Leptin as a risk factor for the development of colorectal cancer
Introduction
Obesity, defined by a body mass index (BMI) ≥30 kg/m2, is a growing epidemic now affecting developing countries, as well as developed nations. The World Health Organization (WHO) reports that globally, an estimated 10% of all men and 14% of all women are obese. Indeed, the WHO estimates that half a billion people over the age of twenty worldwide are obese (1). Obesity is a major cause of global mortality and morbidity: it is well established that people who are obese are at an increased risk for developing comorbidities such as cardiovascular diseases, type 2 diabetes mellitus, end-stage renal disease, respiratory complications, depression and arthritis (2). An increasing number of studies are now demonstrating a strong association between obesity and cancer incidence (3-5). Indeed, obesity is also reported to increase cancer-associated mortality (6). Obesity has well-documented associations with many cancers such as renal and endometrial cancer (7), and those associations also extend to colon cancer. Apart from a sharing a number of common non-modifiable risk factors, obesity and colon cancer are inextricably linked with not only nutrition and metabolism, but also with a variety of hormones associated with excess fat.
Over the past decade, the adipose tissue has gained importance as not only a tissue for energy storage, but also as an endocrine organ (8). Several hormones and cytokines called adipokines are synthesized and released by the adipose tissue. Leptin is the most abundant adipokine, with key roles in controlling hunger and satiety thus regulating food intake, energy balance and body weight (9). Leptin also plays important roles in lipid and glucose metabolism, the gonadal, adrenal, somatotropic and thyroid axes, sympathetic tone, biomarkers of cardiovascular disease, immunity, and brain structure and function (10).
Leptin has been implicated in the pathogenesis of several types of obesity-related cancers, including colon cancer (11). This effect can be explained by leptin’s effect on the regulation of specific intracellular pathways that control cell growth, differentiation, apoptosis and angiogenesis, involved in the pathogenesis of cancer (12). Furthermore, leptin is a crucial inflammatory mediator, owing to its homology with well-characterized cytokines and its ability to stimulate the secretion of other inflammatory factors (13), which also are contributors to colon cancer pathogenesis (12). It is therefore hypothesized that leptin, through its action on the regulation of body weight, specific intracellular pathways, and inflammation, influences the pathogenesis and progression of colon cancer. Whilst maintaining a focus on recent publications, this review will examine the links between obesity and colon cancer, and between leptin and colon cancer, focusing on the molecular mechanisms by which leptin is thought to contribute to cancer etiology. It is hoped that better understanding of the functions of leptin and its involvement in colon cancer pathogenesis will help to unravel novel biomarkers to improve current screening programs, and new potential therapeutic drug targets to prevent or treat the condition.
Obesity-related colon cancer
Colon or colorectal cancer (CRC) is an obesity-related cancer that affects more than 1 million people worldwide. It is associated with a mortality rate of 33% in developed countries, and a 5-year survival rate of less than 60% in most European countries (14). Colon cancer is associated with many risk factors including increasing age, male sex, genetic predisposition, previous colonic polyps or previous incidence of CRC, diabetes mellitus, and inflammatory bowel disease; and with environmental risk factors such as sedentary behavior, consumption of processed red meats, inadequate intake of fiber, tobacco smoking, and heavy alcohol consumption (15-17).
It is widely accepted that obesity supports many adverse hormonal, metabolic and immunological alterations in the body. These alterations, in turn, result in an increased risk for the development and progression of colon cancer. Several studies demonstrate an association between obesity and colon cancer (18-22). In a recent report synthesizing a number of meta-analysis examining obesity and colon cancer risk, Bardou et al. reported that all studies found that obesity was associated with an increased risk of colon cancer in both males and females. The results of the study are summarized in Table 1 (23). These data comprehensively suggest an association between obesity and colon cancer risk.
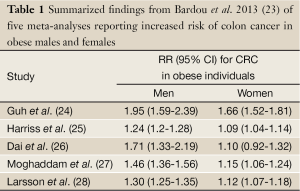
Full Table
The molecular mechanisms by which obesity influences colon cancer development are not completely understood, though several promising streams of investigation are emerging. Obesity is often associated with increased expression of the enzyme fatty acid synthase (FASN), a key regulator of lipogenesis (29) that is upregulated in CRC. In a cohort study comprising 647 CRC patients, the overexpression of FASN in those with a BMI >27 kg/m2 was associated with a poorer outcome (30). Therefore, it has been suggested that FASN plays a role in the pathogenesis of CRC, by maintaining membrane integrity in the endoplasmic reticulum of tumor cells (31).
Obesity is associated with increases in serum concentrations of insulin-like growth factor-1 (IGF-1) (32), which mediates the effects of growth hormone and is a potent inhibitor of apoptosis. In this way, studies have shown that IGF-1 can support tumor cell growth and metastasis, and the prevention of apoptosis (33). It has been shown that IGF-1 levels, as well as its bioavailability (regulated by its binding proteins), are directly associated with CRC risk, by disrupting growth factor regulation and leading to uncontrolled cell proliferation (34). Also, polymorphisms in the IGF-1 gene can regulate the risk for CRC development: in a case-control study of Singaporean-Chinese individuals (as a measure into the effects of the “Western lifestyle”), Wong and colleagues examined polymorphisms in the IGF-1 gene promoter region that affect its viability (35). Of the 298 cases of CRC, a single nucleotide polymorphism in the IGF-1 promoter region “IGF1-2995 C/A” was associated with a 40% decrease in colon cancer risk (35). This suggests that regulation of IGF-1 may be an important mechanism by which colon cancer development is restricted in some cases. Interestingly, the decrease in risk was accentuated in those patients who were physically active. Indeed, in mice on a calorie-restricted diet, decreased systemic IGF-1 resulted in an improved outcome, attributed to the regulation of nuclear factor-κβ (NFκβ) and modulation of inflammatory genes (36).
Estrogen levels are often increased in obese postmenopausal women (37), and can also play a role in the pathogenesis of obesity-related colon cancer, depending on the estrogen receptor (ER) that is predominant in the tissue. In normal colon cells, ER-β is the receptor that is most predominantly expressed, and its activation is protective against colon cancer through the induction of apoptosis. However, in malignant colonic cells, ER-α is the most abundant receptor, and its activation by estrogen promotes cell growth (38). Therefore, increased estrogen in obesity may have protective effect via ER-β activation, whilst activation of ER-α in the later stage of colon cancer may promote cancer development.
An important part of the obesity-associated milieu is the proinflammatory cytokine tumor necrosis factor-alpha (TNF-α), which is overexpressed in obese human and animal adipose tissues (39). In murine models of diet-induced obesity and genetic obesity, Flores and colleagues found that obesity-related colonic inflammation, witnessed through TNF-α overexpression, increased expression and activity of c-jun N terminal kinase (JNK) and inhibitor of nuclear factor κβ kinase (IKK) pathways (40). Stimulation of these pathways resulted in impairment of insulin signaling, and TNF-α neutralization reversed obesity-induced tumor growth. This finding is in accordance with previous epidemiological studies which have demonstrated that hyperinsulinemia and insulin resistance are a potentially crucial mechanism by which obesity increases the risk of colon cancer development, through the activation of the PI3K/Akt pathway (41,42).
The European Prospective Investigation into Cancer and Nutrition (EPIC) yielded many associations between obesity and increased risk of colon cancer development. Obesity is linked to a high-fat diet and alterations in the circulating lipid profile, with decreases in concentrations of high-density lipoproteins (HDL) and increases of low-density lipoproteins (LDL) and triglycerides. As elevated levels of HDL-cholesterol have been associated with a reduced risk of colon cancer, its decrease could potentially predispose to the development of CRC (43).
Obesity is also associated with increased blood glucose and glycated hemoglobin (HbA1c), which is a marker of circulating glucose concentrations. EPIC investigators reported a statistically significant association between high HbA1c and increased colon cancer risk. This suggests that alterations in glucose/insulin homeostasis, most likely due to hyperinsulinemia and insulin resistance, may be an important risk factor for the development of obesity-related cancer (44). The EPIC and other studies also found many associations between other inflammatory factors and cytokines such as IL-6 and IL-17, adipokines (including leptin), IGFs and increased risk of colon cancer (45,46). Moreover, in obesity, circulating levels of the adipokine adiponectin are often decreased, which has also been associated with an increased risk for colon cancer by activating the PI3K/Akt pathway (47). Table 2 summarizes studies associating obesity-related alterations and colon cancer.
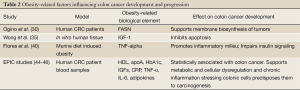
Full Table
Molecular biology of leptin
Leptin is a 16 kDa protein synthesized mainly by the adipose tissue. It is encoded by the ob gene, and shares structural homology with the cytokines IL-6 (an important inflammatory mediator), IL-11, IL-12 and IL-2, indicating its inflammatory roles (13). Leptin regulates feeding behavior by binding to its receptor (Ob-R), expressed in many areas within the central nervous system, mainly in the acuate nucleus of the hypothalamus. Ob-R is a tyrosine kinase-associated receptor that signals through JAK and STAT, and is expressed as at least four different isoforms in humans: Ob-Ra, Ob-Rb, and Ob-Rc (membrane-anchored), and Ob-Re (soluble) (9). In the hypothalamus, the activation of Ob-Rb stimulates the expression of the anorexigenic neurotransmitters pro-opiomelanocortin (POMC) and cocaine- and amphetamine-related transcript (CART). Also, leptin inhibits the orexigenic neurons that express neuropeptide Y (NPY) and Agouti-related peptide (AgRP). Therefore, through its actions on anorexigenic and orexigenic neurons, leptin stimulates satiety and inhibits hunger.
Peripherally, Ob-R and its isoforms are widely expressed in most tissues that have been tested, including the colon (48). Through its central and peripheral actions, leptin is thought to have proinflammatory activities, evidenced by its ability to increase production of TNF and IL-6 in monocytes and to stimulate the production of various CC-motif chemokines (49). The proinflammatory state that is seen in obese individuals can be, at least in part, explained by the high levels of leptin that are seen in those individuals, who do not benefit from the anorexigenic effects of leptin due to central leptin resistance (50). Besides regulating energy balance and having proinflammatory effects, leptin also regulates endocrine systems such as the thyrotropic, gonadotropic and corticotropic axes, and affects glucose homeostasis, hematopoiesis, angiogenesis, osteogenesis, and wound healing (10).
Inflammatory factors increasing leptin
Since there appears to be a connection between leptin and CRC, it is relevant to summarize the factors that contribute to hyperleptinemia. Circulating leptin levels correlate well with body fat, and high levels of circulating leptin is one of the consequences of being obese. Therefore, it is crucial to consider the possible role of leptin in comorbidities related to obesity. As obesity is characterized as a chronic low-inflammatory grade disorder (51,52), contributing factors maintaining and/or enhancing obesity-related inflammation including elevation of circulating leptin levels should be considered. Indeed, it is well known that inflammatory challenges increase leptin synthesis and release (53,54), and that chronic inflammatory conditions promote cancer development (55,56).
High levels of circulating leptin could be deleterious, as leptin has proinflammatory actions. Leptin and its long-isoform functional receptor (Ob-Rb) share tridimensional and sequence homologies respectively, with cytokines of the IL-6 family and gp130, the signal transducing component of the IL-6-type receptor (57,58). Presumably, high circulating leptin levels found in obese individuals could contribute to the low-grade inflammation that characterizes obesity. In fact, circulating leptin levels display a circadian rhythm in parallel to that of NO3/NO2 [measured as an index nitric oxide (NO) synthesis, a powerful oxidant agent] (59). In previous in vitro and in vivo studies, we showed that leptin increased not only NO3/NO2, but also TNF-α, a prototypical proinflammatory cytokine (59). In clinical studies, others have shown that circulating leptin also correlates with proinflammatory factors such as IL-6, a cytokine that has been largely correlated with metabolic syndrome (60). Thus, leptin could play a crucial role bridging the gaps among obesity, inflammation and presumably cancer.
Other two contributing factors to the low-grade inflammatory state occurring during obesity that might also increase leptin synthesis and release are (I) high-calorie intake-induced macrophage infiltration in adipose tissue; and (II) increased intestinal permeability (61). Excessive calorie overload causes hypertrophic adipocytes to release monocyte chemoattractant protein (MCP)-1, which in turn favors increased macrophages infiltration into the adipose tissue (62). Subsequently, infiltrating macrophages increase the synthesis and release of several proinflammatory factors such as TNF-α, IL-1β and IL-6 (63,64), all of which are known to increase leptin synthesis and release.
Gut health plays a key role as a barrier to prevent translocation of intestinal bacteria and bacterial lipopolysaccharide (LPS) into the blood stream. Emerging evidence suggests that obesity causes increased gut permeability, which contributes to the low-grade chronic inflammatory state observed during obesity (65,66). The key role that gut microbiota plays in obesity has been recently shown in preclinical studies (61,67,68). In mice studies, it was shown that high-fat diet increased circulating endotoxin and proinflammatory factors (61). These changes appeared to be due, at least in part, by changing gut microbiota composition (increasing Firmicutes to Bacteroidetes ratio), which favored endotoxin translocation into the bloodstream (61). As leptin synthesis and release can be increased by LPS, this mechanism might account for the increased hyperleptinemia that occurs during obesity.
Other studies carried out in mice mimicking Roux-en Y gastric bypass (RYGB), currently the most effective treatment for obesity, also strengthened the concept that gut microbiota can contribute to the obese/lean phenotype (68). In the latter study, the authors provided support to a new emerging concept: conserved post-operative changes in gut microbiota played a key role to reduced weight and adiposity after RYGB surgery (68).
In summary, leptin shares tridimensional similarities with the cytokine family, it can increase proinflammatory factors, and it might be regulated by gut microbiota and macrophage infiltration in adipose tissue. As a relationship between hyperleptinemia and CRC appears to be evident, inflammatory factors that chronically increase leptin have to be considered for overcoming the deleterious consequeces of hyperleptinemia.
Associations between leptin and colon cancer
Serum leptin levels are markedly increased in obese individuals, where obesity is an important risk factor for colon cancer development. Since the demonstration of leptin’s effect as a growth factor for colonic epithelial cancer (48), several studies have hypothesized an association between increased leptin levels and colon cancer risk. The leptin receptor is found in colonic epithelium, which has functional importance in regulating cell processes (48). Dysregulation of these processes, as a result of the obesity-related hyperleptinemia, can lead to neoplasia.
In vitro colon cancer cell line studies have shown that stimulation of these cells by leptin leads to tyrosine phosphorylation of Ob-R, activating major signal transduction pathway elements including p42/44 mitogen-activated protein kinase, JNK, mitogen-activated protein kinase, Src/phosphoinositide, 3-kinase/protein kinase B and extracellular-signal-regulated kinase (69,70). Leptin stimulation has also been reported to inhibit apoptosis of human CRC cells via several mechanisms involving extracellular-signal-regulated kinase, p38 mitogen-activated protein kinase activation and nuclear translocation of NF-κB (71).
Some rodent studies have contradicted findings from in vitro studies: whilst leptin stimulation in vitro led to increases in signal transduction, in nude mice leptin stimulation failed to promote the growth of cancer xenografts (72). Furthermore, in Apc/Min+ mice (a murine model of colon cancer), leptin stimulation failed to induce tumor growth (72). Interestingly, in the absence of leptin, ob/ob mice showed increased sensitivity to colon cancer carcinogens. In contrast to these negative findings, a high-fat diet promoted colonic epithelium proliferation in mice, which would imply that obesity-associated hyperleptinemia is associated with colon cancer (73). However, carcinogen-induced tumor growth in leptin-deficient mice was slower than in leptin-resistant db/db mice, suggesting the mechanism of carcinogenesis is more complex than simple rises in circulating leptin concentrations. Indeed, Endo and colleagues observed that Ob-R is overexpressed in colonic tumors, and that leptin is linked to the activation of Wingless-related integration site (Wnt) signaling (an important paracrine signaling mechanism) (73). This observation, together with differences in tumor growth seen between substrate and receptor deficient models, reveals a potential molecular mechanism for colon cancer development.
On an inflammatory level, leptin induces the secretion of the inflammatory cytokines IL-6, IL-1β and CXCL1 in humans, which have all been implicated in colon carcinogenesis (74). In particular, the secretion of CXCL1 supports in vitro studies, which reveal that leptin promotes vascular endothelial growth factor (VEGF) activity by epithelial cells, and thus provides a mechanism for tumor-associated angiogenesis, promoting tumor survival and proliferation (70). Indeed, by stimulating angiogenesis, leptin facilitates tumor growth and invasion of adjacent organs (75). Furthermore, Ob-R overexpression suggests that tumors may be sensitive/responsive to leptin, thus providing further means for cancer survival and growth, described below in Figure 1.
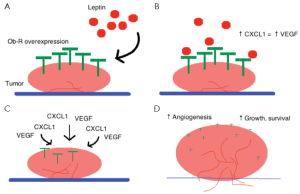
On the other hand, hypoxia (a common finding in malignant tissues), has also been shown to induce cancer epithelial cells to produce endothelial growth factor (EGF), which regulates the levels of leptin and VEGF (70). The effect of hypoxia on leptin levels can also be indirectly mediated by hypoxia-inducible factor 1-alpha (HIF-1α), which binds to target genes that contain a specific hypoxia-responsive element (HRE) (76). The leptin gene contains eight HRE regions, and thus it is likely to be regulated by hypoxia through HIF-1α. Koda and colleagues explored this relationship in a cohort of CRC patients, and found a significant positive correlation between leptin levels and the amount of HIF-1α (r=0.243, P=0.005), and between Ob-R and HIF-1α (r=0.325, P<0.001). As expected, leptin and Ob-R also shared a positive correlation (r=0.426, P<0.001) (75). These results further support a role for hypoxia in neoplasia, and demonstrate that leptin has a role in cancer progression through an auto-/paracrine mechanism. The co-expression of leptin with Ob-R suggests that local activity of the leptin/Ob-R axis is responsible for colon cancer cell responses to a hypoxic environment.
Leptin affects many cellular signal transduction pathways, and can act as an important gene expression regulator. Nowakowska-Zajdel performed a microarray analysis using samples obtained from 11 CRC patients, targeted at analyzing genes that encode proteins that are affected by leptin. The genes AKT1, STAT3 and MCL1 were upregulated at the early stage of disease, and the gene STAT5B was silenced. Furthermore, the genes VEGFC and CCND1 were overexpressed and the VEGFA gene was silenced (77). Differences in the gene expression profile between early and late stage cancers suggest that leptin plays a role in the dynamic and changing system of the neoplasm, and that leptin may, at least in part, be responsible for tumor progression by means of transcription activation and repression or silencing. Of clinical value, the genes revealed to be overexpressed at an early stage of the disease may have the potential to be used as part of a colon cancer screening program, in an effort to identify patients for treatment at an earlier stage, thereby improving the prognosis.
Similar to Nowakowska-Zajdel’s study, in a mouse model of colon cancer, leptin was found to upregulate the proinflammatory cytokine gene profile (74). Real-time polymerase chain reaction (RT-PCR) assays were performed on colonic tissue harvested from wild-type and leptin-deficient mice. Compared to basal gene expression, a few genes were differentially expressed. Following leptin administration, several more genes encoding products affected by leptin were significantly upregulated, summarized in Table 3.
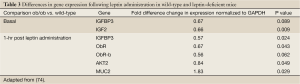
Full Table
Additionally, cytokines, including those previously mentioned to promote colonic neoplasia, were significantly altered. Leptin administration altered the proinflammatory cytokine profile more substantially in ob/ob mice than in wild-type mice (summarized in Table 4) (74). These findings fit with the growing hypothesis that leptin is a major immune regulator, and substantiates the notion of adipose tissue as an immunoendocrine organ. In the presence of increase leptin sensitivity (i.e., in the leptin-deficient mouse), leptin’s effects on the upregulation of proinflammatory markers are enhanced.
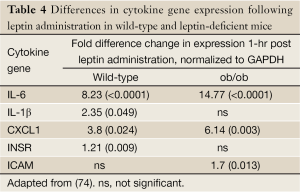
Full Table
The same study has shown that IL-6 and CXCL1 were rapidly upregulated 1-hr after leptin administration, and returned to near basal levels after a further three hours (74). This time-dependent response indicates that these genes may be involved in an early response. Time-dependent changes, though over a longer time period, were also seen by Nowakowska-Zajdel and colleagues (44). Interestingly, the authors have not observed the localization of leptin-regulated proinflammatory cytokines with macrophage markers (F4/80 and CD11c) (74). This suggests that leptin stimulation may directly or indirectly result in an upregulation of IL-6, IL-1β and CXCL1 in cells already resident within colonic tissue, possibly independent of other inflammatory mechanisms. Furthermore, Padidar and colleagues showed visible changes in response to leptin in cells embedded in the epithelium, lamina propria and muscularis layers of the colon (74). As previously mentioned, CXCL1 is an important angiogenic factor and, together with VEGF (a powerful pro-angiogenic growth factor), this could be a potential mechanism by which Ob-R–expressing tumors support their growth and survival, when stimulated by leptin. Overall, this study provides in vivo evidence of the direct effect of leptin on colon cancer pathogenesis.
Human epidemiological studies have further demonstrated the association between hyperleptinemia and colon cancer. Epidemiological studies carried out in two different cohorts, one from Norway (78) and another from Sweden (79), have shown increased risk of colon cancer in individuals with high levels of leptin. In a case-control study of more than 100 volunteers, Hillenbrand and colleagues examined the adipokine profile of CRC patients, morbidly obese (MO) patients and healthy blood donor (BD) participants. As expected, CRC and MO patients had a systemic increase in inflammatory mediators, in line with the theory that inflammation contributes to obesity and colon cancer (80). However, there were significant differences between CRC and MO adipokine profiles. Median leptin concentrations were lower in CRC patients as compared with MO. In contrast to the leptin findings, adiponectin, another adipose tissue-derived cytokine, was increased in CRC patients as compared with MO. Furthermore, there was no difference in adiponectin levels between CRC and BD individuals. These differences were sex-dependent, where females tended to have higher levels of both leptin and adiponectin compared to males in all three groups of volunteers. Overall, this study suggested that CRC and MO individuals have similar cytokine profiles, but with discrepancies in the concentration of leptin, suggesting that leptin does contribute to CRC risk, independent of obesity. However, this study failed to take into account the possible role for soluble leptin receptor (Ob-Re) as a potential mechanism of circulating leptin sequestration thus reducing its bioavailability (80).
The role of Ob-Re on colon cancer was addressed by Aleksandrova and colleagues (46). In a large prospective study of approximately 520,000 participants, leptin was negatively correlated with Ob-Re. Furthermore, leptin was not significantly associated with an increased risk of CRC, but Ob-Re was strongly inversely associated with CRC, meaning that CRC is associated with a low circulating concentration of Ob-Re (which lead to higher bioavailable leptin levels) (46). Indeed, higher levels of Ob-Re were found to be associated with an advanced stage of tumor development (81). These studies do, however, report cancer site specific differences in adipokine concentrations. Hillenbrand and colleagues report higher levels of leptin in patients with colonic cancer as compared with rectal cancer in both males and females, and Aleksandrova and colleagues report an increased risk of colonic cancer at the highest quintile of leptin concentration compared to no significant increases in risk in developing rectal cancer (46,80). It is important to note that the study by Alesandrova and colleagues was a multi-center trial which included over half a million participants from nine countries, and thus greater emphasis should be placed on their conclusions. Taken together, these studies indicate a role for the leptin/Ob-Re axis in colon cancer development, and highlight the need to establish the action of leptin in colon cancer pathogenesis. Furthermore, these studies demonstrate the need to clarify the role of Ob-Re in either stimulating leptin signaling or sequestering leptin and reducing its bioavailability. Finally, it would be of clinical value to determine the reasons for gender-related differences in leptin levels and colon cancer risk. The effects of leptin in the development and progression of colon cancer in cell, animal and humans studies are summarized in Table 5.
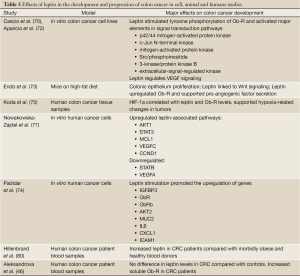
Full Table
Conclusions and future directions
Obesity is a risk factor for several cancer types, including colon cancer. This can be explained by several changes in hormonal and cytokine profiles that stimulate cell growth, inhibit apoptosis, and promote angioneogenesis. Leptin is increased in obesity, and has been shown to play an important role in the pathophysiology of obesity-related colon cancer by affecting cell growth, apoptosis and angioneogenesis. Several human and animal trials have explored the possible association between leptin and colon cancer, though the exact mechanisms remain unclear. Some human studies have yielded contradictory findings in terms of a clear association between the adipokine and increased CRC risk (46,80), and it is possible that the links between obesity, inflammation and colon cancer extend beyond the traditional adipokines leptin and adiponectin. The adipose tissue is emerging as a major endocrine organ and with more research focusing into the immunoendocrine nature of that tissue, many novel adipokines have been discovered. These adipokines, for example visfatin, omentin-1 and vaspin have now also been associated with CRC in an obesity-independent manner (82).
Like any cancer, CRC has a multifactorial etiology, and several factors affecting cancer development and progression need to be taken into account. As colon cancer develops and progresses over several decades, lifestyle interventions can be an important adjunct to medical therapies to effectively treat and suppress cancer development and metastasis. Better understanding of the mechanisms by which leptin is associated with CRC can potentially lead to the development of novel approaches for the diagnosis, risk stratification, and treatment of colon cancer.
Acknowledgements
This review was supported by The Australian National University.
Disclosure: The authors declare no conflict of interest.
References
- WHO. Obesity and overweight. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/index.html
- Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol 2013;9:13-27. [PubMed]
- Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist 2010;15:556-65. [PubMed]
- Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer 2011;11:886-95. [PubMed]
- Renehan AG, Soerjomataram I, Tyson M, et al. Incident cancer burden attributable to excess body mass index in 30 European countries. Int J Cancer 2010;126:692-702. [PubMed]
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625-38. [PubMed]
- Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008;371:569-78. [PubMed]
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 2004;89:2548-56. [PubMed]
- Boguszewski CL, Paz-Filho G, Velloso LA. Neuroendocrine body weight regulation: integration between fat tissue, gastrointestinal tract, and the brain. Endokrynol Pol 2010;61:194-206. [PubMed]
- Paz-Filho G, Wong ML, Licinio J. Ten years of leptin replacement therapy. Obes Rev 2011;12:e315-23. [PubMed]
- Paz-Filho G, Lim EL, Wong ML, et al. Associations between adipokines and obesity-related cancer. Front Biosci (Landmark Ed) 2011;16:1634-50. [PubMed]
- Drew JE. Symposium 3: obesity-related cancers molecular mechanisms linking adipokines to obesity-related colon cancer: focus on leptin. Proc Nutr Soc 2012;71:175. [PubMed]
- Paz-Filho G, Mastronardi C, Franco CB, et al. Leptin: molecular mechanisms, systemic pro-inflammatory effects, and clinical implications. Arq Bras Endocrinol Metabol 2012;56:597-607. [PubMed]
- Cunningham D, Atkin W, Lenz HJ, et al. Colorectal cancer. Lancet 2010;375:1030-47. [PubMed]
- Chan DS, Lau R, Aune D, et al. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One 2011;6:e20456. [PubMed]
- Wei EK, Giovannucci E, Wu K, et al. Comparison of risk factors for colon and rectal cancer. Int J Cancer 2004;108:433-42. [PubMed]
- Chen K, Qiu JL, Zhang Y, et al. Meta analysis of risk factors for colorectal cancer. World J Gastroenterol 2003;9:1598-600. [PubMed]
- Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS One 2013;8:e53916. [PubMed]
- Vazzana N, Riondino S, Toto V, et al. Obesity-driven inflammation and colorectal cancer. Curr Med Chem 2012;19:5837-53. [PubMed]
- Cohen SS, Murff HJ, Signorello LB, et al. Obesity and colorectal cancer screening among black and white adults. Cancer Causes Control 2012;23:709-16. [PubMed]
- Whitlock K, Gill RS, Birch DW, et al. The association between obesity and colorectal cancer. Gastroenterol Res Pract 2012;2012:768247.
- Lund EK, Belshaw NJ, Elliott GO, et al. Recent advances in understanding the role of diet and obesity in the development of colorectal cancer. Proc Nutr Soc 2011;70:194-204. [PubMed]
- Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut 2013;62:933-47. [PubMed]
- Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009;9:88. [PubMed]
- Harriss DJ, Atkinson G, George K, et al. Lifestyle factors and colorectal cancer risk (1): systematic review and meta-analysis of associations with body mass index. Colorectal Dis 2009;11:547-63. [PubMed]
- Dai Z, Xu YC, Niu L. Obesity and colorectal cancer risk: a meta-analysis of cohort studies. World J Gastroenterol 2007;13:4199-206. [PubMed]
- Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: A meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev 2007;16:2533-47. [PubMed]
- Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am J Clin Nutr 2007;86:556-65. [PubMed]
- Johnson IT, Lund EK. Review article: nutrition, obesity and colorectal cancer. Aliment Pharm Ther 2007;26:161-81. [PubMed]
- Ogino S, Nosho K, Meyerhardt JA, et al. Cohort Study of Fatty Acid Synthase Expression and Patient Survival in Colon Cancer. J Clin Oncol 2008;26:5713-20. [PubMed]
- Fuchs CD, Claudel T, Kumari P, et al. Absence of adipose triglyceride lipase protects from hepatic endoplasmic reticulum stress in mice. Hepatology 2012;56:270-80. [PubMed]
- Song M, Wu K, Ogino S, et al. A prospective study of plasma inflammatory markers and risk of colorectal cancer in men. Br J Cancer 2013;108:1891-8. [PubMed]
- Cao H, Jin C, Huang D, et al. Changes in serum IGF-1 level and tumor VEGF expression in mice with colorectal cancer under hyperglycemic conditions. Mol Med Rep 2013;7:1361-5. [PubMed]
- Feik E, Baierl A, Hieger B, et al. Association of IGF1 and IGFBP3 polymorphisms with colorectal polyps and colorectal cancer risk. Cancer Causes Control 2010;21:91-7. [PubMed]
- Wong HL, Koh WP, Probst-Hensch NM, et al. Insulin-like growth factor-1 promoter polymorphisms and colorectal cancer: a functional genomics approach. Gut 2008;57:1090-6. [PubMed]
- Harvey AE, Lashinger LM, Otto G, et al. Decreased systemic IGF-1 in response to calorie restriction modulates murine tumor cell growth, nuclear factor-kappaB activation, and inflammation-related gene expression. Mol Carcinog 2012. [Epub ahead of print]. [PubMed]
- Simpson ER, Brown KA. Minireview: obesity and breast cancer: a tale of inflammation and dysregulated metabolism. Mol Endocrinol 2013;27:715-25. [PubMed]
- Chen J, Iverson D. Estrogen in obesity-associated colon cancer: friend or foe? Protecting postmenopausal women but promoting late-stage colon cancer. Cancer Causes Control 2012;23:1767-73. [PubMed]
- Hotamisligil GS, Arner P, Caro JF, et al. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest 1995;95:2409-15. [PubMed]
- Flores MB, Rocha GZ, Damas-Souza DM, et al. Obesity-induced increase in tumor necrosis factor-alpha leads to development of colon cancer in mice. Gastroenterology 2012;143:741-53.e1-4.
- Giovannucci E. Metabolic syndrome, hyperinsulinemia, and colon cancer: a review. Am J Clin Nutr 2007;86:s836-42. [PubMed]
- Chen J, Katsifis A, Hu C, et al. Insulin decreases therapeutic efficacy in colon cancer cell line HT29 via the activation of the PI3K/Akt pathway. Curr Drug Discov Technol 2011;8:119-25. [PubMed]
- Murphy N, Norat T, Ferrari P, et al. Dietary fibre intake and risks of cancers of the colon and rectum in the European prospective investigation into cancer and nutrition (EPIC). PLoS One 2012;7:e39361. [PubMed]
- Rinaldi S, Rohrmann S, Jenab M, et al. Glycosylated hemoglobin and risk of colorectal cancer in men and women, the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev 2008;17:3108-15. [PubMed]
- Rinaldi S, Cleveland R, Norat T, et al. Serum levels of IGF-I, IGFBP-3 and colorectal cancer risk: results from the EPIC cohort, plus a meta-analysis of prospective studies. Int J Cancer 2010;126:1702-15. [PubMed]
- Aleksandrova K, Boeing H, Jenab M, et al. Leptin and soluble leptin receptor in risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Cancer Res 2012;72:5328-37. [PubMed]
- Huang XF, Chen JZ. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes Rev 2009;10:610-6. [PubMed]
- Hardwick JC, Van Den Brink GR, Offerhaus GJ, et al. Leptin is a growth factor for colonic epithelial cells. Gastroenterology 2001;121:79-90. [PubMed]
- Kiguchi N, Maeda T, Kobayashi Y, et al. Leptin enhances CC-chemokine ligand expression in cultured murine macrophage. Biochem Biophys Res Commun 2009;384:311-5. [PubMed]
- Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab 2009;297:E1247-59. [PubMed]
- Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol 2004;15:2792-800. [PubMed]
- Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest 2003;112:1785-8. [PubMed]
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 2005;115:911-9. [PubMed]
- Mastronardi CA, Yu WH, Srivastava VK, et al. Lipopolysaccharide-induced leptin release is neurally controlled. Proc Natl Acad Sci U S A 2001;98:14720-5. [PubMed]
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7. [PubMed]
- Sussman DA, Santaolalla R, Strobel S, et al. Cancer in inflammatory bowel disease: lessons from animal models. Curr Opin Gastroenterol 2012;28:327-33. [PubMed]
- Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature 1994;372:425-32. [PubMed]
- Tartaglia LA, Dembski M, Weng X, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995;83:1263-71. [PubMed]
- Mastronardi CA, Yu WH, McCann SM. Resting and circadian release of nitric oxide is controlled by leptin in male rats. Proc Natl Acad Sci U S A 2002;99:5721-6. [PubMed]
- Stelzer I, Zelzer S, Raggam RB, et al. Link between leptin and interleukin-6 levels in the initial phase of obesity related inflammation. Transl Res 2012;159:118-24. [PubMed]
- Kim KA, Gu W, Lee IA, et al. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One 2012;7:e47713. [PubMed]
- Teixeira TF, Collado MC, Ferreira CL, et al. Potential mechanisms for the emerging link between obesity and increased intestinal permeability. Nutr Res 2012;32:637-47. [PubMed]
- Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch 2007;455:479-92. [PubMed]
- van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev 2009;18:2569-78. [PubMed]
- Frazier TH, DiBaise JK, McClain CJ. Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury. JPEN J Parenter Enteral Nutr 2011;35:14S-20S. [PubMed]
- Cani PD, Possemiers S, Van de Wiele T, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009;58:1091-103. [PubMed]
- Moreira AP, Texeira TF, Ferreira AB, et al. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr 2012;108:801-9. [PubMed]
- Liou AP, Paziuk M, Luevano JM Jr, et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 2013;5:178ra41.
- Jaffe T, Schwartz B. Leptin promotes motility and invasiveness in human colon cancer cells by activating multiple signal-transduction pathways. Int J Cancer 2008;123:2543-56. [PubMed]
- Cascio S, Ferla R, D’Andrea A, et al. Expression of angiogenic regulators, VEGF and leptin, is regulated by the EGF/PI3K/STAT3 pathway in colorectal cancer cells. J Cell Physiol 2009;221:189-94. [PubMed]
- Hoda MR, Keely SJ, Bertelsen LS, et al. Leptin acts as a mitogenic and antiapoptotic factor for colonic cancer cells. Br J Surg 2007;94:346-54. [PubMed]
- Aparicio T, Kotelevets L, Tsocas A, et al. Leptin stimulates the proliferation of human colon cancer cells in vitro but does not promote the growth of colon cancer xenografts in nude mice or intestinal tumorigenesis in Apc(Min/+) mice. Gut 2005;54:1136-45. [PubMed]
- Endo H, Hosono K, Uchiyama T, et al. Leptin acts as a growth factor for colorectal tumours at stages subsequent to tumour initiation in murine colon carcinogenesis. Gut 2011;60:1363-71. [PubMed]
- Padidar S, Farquharson AJ, Williams LM, et al. Leptin up-regulates pro-inflammatory cytokines in discrete cells within mouse colon. J Cell Physiol 2011;226:2123-30. [PubMed]
- Koda M, Sulkowska M, Kanczuga-Koda L, et al. Expression of the obesity hormone leptin and its receptor correlates with hypoxia-inducible factor-1 alpha in human colorectal cancer. Ann Oncol 2007;18 Suppl 6:vi116-9. [PubMed]
- Giatromanolaki A, Harris AL. Tumour hypoxia, hypoxia signaling pathways and hypoxia inducible factor expression in human cancer. Anticancer Res 2001;21:4317-24. [PubMed]
- Nowakowska-Zajdel E, Mazurek U, Stachowicz M, et al. Cellular signal transduction pathways by leptin in colorectal cancer tissue: preliminary results. ISRN Endocrinol 2011;2011:575397.
- Stattin P, Lukanova A, Biessy C, et al. Obesity and colon cancer: does leptin provide a link? Int J Cancer 2004;109:149-52. [PubMed]
- Stattin P, Palmqvist R, Soderberg S, et al. Plasma leptin and colorectal cancer risk: a prospective study in Northern Sweden. Oncol Rep 2003;10:2015-21. [PubMed]
- Hillenbrand A, Fassler J, Huber N, et al. Changed adipocytokine concentrations in colorectal tumor patients and morbidly obese patients compared to healthy controls. BMC Cancer 2012;12:545. [PubMed]
- Tutino V, Notarnicola M, Guerra V, et al. Increased soluble leptin receptor levels are associated with advanced tumor stage in colorectal cancer patients. Anticancer Res 2011;31:3381-3. [PubMed]
- Fazeli MS, Dashti H, Akbarzadeh S, et al. Circulating levels of novel adipocytokines in patients with colorectal cancer. Cytokine 2013;62:81-5. [PubMed]


