification of a signaling pathway mediated by protein kinase C. Peptides 1993;14:1119-24. [PubMed]
- Quintero E, Ohning GV, Del Rivero M, et al. Gastrin mediates the increase in gastric cell growth in uremic rats. Am J Physiol 1995;268:G586-91. [PubMed]
- Waldum HL, Brenna E, Kleveland PM, et al. Gastrin-physiological and pathophysiological role: clinical consequences. Dig Dis 1995;13:25-38. [PubMed]
- Chen D, Ding XQ, Rehfeld JF, et al. Endogenous gastrin and cholecystokinin do not promote growth of rat liver. Scand J Gastroenterol 1994;29:688-92. [PubMed]
- Detjen K, Fenrich MC, Logsdon CD. Transfected cholecystokinin receptors mediate growth inhibitory effects on human pancreatic cancer cell lines. Gastroenterology 1997;112:952-9. [PubMed]
- Seva C, Dickinson CJ, Yamada T. Growth-promoting effects of glycine-extended progastrin. Science 1994;265:410-2. [PubMed]