Systematic review and meta-analysis in oncology: topic selection and scientific writing
Evidence based medicine (EBM) is a useful research method and brand new theory in medical science, which has developed since the 1970s. EBM has been defined as “the conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients. The practice of evidence based medicine means integrating individual clinical expertise with the best available external clinical evidence from systematic research” (1). More recently it has been described as the “integration of best research evidence with clinical expertise and patient values” (2). The 3 key phrases in this definition are “best research evidence”, “clinical expertise” and “patient values”.
A systematic review is a literature review focused on a research question that tries to identify, appraise, select and synthesize all high quality research evidence relevant to that question. Systematic reviews of high-quality randomized controlled trials are crucial to EBM (3). Meta-analysis is the quantitative assessment of the pooled results of multiple clinical trials. It is ideally suited for use in assessing results of trials, are often under-powered to detect small differences in primary endpoints, let alone subgroup analyses. While systematic review is a more extensive concept, meta-analysis is a systematic review that uses quantitative methods. Naturally, the titles of EBM articles often use “systematic review and meta-analysis” as their endings.
Why should we introduce the concept of EBM? What are its implications for the clinical practices? There are two useful terms: strength of evidence and level of evidence. The strength of evidence refers to the authenticity and applicability of the research findings. On the contrary, the level of evidence is graded in accordance with the strength of evidence. Grading of the mass research findings can help us find the best evidences. The most well-known grading system is the one developed by Canadian Task for Preventive Health Care (CTFPHC). It divides the evidences into five levels: level I, large double blind randomized, control trials (RCTs), or meta analyses of smaller RCTs, clinically relevant outcomes; level II, small RCTs, non-blinded RCTs, or RCTs using valid surrogate markers; level III, non-randomized controlled studies, observational (cohort) studies, case-control studies, or cross-sectional studies; level IV, opinion of expert committees or respected authorities; and level V, expert opinion. Thus, it has been well recognized that EBM is the basis for clinical decision-making.
Then, how to find a good topic for meta-analysis? Actually, this is a particularly important question for young doctors who wish to publish scientific articles but have no research grant. Here I would like to share my experience in finding a meta-analysis topic—postoperative adjuvant chemotherapy for gastric cancer, which had confused me a lot before I wrote the article. Firstly, we searched the relevant studies in PubMed and found 404 articles. Of course, not all of them were read by us thoroughly. We further searched the meta analyses on this controversial topic and found that a meta-analysis had already been published in JAMA in 2010 (4), which showed that adjuvant chemotherapy after surgery was more favorable for the patients in terms of overall survival (OS) and progression-free survival (PFS) when compared with the surgery alone. The HR value of the OS was 0.82; in other words, post-operative adjuvant chemotherapy could lower the risk of death by about 18%. Subgroup analysis based on different chemotherapy regimens produced consistent results. This meta-analysis was sufficient to demonstrate the role of post-operative adjuvant chemotherapy for gastric cancer since it is the largest individual patient-based (IPB) meta-analysis ever. Notably, the authors of this article asked the authors of all included articles to provide the details of individual patients and then performed pooled analysis. By doing so, it had higher statistical power, which may explain why it could be published in JAMA. Then, how about the neoadjuvant chemotherapy? Similarly, by reviewing five RCTs, a meta-analysis in 2008 concluded that neoadjuvant chemotherapy had no benefit for patients. Clinically, neoadjuvant chemotherapy is not recommended for gastric cancer patients, unless more data are obtained from large-scale RCTs.
As demonstrated in these two examples, high-quality meta-analyses are helpful to resolve clinical concerns and guide clinical decision-making. Art comes from life and goes beyond it; similarly, a good EBM topic comes from clinical settings and can be applied to guide clinical practices. Therefore, the research topic comes from clinical concerns, and it is particularly important to identify the clinically important and feasible issues. Then, I searched for the relevant literature, evaluated the strength and level of evidences, and finally decided whether this topic is feasible for meta-analysis.
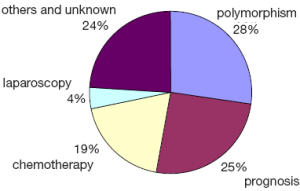
I performed a simple analysis on these published articles to frame my topic. By searching PubMed, I found 422 meta-analyses on gastric cancer; further search and analysis showed that more than half of these articles were on the gene polymorphisms and prognosis factors and some other articles were focused on chemotherapy and laparoscopic techniques (Figure 1). To make things easier, I classified them into five directions: etiology, prevention, treatment, diagnosis, and prognosis. The statistical methods applied for the meta-analysis of these five directions differed, so did their clinical implications. The meta-analyses on treatment were of the highest clinical implications, but were often more difficult to be published.
Now let’s see some meta-analyses on treatment of gastric cancer. This is a study published in JCO (5). It evaluated the role of systemic chemotherapy in managing the advanced gastric cancer by comparing the chemotherapy and optimal supportive treatment and by comparing the combined therapy and single-drug treatment; also, it carried subgroup analysis on the efficacies of various drugs including fluorouracil, anthracycline, and irinotecan. In addition, some other articles evaluated the roles of radiotherapy, intraperitoneal chemotherapy, and laparoscopy on gastric cancer (6-8).
Quite a few articles were focused on the prevalence (i.e., etiology) of gastric cancer. This is a meta-analysis published in Ann Intern Med (9). The authors evaluated the effectiveness of Helicobacter pylori (HP) eradiation in lowering the risk of gastric cancer. Although only six RCTs were included, the author concluded a positive correlation. The potential associations of non-steroidal anti-inflammatory drugs, smoking, and body mass index with the risk of gastric cancer had also been analyzed (10-12). In recent years, quite a few meta analyses have been performed on the gene polymorphisms (e.g., ERCC1/CYP2E1) of gastric cancer (13,14).
In terms of gastric cancer prevention, this is a very interesting meta-analysis published in Gastroenterology (15). The authors found that garlics could decrease the risk of gastric cancer. “Garlic prevents cancer”. Here we see its scientific evidences. This article, published in Lancet (16), brings a negative result. The authors found that the antioxidants can not decrease the risk of gastric cancer; rather, they increase the risk of death.
Fewer meta-analyses were on the diagnosis. This is an article on the role of EUS for the staging of gastric cancer (17), which concluded that both the sensitivity and the specificity of EUS were about 90%. The statistical analysis of the diagnosis could be performed using special software, and somehow different from other directions.
Quite a few meta-analyses on prognosis have been published. This article was recently published in PLOS ONE. It proposed a somehow astonishing conclusion that gastric cancer patients with HP infection might have a better prognosis (18). The prognostic values of HER3, HER2, E-cadherin, and vascular endothelial growth factor (VEGF) have also been evaluated, and all of them were positively associated with the prognosis of gastric cancer (19-22).
Among these five directions, the largest proportions of meta-analyses were focused on prognosis and gene polymorphisms, which have relatively fixed and uniform methodologies and certain clinical implications, and therefore are more likely to be published. Meta-analysis on treatment is most difficult to be published because there are fewer eligible clinical trials and IPB data are often not available. However, meta-analysis on this direction has the highest clinical value. Therefore, we should be more alerted of our daily work and keep a strong willingness to clarify any misconceptions or confusions. By doing so, we may find a good EBM topic.
The process of systematic review, or the writing of a meta-analysis article, has its fixed mode. The statistical methods for meta-analyses on different directions are somehow different, but their general ideas are unified. According to the Cochrane Collaboration (23), the steps of a systematic review on the treatment may include: (I) Framing questions for a review and developing a plan: four components must be identified when defining the review question: subjects, interventions, outcomes, and study designs; (II) Identifying relevant work: all the relevant literature must be collected in a systematic and extensive manner; (III) Selecting literature: articles should be selected based on the pre-defined inclusion and exclusion criteria. Literature that can answer the review question should be screened from the collected articles; (IV) Assessing the quality of studies: study quality assessment should include the following three components: internal validity (bias), external validity (suitability), and confounding factors; (V) Summarizing the evidence: all data must be input into a systematic review management software such as Revman software (Cochrane website); (VI) Interpreting the findings: Qualitative or quantitative methods should be applied to interpret the collected data to yield a corresponding result; (VII) Drawing conclusions: conclusions may include the implications of the systematic review, strength of evidence, applicability, advantages and disadvantages of interventions, and health economics; (VIII) Updating the systematic review: The results should be updated regularly.
In summary, the methodologies of systematic review are based on a fixed mode, and therefore are not as important as the topic selection. In fact, a good topic is often originated from in-depth investigations, and clinically important and feasible issues can often yield high-quality systematic review.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ 1996;312:71-2. [PubMed]
- Sackett DL, Richardson WS, Rosenberg W, et al. eds. Evidence - based medicine: How to practice and teach EBM. 2nd ed. London: Churchill Livingstone, 2000:1-10.
- “What is EBM?”. Centre for Evidence Based Medicine. 2009-11-20. Retrieved 2011-06-17.
- GASTRIC (Global Advanced/Adjuvant Stomach Tumor Research International Collaboration) Group, Paoletti X, Oba K, et al. Benefit of adjuvant chemotherapy for resectable gastric cancer: a meta-analysis. JAMA 2010;303:1729-37. [PubMed]
- Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006;24:2903-9. [PubMed]
- Fiorica F, Cartei F, Enea M, et al. The impact of radiotherapy on survival in resectable gastric carcinoma: a meta-analysis of literature data. Cancer Treat Rev 2007;33:729-40. [PubMed]
- Yan TD, Black D, Sugarbaker PH, et al. A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol 2007;14:2702-13. [PubMed]
- Ye LY, Liu DR, Li C, et al. Systematic review of laparoscopy-assisted versus open gastrectomy for advanced gastric cancer. J Zhejiang Univ Sci B 2013;14:468-78. [PubMed]
- Fuccio L, Zagari RM, Eusebi LH, et al. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med 2009;151:121-8. [PubMed]
- Abnet CC, Freedman ND, Kamangar F, et al. Non-steroidal anti-inflammatory drugs and risk of gastric and oesophageal adenocarcinomas: results from a cohort study and a meta-analysis. Br J Cancer 2009;100:551-7. [PubMed]
- Ladeiras-Lopes R, Pereira AK, Nogueira A, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control 2008;19:689-701. [PubMed]
- Kubo A, Corley DA. Body mass index and adenocarcinomas of the esophagus or gastric cardia: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2006;15:872-8. [PubMed]
- Zhuo W, Zhang L, Wang Y, et al. CYP2E1 RsaI/PstI polymorphism and gastric cancer susceptibility: meta-analyses based on 24 case-control studies. PLoS One 2012;7:e48265. [PubMed]
- Xue H, Lu Y, Lin B, et al. The effect of XPD/ERCC2 polymorphisms on gastric cancer risk among different ethnicities: a systematic review and meta-analysis. PLoS One 2012;7:e43431. [PubMed]
- Zhou Y, Zhuang W, Hu W, et al. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology 2011;141:80-9. [PubMed]
- Bjelakovic G, Nikolova D, Simonetti RG, et al. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet 2004;364:1219-28. [PubMed]
- Mocellin S, Marchet A, Nitti D. EUS for the staging of gastric cancer: a meta-analysis. Gastrointest Endosc 2011;73:1122-34. [PubMed]
- Wang F, Sun G, Zou Y, et al. Protective role of Helicobacter pylori infection in prognosis of gastric cancer: evidence from 2,454 patients with gastric cancer. PLoS One 2013;8:e62440. [PubMed]
- Ocana A, Vera-Badillo F, Seruga B, et al. HER3 overexpression and survival in solid tumors: a meta-analysis. J Natl Cancer Inst 2013;105:266-73. [PubMed]
- Wang S, Zheng G, Chen L, et al. Effect of HER-2/neu over-expression on prognosis in gastric cancer: a meta-analysis. Asian Pac J Cancer Prev 2011;12:1417-23. [PubMed]
- Xing X, Tang YB, Yuan G, et al. The prognostic value of E-cadherin in gastric cancer: a meta-analysis. Int J Cancer 2013;132:2589-96. [PubMed]
- Peng L, Zhan P, Zhou Y, et al. Prognostic significance of vascular endothelial growth factor immunohistochemical expression in gastric cancer: a meta-analysis. Mol Biol Rep 2012;39:9473-84. [PubMed]
- Higgins JPT, Green S. eds. Cochrane handbook for systematic reviews of interventions. USA: Wiley, 2008.