Glucose intolerance and the risk of pancreatic cancer
Introduction
Mortality due to pancreatic cancer is increasing globally in most industrialized countries including Japan, with an estimated 227,000 deaths per year worldwide (1). According to the national statistics of 2011 in Japan, there were 28,829 deaths due to pancreatic cancer, ranking it fifth after lung cancer, gastric cancer, colorectal cancer, and liver cancer (2-4). The prognosis of pancreatic cancer is still extremely poor despite advances in various diagnostic imaging techniques and medical treatment, with a 5-year survival rate of less than 10% (5,6). Early-stage pancreatic cancer is usually clinically silent, and the disease only becomes apparent after the tumor invades into surrounding tissues or metastasizes to distant organs. Early detection of pancreatic cancer is required for improving the outcome, and recognition of high-risk patients is a major issue.
Risk factors for pancreatic cancer
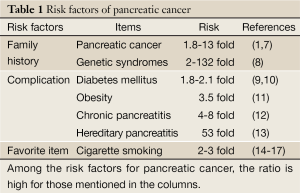
Certain factors can increase the risk of acquiring the genetic mutations that may potentially result in pancreatic cancer (Table 1). Risk factors for this malignant disease include cigarette smoking (14-17), family history (7,8,18-21), advancing age, male sex, diabetes mellitus, chronic pancreatitis (12), hereditary pancreatitis (13), obesity (11,22-27), non-O blood group (28,29), a high-fat diet, diets high in meat and low in vegetables, and folate deficiency (1).
Full Table
Cigarette smoking is one of the biggest risk factors for the development of pancreatic cancer. Heavy smokers have a 2-3-fold increased risk of death due to pancreatic cancer compared with non-smokers.
A family history of pancreatic cancer is also an important risk factor (7,8,18-21); about 3-9% of pancreatic cancer patients have such a family history. Ghadirian et al., found that 7.8% of all patients with pancreatic cancer and only 0.6% of controls had a family history of pancreatic cancer, i.e., a 13-fold difference, with no differences in environmental risk factors between the two groups (18). In a meta-analysis of familial risks in pancreatic cancer, Permuth-Wey et al. concluded that results from case-control [RR =2.82; 95% confidence interval (CI): 1.99-3.66] and cohort (RR =1.62; 95% CI: 1.28-1.97) studies showed a significant increase in pancreatic cancer risk if a relative had been affected, with an overall summary RR =1.80 (95% CI: 1.48-2.12) (8). Familial pancreatic cancer has been defined in most studies as the presence of pancreatic tumors in a pair of first-degree relatives. Prospective analysis of families with this malignant disease shows that first-degree relatives of individuals with familial pancreatic cancer have a 9-fold increased risk of this neoplasm over the general population (18). This risk rises to 32-fold in kindred with three or more first-degree relatives with pancreatic cancer.
Diabetes is a very important risk factor for disease, as described in detail later.
Obesity and being overweight increase the risk of pancreatic cancer significantly. According to a large-scale cohort study performed in Japan (11), men with a BMI of 30 kg/m2 or more at age 20 years had a 3.5-fold higher risk than men with a normal BMI. Women with a BMI of 27.5-29.9 at the baseline had a ~60% increased risk compared with women with a BMI of 20.0-22.4. In men, weight loss of 5 kg or more between 20 years of age and the baseline age was associated with an increased risk of pancreatic cancer death.
On the other hand, no correlation has been observed between pancreatic cancer and BMI in two other cohort studies (22,23). One report has indicated that the estimated summary RR of pancreatic cancer per 5 kg/m2 increase in BMI was 1.12 (95% CI: 1.06-1.17) in men and women combined (24). Compared with those with a BMI of 18.5-<25, individuals with a BMI of ≥35 had a 45% greater pancreatic cancer risk (95% CI: 1.04-2.02) (25). Being overweight or obese during early adulthood is associated with a greater risk of pancreatic cancer and a younger age at disease onset (26).
Complex relationship between diabetes and pancreatic cancer
Glucose intolerance is a pre-diabetic state of hyperglycemia associated with insulin resistance and increased risk of future diabetes and adverse outcomes. According to the criteria of the World Health Organization and the American Diabetes Association, glucose intolerance is defined as a two-hour glucose level of 140-199 mg/dL in the 75-gram oral glucose tolerance test.
Diabetes is a chronic disease that occurs either when the pancreas does not produce enough insulin or when the body cannot effectively use the insulin it produces. Insulin is a hormone that regulates blood sugar levels. Hyperglycemia, or raised blood sugar, is a common effect of uncontrolled diabetes, and this leads to serious damage to many body’s systems over time, especially the nerves and blood vessels. The classification of glucose metabolism disorders is principally derived from etiology, and includes staging of pathophysiology based on the degree of deficiency of insulin action. These disorders are classified into four groups: (I) type 1 diabetes mellitus; (II) type 2 diabetes mellitus; (III) diabetes mellitus due to other specific mechanisms or diseases; and (IV) gestational diabetes mellitus. Type 1 diabetes is characterized by destruction of pancreatic β-cells. Type 2 diabetes is characterized by combinations of decreased insulin secretion and decreased insulin sensitivity (insulin resistance) (30).
In medical practice, many cases of pancreatic cancer are diagnosed as a result of worsening glycemic control. Diabetes has the highest incidence among diseases that are complicated by pancreatic cancer, with a rate as high as 25.9% according to the pancreatic cancer registry report of 2007 (Committee for Pancreatic Cancer Registry, Japan Pancreas Society) (31). There have been many arguments regarding whether or not diabetes is the cause or result of pancreatic cancer (9,10,32-39); however, details of the molecular biologic mechanism itself have not yet been clarified. Understanding the effect of the pathophysiology of diabetes on the pancreatic duct epithelium is believed to be very important for achieving early detection of pancreatic cancer.
In 1994, the Italian Pancreatic Cancer Study Group published a case control study of 720 patients with pancreatic cancer. This study concluded that the increased prevalence of diabetes mellitus in these patients was likely related to the diabetes caused by the tumor (34).
Mizuno et al. reported a retrospective study of 540 pancreatic cancer patients that showed that the prevalence of diabetes in different stages of pancreatic cancer was 45%, of which more than half were less than 2 years in duration (35). Their data showed that even though the prognosis of pancreatic cancer patients complicated by diabetes was the same as that of patients without diabetes, outcome and survival were better if they were diagnosed in association with diabetes alone (median survival time: 20.2 months), compared to patients diagnosed on the basis of symptoms such as pain, jaundice, and/or appetite loss (10.2 months, P<0.01).
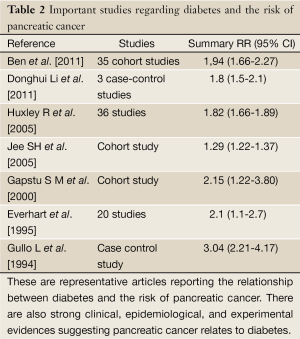
There is also strong clinical, epidemiological, and experimental evidence that pancreatic cancer causes diabetes (Table 2). Hyperglycemia and diabetes mellitus occur in approximately 85% of patients with pancreatic cancer, diabetes being present in 45-67% of patients with pancreatic cancer, depending on how the presence of diabetes is ascertained. The majority (approximately 75%) of diabetes in patients with pancreatic cancer is new-onset (i.e., less than 3 years in duration). New-onset diabetes often resolves when the cancer is resected (36).
Full Table
Huxley et al. performed a meta-analysis of 9,220 cases of pancreatic cancer in 36 reports published between 1966 and 2005 (19 cohort studies and 17 case-control studies). They reported that the relative risk of developing pancreatic cancer in diabetes patients was 1.82 (95% CI: 1.66-1.89) (9). Everhart et al., performed a meta-analysis of 20 reports published between 1975 and 1994 (9 cohort studies and 11 case-control studies) of patients suffering from diabetes from one year or more prior to diagnosis of pancreatic cancer in which the relative risk of pancreatic cancer was appropriately calculated. They reported that the relative risk of pancreatic cancer in diabetes patients was 2.1 (95% CI: 1.1-2.7) (10). Moreover, Gapstur et al. extracted 35,640 men and women (average age: 40 years) and performed a 25-year prospective study of the relationship between the blood glucose level at one hour in the 50-g oral glucose tolerance test (OGTT) and the onset of pancreatic cancer. They reported that the relative risk of pancreatic cancer was 1.65 (95% CI: 1.05-2.60) in the group with a mild blood glucose increase of 120-159 mg/dL, 1.60 (95% CI: 0.95-2.70) in the group with a glucose level of 160-199 mg/dL, and 2.15 (95% CI: 1.22-3.80) in the group with a glucose level of 200 mg/dL or more compared with control cases in which the blood glucose level at one hour was 119 mg/dL or less. There was a significant relationship between the increase in blood glucose and the onset of pancreatic cancer (37).
The complex relationship between the two diseases has been the subject of numerous clinical, epidemiological, and experimental studies. Epidemiologic studies have suggested that long-standing type 2 diabetes is a modest risk factor for the development of pancreatic cancer. Meta-analysis of multiple cohort and case control studies has shown that the risk of pancreatic cancer in patients who have had diabetes for more than 5 years is 1.5- to 2-fold higher. This is not fully explained by risk factors such as obesity that are shared between the two diseases (38).
Possible mechanism of carcinogenesis in obesity and diabetes
Previous reports have indicated that hyperinsulinemia (40), insulin resistance (41) and insulin-like growth factor (IGF) gene polymorphisms (42) affect the onset of pancreatic cancer.
Insulin analogs and stimulators of insulin secretion used for treatment of diabetes increase the risk of pancreatic cancer, whereas metformin reduces the onset and death rate of pancreatic cancer (43).
It has been reported that a high insulin level promotes the growth of human pancreatic cancer cell lines (44,45), and that hyperglycemia and a high fatty acid level promote the growth of pancreatic cancer cells (46).
Butler et al. classified 45 autopsied samples of pancreas tissue from patients into 4 groups depending on BMI and the presence of type 2 diabetes, then histologically compared and investigated the proliferation of pancreatic duct epithelium using Ki67 immunostaining (47). They found that the Ki67 positivity rate in the pancreatic duct epithelium was significantly (4-fold) higher in the diabetic group with BMI <25 than in the non-diabetic group with BMI <25, while the Ki67 positivity rate was approximately 10-fold higher in the non-diabetic group with BMI >27, and 14.3-fold higher in the diabetic group with BMI >27. These results suggest that the proliferation of pancreatic duct epithelium is accelerated in diabetic and obese patients. Accordingly, it is surmised that hyperglycemia due to diabetes is involved in the accelerated proliferation of pancreatic duct epithelium, and that furthermore, hyperinsulinemia, which is observed in insulin-resistant obese patients, is also involved in the accelerated proliferation of pancreatic duct epithelial cells.
Recent findings from both epidemiologic investigations and experimental systems suggest that metformin, a hypoglycemic agent used in the management of diabetes, may be a potential chemopreventive agent for pancreatic cancer. Two epidemiologic investigations in patients with type II diabetes found that patients taking metformin had a reduced risk of cancer (48,49). These results were significant both before and after adjusting for BMI. Evans et al., reported that metformin use among 11,876 diabetic patients, including 923 cancer cases, was associated with a 21% reduced risk for all types of malignancies, and a dose-response relationship was observed. Currie et al., reported that 2,109 of 62,809 diabetic patients developed cancer. Compared with patients treated with metformin monotherapy, those treated with sulfonylurea and insulin had 1.36- and 1.42-fold higher risks of cancer, respectively.
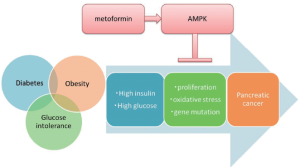
Li et al., compared and investigated the treatment regimen of diabetes and the pancreatic carcinogenesis rate, and found that while insulin analog and insulin secretagogue respectively increased the risk of pancreatic cancer onset in diabetic patients by approximately 4.99- and 2.52-fold, metformin, which is an insulin resistance-improving drug that does not increase the insulin concentration in blood, reduced the risk of pancreatic cancer by 62%, and even when metformin treatment was continued for 5 years or longer (50). Metformin is known to have a direct effect on the activation of AMP-activated protein kinase (AMPK), and mediates cell proliferation and apoptosis via p53 and p27kip1. Furthermore, protein synthesis and cell growth are inhibited due to inhibition of the mammalian target of rapamycin (mTOR) (51). Yang et al., reported that the molecular mechanism involved in cell proliferation via AMPK and mTOR is involved in the carcinogenesis of pancreatic cancer against a background of diabetes (52). As is evident from these reports, it is believed that various molecular mechanisms are involved in the increased cell proliferation due to hyperglycemia and hyperinsulinemia, and that clarifying these mechanisms will lead to the future prevention and treatment of pancreatic cancer.
In addition, one of the possible mechanisms of carcinogenesis resulting from obesity and diabetes is oxidative stress. In a study of the mechanism of oxidative stress in diabetics, Giardino et al. cultured vascular endothelial cells in the presence of a high sugar concentration and found that reactive oxygen species (ROS) did not increase in the culture medium, whereas in the cells oxidative stress increased due to diabetes, rather than an increase in ROS (53). Moreover, Nishikawa et al. investigated the involvement of the mitochondrial electron transport system as a source of intracellular ROS production in diabetes, and found that the generation of mitochondria-mediated ROS played a major role in the expression of intracellular metabolic disorder due to high glucose (54). Furthermore, it has been reported that the ROS generated in this manner damage the genomic DNA involved in cell proliferation in various ways, and may be involved in carcinogenesis (55). It is believed that hyperglycemia damages the DNA of pancreatic duct epithelia through oxidative stress, leading to the onset of pancreatic cancer. In this way, it is considered that hyperglycemia and hyperinsulinemia due to diabetes, obesity and glucose intolerance are involved in accelerated cell proliferation in the pancreatic duct epithelium cell. Various molecular mechanisms are involved in carcinogenesis due to hyperglycemia and hyperinsulinemia, and clarification of these mechanisms will lead to methods for prevention and treatment of pancreatic cancer (Figure 1).
Conclusions
In the future, for early detection and treatment of pancreatic cancer, we believe that it is critical to share consensus with diabetologists, and perform adequate screening for pancreatic cancer in patients with glucose intolerance. As described above, it is important to consider the relationship of diabetes with pancreatic cancer, and to bear in mind that diabetes is an important factor for early detection of pancreatic cancer. However, pancreatic cancer screening for all diabetes patients is inefficient, because diabetic morbidity is very high. Therefore the determination of specific risk factors and an appropriate time point for screening in diabetes patients is required.
Acknowledgements
We wish to take this opportunity to thank the many people who have made it possible for me to complete this review article.
Disclosure: The authors declare no conflict of interest.
References
- Raimondi S, Maisonneuve P, Lowenfels AB. Epidemiology of pancreatic cancer: an overview. Nat Rev Gastroenterol Hepatol 2009;6:699-708. [PubMed]
- Ministry of Health, Labour and Welfare. Vital Statistics in Japan, 2010:1970-2010.
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225-49. [PubMed]
- Bouvier AM, David M, Jooste V, et al. Rising incidence of pancreatic cancer in France. Pancreas 2010;39:1243-6. [PubMed]
- Matsuda T, Ajiki W, Marugame T, et al. Population-based survival of cancer patients diagnosed between 1993 and 1999 in Japan: a chronological and international comparative study. Jpn J Clin Oncol 2011;41:40-51. [PubMed]
- Center for Cancer Control and Information Services, National Cancer Center, 2011: Monitoring of Cancer Incidence in Japan - Survival 2000-2002 Report.
- Petersen GM, de Andrade M, Goggins M, et al. Pancreatic cancer genetic epidemiology consortium. Cancer Epidemiol Biomarkers Prev 2006;15:704-10. [PubMed]
- Permuth-Wey J, Egan KM. Family history is a significant risk factor for pancreatic cancer: results from a systematic review and meta-analysis. Fam Cancer 2009;8:109-17. [PubMed]
- Huxley R, Ansary-Moghaddam A, Berrington de González A, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer 2005;92:2076-83. [PubMed]
- Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer. A meta-analysis. JAMA 1995;273:1605-9. [PubMed]
- Lin Y, Kikuchi S, Tamakoshi A, Yagyu K, et al. Obesity, physical activity and the risk of pancreatic cancer in a large Japanese cohort. Int J Cancer 2007;120:2665-71. [PubMed]
- Raimondi S, Lowenfels AB, Morselli-Labate AM, et al. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol 2010;24:349-58. [PubMed]
- Whitcomb DC, Applebaum S, Martin SP. Hereditary pancreatitis and pancreatic carcinoma. Ann N Y Acad Sci 1999;880:201-9. [PubMed]
- Larsson SC, Permert J, Håkansson N, et al. Overall obesity, abdominal adiposity, diabetes and cigarette smoking in relation to the risk of pancreatic cancer in two Swedish population-based cohorts. Br J Cancer 2005;93:1310-5. [PubMed]
- Qiu D, Kurosawa M, Lin Y, et al. Overview of the epidemiology of pancreatic cancer focusing on the JACC Study. J Epidemiol 2005;15 Suppl 2:S157-67. [PubMed]
- Gallicchio L, Kouzis A, Genkinger JM, et al. Active cigarette smoking, household passive smoke exposure, and the risk of developing pancreatic cancer. Prev Med 2006;42:200-5. [PubMed]
- Iodice S, Gandini S, Maisonneuve P, et al. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbecks Arch Surg 2008;393:535-45. [PubMed]
- Ghadirian P, Boyle P, Simard A, et al. Reported family aggregation of pancreatic cancer within a population-based case-control study in the Francophone community in Montreal, Canada. Int J Pancreatol 1991;10:183-96. [PubMed]
- Klein AP, Brune KA, Petersen GM, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer Res 2004;64:2634-8. [PubMed]
- Shi C, Hruban RH, Klein AP. Familial pancreatic cancer. Arch Pathol Lab Med 2009;133:365-74. [PubMed]
- Brune KA, Lau B, Palmisano E, et al. Importance of age of onset in pancreatic cancer kindreds. J Natl Cancer Inst 2010;102:119-26. [PubMed]
- Luo J, Iwasaki M, Inoue M, et al. Body mass index, physical activity and the risk of pancreatic cancer in relation to smoking status and history of diabetes: a large-scale population-based cohort study in Japan--the JPHC study. Cancer Causes Control 2007;18:603-12. [PubMed]
- Nakamura K, Nagata C, Wada K, et al. Cigarette smoking and other lifestyle factors in relation to the risk of pancreatic cancer death: a prospective cohort study in Japan. Jpn J Clin Oncol 2011;41:225-31. [PubMed]
- Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: A meta-analysis of prospective studies. Int J Cancer 2007;120:1993-8. [PubMed]
- Stolzenberg-Solomon RZ, Adams K, Leitzmann M, et al. Adiposity, physical activity, and pancreatic cancer in the National Institutes of Health-AARP Diet and Health Cohort. Am J Epidemiol 2008;167:586-97. [PubMed]
- Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA 2009;301:2553-62. [PubMed]
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625-38. [PubMed]
- Amundadottir L, Kraft P, Stolzenberg-Solomon RZ, et al. Genome-wide association study identifies variants in the ABO locus associated with susceptibility to pancreatic cancer. Nat Genet 2009;41:986-90. [PubMed]
- Wolpin BM, Chan AT, Hartge P, et al. ABO blood group and the risk of pancreatic cancer. J Natl Cancer Inst 2009;101:424-31. [PubMed]
- Kuzuya T, Nakagawa S, Satoh J, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract 2002;55:65-85. [PubMed]
- Egawa S, Toma H, Ohigashi H, et al. Japan Pancreatic Cancer Registry; 30th year anniversary: Japan Pancreas Society. Pancreas 2012;41:985-92. [PubMed]
- Magruder JT, Elahi D, Andersen DK. Diabetes and pancreatic cancer: chicken or egg? Pancreas 2011;40:339-51. [PubMed]
- Chari ST, Leibson CL, Rabe KG, et al. Pancreatic cancer-associated diabetes mellitus: prevalence and temporal association with diagnosis of cancer. Gastroenterology 2008;134:95-101. [PubMed]
- Gullo L, Pezzilli R, Morselli-Labate AM, et al. Diabetes and the risk of pancreatic cancer. N Engl J Med 1994;331:81-4. [PubMed]
- Mizuno S, Nakai Y, Isayama H, et al. Diabetes is a useful diagnostic clue to improve the prognosis of pancreatic cancer. Pancreatology 2013;13:285-9. [PubMed]
- Pannala R, Basu A, Petersen GM, et al. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol 2009;10:88-95. [PubMed]
- Gapstur SM, Gann PH, Lowe W, et al. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA 2000;283:2552-8. [PubMed]
- Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: A meta-analysis of cohort studies. Eur J Cancer 2011;47:1928-37. [PubMed]
- Li D, Tang H, Hassan MM, et al. Diabetes and risk of pancreatic cancer: a pooled analysis of three large case-control studies. Cancer Causes Control 2011;22:189-97. [PubMed]
- Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009;9:88. [PubMed]
- Stolzenberg-Solomon RZ, Graubard BI, Chari S, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA 2005;294:2872-8. [PubMed]
- Suzuki H, Li Y, Dong X, Hassan MM, et al. Effect of insulin-like growth factor gene polymorphisms alone or in interaction with diabetes on the risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev 2008;17:3467-73. [PubMed]
- Decensi A, Puntoni M, Goodwin P, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451-61. [PubMed]
- Wang F, Larsson J, Adrian TE, et al. In vitro influences between pancreatic adenocarcinoma cells and pancreatic islets. J Surg Res 1998;79:13-9. [PubMed]
- Ding XZ, Fehsenfeld DM, Murphy LO, et al. Physiological concentrations of insulin augment pancreatic cancer cell proliferation and glucose utilization by activating MAP kinase, PI3 kinase and enhancing GLUT-1 expression. Pancreas 2000;21:310-20. [PubMed]
- Schneider MB, Matsuzaki H, Haorah J, et al. Prevention of pancreatic cancer induction in hamsters by metformin. Gastroenterology 2001;120:1263-70. [PubMed]
- Butler AE, Galasso R, Matveyenko A, et al. Pancreatic duct replication is increased with obesity and type 2 diabetes in humans. Diabetologia 2010;53:21-6. [PubMed]
- Evans JM, Donnelly LA, Emslie-Smith AM, et al. Metformin and reduced risk of cancer in diabetic patients. BMJ 2005;330:1304-5. [PubMed]
- Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia 2009;52:1766-77. [PubMed]
- Li D, Yeung SC, Hassan MM, et al. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology 2009;137:482-8. [PubMed]
- Zakikhani M, Dowling R, Fantus IG, et al. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res 2006;66:10269-73. [PubMed]
- Yang YX. Do diabetes drugs modify the risk of pancreatic cancer? Gastroenterology 2009;137:412-5. [PubMed]
- Giardino I, Edelstein D, Brownlee M. BCL-2 expression or antioxidants prevent hyperglycemia-induced formation of intracellular advanced glycation endproducts in bovine endothelial cells. J Clin Invest 1996;97:1422-8. [PubMed]
- Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000;404:787-90. [PubMed]
- Cowey S, Hardy RW. The metabolic syndrome: A high-risk state for cancer? Am J Pathol 2006;169:1505-22. [PubMed]


