Recent advances in endoscopic management of gastrointestinal cancers
Introduction
Endoscopy was developed in the 19th century although numerous attempts were made previously for the exploration of hidden cavities in the human body. Since its introduction, endoscopy has gained more impact in the clinical setting as a consequence of technological improvement. At the beginning, a challenge was to find a safe source of light that did not damage tissue by generating heat. The first gastroscopy was performed in 1868. Later on, Thomas Edison solved the light problem but it was only after 30 years that the light source was fitted into endoscopes. The semi-flexible gastroscope was created by Wolf, a fabricator of medical instruments, and Schindler, a physician, around 1930 (1). In 1954 Hopkins generated a prototype of flexible fibre imaging device (2). In 1958, Basil Hirschowitz and Larry Curtiss built the flexible fiberoptic endoscope thanks to highly transparent optical quality glass (3). The creation of an electronic image that soon became digital led to the invention of an interface between endoscope and computer. From the development of fiberoptics the improvements that have occurred in equipment have transformed the field of gastroenterology. Endoscopic innovations derive from the explosion of technical achievements through the interaction between physicians and artisan-engineers and the incorporation of technology from other fields. One of the greatest technical development, was the discovery of endoscopic ultrasonography (EUS) in the 80s (4). By combining ultrasonography and endoscopy the endoscopic diagnosis completely changed. Digestive endoscopy, including endoscopic ultrasound, plays actually an important role in oncology regarding early diagnosis, tumor staging, and therapeutic procedures (Table 1). Indeed, improvement of endoscope and dedicated accessories allows increasing applications of therapeutic endoscopy in oncologic indications: curative resection of early carcinoma and submucosa tumor and palliative treatment of tumoral bilio-digestive obstruction. The possibility to resect sessile or flat polyps allows us to treat curatively well-differentiated carcinoma without infiltration of the muscularis mucosae, with the risk of invasion of lymph nodes being null in these cases. In case of invasion of muscularis mucosae, this risk is inferior to 1% for colorectal cancer when submucosal invasion does not exceed 1,000 microm, but this risk is between 6% and 22% in case of esophagogastric carcinoma invading the third part of the submucosa (5). The mortality of endoscopic resection was null in almost all published series. Morbidity was 15-20% for colorectal resection with 5-6% of severe complications and up to 23% after esophageal tumor ablation (5). Moreover, improvement of echoendoscope dedicated to therapeutic procedures allows from now to achieve non-anatomic pancreatic or biliary drainage through the gastric wall when the retrograde route is not suitable (whipple resection, duodenal stricture) or when drainage of the left hepatic lobe is difficult via the retrograde approach (6). A new technique was able to realize an anastomosis between the left hepatic duct and the stomach by the insertion of one or two stents. The efficacy and safety of this procedure were recently retrospectively evaluated with a technical success in 91% of the cases. Therapeutic endoscopy made many progress during the last years and development of new generation of endoscope and accessories would allow a real endoluminal surgical approach for superficial tumor, bilio-digestive anastomosis or gastro-enteroanastomosis. In addition the development of technologies based on light-tissue interactions [e.g., narrow band imaging (NBI)], computer aided diagnosis, injection pharmacotherapy (with particular reference to EUS-guided injection), photodynamic diagnosis and therapy have made endoscopy a keystone in modern gastroenterology.

Full Table
New technology in the diagnosis of gastrointestinal (GI) cancers
Confocal laser endomicroscopy (CLE)
Since endoscopes can go everywhere but cannot see everything while microscopes can see everything but cannot reach every place, a new technology called CLE was introduced in 2004 (7). This is a novel endoscopic imaging technique that allows for instant in vivo histology during ongoing endoscopy. Two probe devices are actually approved, one is integrated into the distal tip of a high-resolution endoscope (iCLE; Pentax, Tokyo, Japan) and the other is a standalone probe, which is introduced through the instrument channel of standard endoscopes (pCLE; Cellvizio, Mauna Kea Technologies, Paris, France). Once the suspect area is seen, the operator put the probe in contact with the mucus thus performing an “optic biopsy”. Thus, thanks to light property it could be not mandatory to take a sample for histology or cytology saving time and the risk of false negatives and seeding. The end-result images, in fact, are approximately a 1,000-fold magnification of in vivo tissue (8-10). One of the major limitations of CLE is that it only covers a limited field within the mucosa rendering a pan-endomicroscopy of the GI tract virtually impossible, so this technique is used more to perform targeted biopsy. CLE also allows us to choose the best treatment immediately, to correctly identify lesions margins, and to follow-up treatment response (8-10). Multiple high-quality studies evaluated the role of CLE; all these studies demonstrated that CLE was able to distinguish between normal tissue and regenerative or neoplastic tissue with very high accuracy (8-11). CLE is well established in most recent guidelines as an excellent choice for dysplasia surveillance in patients with UC instead of random biopsy with a considerable cost saving (12-15). However, the procedure tends to be time-consuming and the operational equipment is costly. Furthermore, it requires additional training and there is also a medical-legal issue in the endoscopists making a histological diagnosis without confirmation by a pathologist.
Narrow band imaging (NBI)
NBI utilizes green and blue light to enhance blood vessels and consequently tumour lesions that are more vascularized. NBI allows to correctly identifying lesions margins that cannot be seen so clearly by standards endoscopes. Recently a multicenter randomized controlled trial showed that NBI improves the detection of subtle gastric lesions (e.g., early gastric cancer or dysplastic lesions) and intestinal metaplasia compared with white light endoscopy WLE (16). This study suggested that NBI could open new screening for gastric cancer. Another challenge is the detection of flat dysplasia in Barrett’s esophagus. Dysplasia is difficult to detect showing a similar appearance to nondysplastic mucosa on WLE, and therefore random biopsy sampling is currently recommended. A recent meta-analysis provided evidence for the use of advanced imaging in Barrett’s esophagus surveillance (17) suggesting that newer imaging modalities could help target biopsies to evaluate for dysplasia.
Another challenge is to improve the adenoma detection rate of the endoscopist in screening colonoscopy thus reducing the risk of interval cancer. With the discovery of the serrated adenoma pathway, it would be important that endoscopists would be able to adequately identify these precancerous polyps. Kumar et al. (18) found that sessile serrated adenomas were more likely to resemble adenomas on NBI features than were hyperplastic polyps (odds ratio 0.84 vs. 0.59) and concluded that NBI optical biopsy eliminate misclassification of these high risk polyps as hyperplastic polyps.
Low coherence-enhanced backscattering spectroscopy freestanding fiberoptic probe
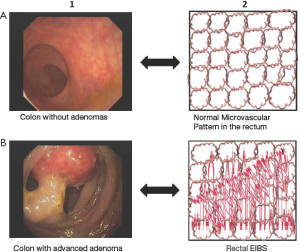
Colonoscopy as a colorectal cancer screening technique is not efficient given the low prevalence of advanced adenomas. Recent findings could make colorectal screening program more cost-effective allowing the identification of patients who are at a high risk of adenomas. Microvascular blood content is increased in early carcinogenesis and is a robust marker of field carcinogenesis in humans. Rectal mucosal microvasculature endoscopic increased blood supply (EIBS) is altered in patients harboring advanced adenomas elsewhere in the colon (Figure 1) (19). The quantitative measurement of mucosal microvasculature is feasible in vivo by a 2 mm fiberoptic probe that can be used either as an endoscopically compatible device or a stand-alone device for detection of EIBS in rectal mucosa (19). Roy et al. (20) recently confirmed a robust performance of this minimally invasive test in the identification of patients with colon polyps allowing risk-stratification of patients for screening colonoscopy.
Full-spectrum view colonoscopies
The most important problem in colonoscopy is the adenoma detection rate because a lesion could lie behind folds. A new colonoscope was introduced this year to increase the adenoma detection rate thanks to its ability to see behind folds. It is called the PeerScope (PeerMedical Ltd, Caesarea, Israel) (21). It maintains the standard features of a colonoscope but has two viewing modes: a 160-degree forward-viewing mode and a 330-degree or greater full-spectrum view. Recent data suggest that it is functionally comparable to standard colonoscopes, but further studies are needed to assess the improvement of the adenoma detection rate.
Radio-controlled motor-driven capsule
The ability to screen the GI tract in a non-invasive way is another important challenge. To this purpose, a new capsule technology able to control both the direction and transit speed resulting in a more adequate visualization of the GI tract was introduced this year (radio-controlled motor-driven capsule) (22). The device was tested on dogs showing adequate maneuverability but some difficulties exist in maintaining its position with peristalsis and postural changes and a limited life battery and poor motor force. Nevertheless the evaluation of the stomach and colon was feasible. If refined the remote-control capsule system will increase the ability to screen the GI tract without invasion.
Endoscopic ultrasound (EUS)
With the advent of EUS, new frontiers in GI tumors diagnosis were gained. By combining endoscopy and ultrasound an accurate stadiation of GI cancer was possible and thanks to linear echoendoscope also tissue sampling was possible. Recently, the use of contrast enhanced imaging and elastography increased the diagnostic performance of EUS. At the same time the role of other techniques such as the radiologic approaches and even endoscopic retrograde cholangiopancreatography (ERCP) progressively declined. EUS has been established as a valuable diagnostic modality in detecting and staging malignancies. In addition EUS-guided fine needle aspiration (EUS-FNA) is routinely used for the evaluation of pancreatic masses. Recently Wani et al. (23) showed that EUS-FNA diagnostic yield of pancreatic masses is improved by the presence of an on-site cytopathologist. EUS is also employed for the evaluation of pancreatic cystic lesions that have a poorly understood natural history and a controversial management. Regarding this latter in 2012 the International Association of Pancreatology published modified consensus guidelines (e.g., modified Sendai guidelines), for the management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms. Two studies attempting to validate the Sendai guidelines performing EUS produced conflicting conclusions. The first by Palta et al. (24) concluded that the EUS findings of Sendai guidelines lack sensitivity for the detection of malignancy in pancreatic cystic neoplasms. The second study (25) concluded that the patients with “high-risk” features as defined by the Sendai guidelines (jaundice, pancreatic duct 35 mm in diameter, cyst 330 mm in size, and the presence of a mural nodule), have a high rate of the development of pancreatic cancer. These two studies produced conflicting conclusions and new data are needed.
Single-incision needle-knife (SINK) biopsy
The diagnostic ability of EUS-FNA is often limited by insufficient tissue for immunohistochemistry. Additional experiences with established endoscopic techniques to obtain tissue in submucosal tumors of the stomach were recently reported. De la Serna et al. (26) evaluated the role of SINK biopsy for histopathological examination of gastric subepithelial tumors. The lesions first underwent EUS for evaluation of size and morphological characteristics. A pulsed Doppler scan was performed to scan for blood vessels in the area of the tumor. A 6- to 12- mm linear incision was made on the highest protrusion of the subepithelial tumor using a needle-knife. A regular biopsy forceps was introduced through this incision to obtain 3 to 5 biopsy bites for histopathological evaluation. Prophylactic clips were placed over the incision only in the first patients. SINK biopsy was diagnostic in 93% while FNA was diagnostic in only 12.5% of the patients, the authors concluded that SINK biopsy is easy and safe, has a high histological yield and may represent a reliable alternative to EUS-FNA in smaller subepithelial lesions.
Tumor suck ligate unroof biopsy
Another technique to diagnose and treat small subepithelial lesions was described in 16 patients by Binmoeller et al. (27). This approach involves the suction of the lesion into a cap, ligation below the tumor, unroofing of the mucosa overlying the subepithelial tumor with a needle-knife, and biopsies from exposed tumor suck ligate unroof biopsy. One patient suffered of abdominal pain. No other adverse events were noted. This pilot study shows that this new technique may be safe and effective in obtaining sufficient tissue for immunohistochemistry.
New technology in therapy of GI cancers
Natural orifice transluminal endoscopic surgery technique (NOTES)
Pure NOTES has attracted great interest from both surgeons and gastroenterologists. For this reason, in 2005 the American Society for Gastrointestinal Endoscopy (ASGE) and the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) came together in the Natural Orifice Surgery Consortium for Assessment and Research (NOSCAR). This minimally invasive surgery can be performed with an endoscope passed through a natural orifice (e.g., mouth, anus) then through an internal incision in the stomach, vagina, bladder or colon, thus completely eliminating the need for skin incision. Potential main advantages of NOTES include lower anaesthesia requirements, faster recovery and shorter hospital stays, avoidance of the potential complications of transabdominal wound infections, better postoperative pulmonary and diaphragmatic function. Critics challenge the safety and advantages of this technique in the face of effective minimally invasive surgical options such as laparoscopic surgery. NOTES was originally performed in animals by researchers at Johns Hopkins University and was recently used for transgastric appendectomy in humans (28). In 2007 Swanstrom and colleagues reported the first human transgastric cholecystectomy (29). In the last years, NOTES has ranged from diagnostic explorations of the peritoneal cavity to complex organ resections including pancreatectomy, splenectomy, cholecystectomy and nephrectomy. Technologies growing from the concept of NOTES may resolve an array of challenges currently associated with endoscopic surgery and flexible endoscopy. The feasibility of endoscopic transgastric gastrojejunostomy (30) and pure NOTES rectosigmoidectomy using transgastric endoscopic inferior mesenteric artery (IMA) dissection and transanal rectal mobilization were showed feasible in animal models (31). To improve NOTES a critical need is the ability to triangulate. This year the Endomina system (MEDI-LINE SA, Liege, Belgium), a new universal triangulation platform adaptable to a flexible endoscope was created to allow an easier endoscopic submucosal dissection (ESD) by lift and cut and oppose and suture tissue (32). The system has been tested in animal models, and recently in humans. In addition, a wireless tissue palpation system for tactile and kinaesthetic feedback created a mechanical link between the external platform and the target region (33). Finally an endoscopic suturing device, capable of performing interrupted or continuous sutures by using a double-channel endoscope (Overstitch, Apollo Endosurgery, Austin, TX, USA), has been tested in humans to close large mucosal defects after ESD eliminating the need for hospital admission with encouraging results (34).
Submucosal tunneling
The development of peroral endoscopic myotomy (POEM) has opened up a new discipline of submucosal endoscopic surgery. With this approach, there is feasibility of removing submucosal tumors arising from the muscularis propria by using the submucosal tunneling technique. Several studies suggest that by using the submucosal tunneling technique it’s feasible to remove submucosal tumors arising from the muscularis propria such as tumors at the esophagogastric (EG) junction. A preliminary study (35) has shown a 100% successful en bloc resection of the EG junction for leiomyomas or GI stromal tumors with the submucosal tunneling technique (negative lateral and deep tumor-free margins in all cases). The technique was showed safe (the only complication was pneumothorax in two patients treated by chest tubes) and there was no local recurrence or distant metastases during 12 months follow-up. These results were confirmed by another study described tunneling to resect submucosal tumors in 151 consecutive patients with GI stromal tumors and leiomyomas, mostly located in the esophagus or EG junction (36). The en bloc resection rate was 86% with complete R0 resection in 83% and an adverse event rate of 35.5% (pneumothorax subcutaneous/mediastinal air and pneumoperitoneum). The mean procedure time was one hour. This technique offers a less invasive technique compared with surgery but needs to be further investigated.
EUS-guided therapies
Interventional EUS is a very promising technique with many potential applications. Since the introduction of EUS-FNA in 1992 (37), numerous novel EUS-based interventions and techniques have emerged. Currently, established interventional EUS techniques include celiac plexus block and neurolysis, drainage of pancreatic pseudocysts and pelvic fluid collections. Emerging EUS-guided experimental techniques include antitumor injection, ablation of tumors, and vascular access. The use of EUS to guide novel therapies is gaining momentum. There have been multiple trials investigating EUS-guided injection therapy (fine-needle injection) primarily in pancreatic cancer, but all have failed to show benefit in survival. Recently new encouraging data have been shown, but we must wait for results from an ongoing phase 2 study (38). New results in EUS-guided intravascular therapy have been shown for the treatment of liver metastases secondary to colorectal cancer (39) by the injection of 5-flurouracil or 5-fluorodeoxymidina into the hepatic artery under EUS guidance. The future holds promise for substantial progress in EUS-guided therapeutic interventions and their applications in clinical gastroenterology.
Endobiliary radiofrequency ablation (RFA)
Until recently, endoscopic palliation of malignant biliary obstruction included placement of plastic stents or self-expandable metal stents (SEMSs). Endobiliary RFA is a recent therapeutic modality that can be used as primary therapy in unresectable biliary malignancies or to treat occluded uncovered biliary SEMSs because of tumor ingrowth. Kahaleh et al. (40) suggest that endobiliary RFA has a defined role in the palliation of malignant biliary strictures, although further long-term trials are needed. They performed endobiliary RFA with the Habib EndoHPB catheter (EMcision, London, UK) in 40 patients with malignant biliary obstruction. After RFA plastic stents or SEMSs were placed on the strictures. RFA appeared to be efficacious and safe (the major postprocedural complications were pancreatitis/cholecystitis).
Although endobiliary RFA appears efficacious and safe, it is unknown whether this provides any survival benefit for patients. A recent study by Kallis et al. (41) suggests that endobiliary RFA may have a potential early survival benefit in patients with biliary obstruction secondary to unresectable pancreatic cancer. The addition of endobiliary RFA to the therapeutic armamentarium for treatment of malignant biliary strictures would certainly be very important since the photodynamic therapy (PDT) for hilar cholangiocarcinoma showed some advantages but also important limitations such as costs and availability, photosensitivity, and repeated treatment sessions. A retrospective small size study by Strand et al. (42) suggested that there is no survival benefit in patients with unresectable cholangiocarcinoma who undergo ERCP-directed RFA compared with similar patients who undergo ERCP-directed PDT but a follow-up randomized, controlled trial to validate these results is needed. The chosen therapeutic intervention for unresectable cholangiocarcinoma, either PDT or RFA, may ultimately be institution or physician dependent; however, these preliminary results suggest that survival is similar.
A recent pilot study (43) successfully demonstrates the safety and efficacy of EUS-guided RFA of pancreatic cysts in a small set of patients with pancreatic cystic neoplasms and neuroendocrine tumors. Using a 19- or 22-gauge needle performed EUS-FNA, and then a novel RFA probe was passed through the needle and then used to treat with varying wattages in different patients. The study showed a decrease in cyst size (38.8 vs. 20 mm) after RFA and a change in vascularity or an area of necrosis in the neuroendocrine tumors. The only complication was abdominal pain that resolved in 3 days.
RFA of esophageal tumors
RFA was recently advocated as treatment for early squamous cell cancer (SCC) of the esophagus but the results are not conclusive. RFA as a single treatment seems to be insufficient for SCC/squamous cell dysplasia and should only be performed after endoscopic resection of the suspicious lesion. SCC in fact is a very aggressive disease, and endoscopic resection of SCC or squamous cell dysplasia is important for staging purposes. Additional RFA might reduce the risk of recurrence or metachronous neoplasia.
Previous studies showed that RFA of high-grade dysplasia and intramucosal cancer in people with Barrett’s esophagus is safe and effective. However, the durability of RFA therapy has not been well understood. The risk of esophageal adenocarcinoma (EAC) in this set of patients is about 0.5 percent per year (44). Typically, before EAC develops, precancerous cells [low grade dysplasia (LGD) or high grade dysplasia (HGD)] appear in the Barrett’s tissue. Although endoscopic eradication therapy has become the standard of care for patients with HGD and intramucosal cancer, endoscopic therapy of all patients with low-grade dysplasia LGD remains controversial. Recent guidelines suggest that the option to endoscopically treat patients with LGD should be a shared decision between the patient and the physician after thoroughly weighing the risks and benefits of the procedure. A multicenter, randomized, controlled trial by Phoa et al. compared surveillance with RFA in the management of patients with confirmed LGD (45). The RFA group underwent treatment every two to three months with a maximum of five sessions with subsequent post ablation yearly endoscopic surveillance for three years. The authors conclude that RFA is effective in reducing progression to HGD/EAC.
Endoscopic full-thickness resection (EFTR) of colonic submucosal tumors originating from the muscularis propria
Endoscopic mucosal resection (EMR) and ESD are widely used techniques for en bloc resection of superficial GI carcinomas and premalignant lesions from, respectively, the mucosal and submucosal layers (46). However, these techniques are suboptimal for resecting subepithelial tumors originating from the muscularis propria. Colonic ESD is considered technically even more difficult than gastric ESD because of the thin walls, narrow lumen and acute angulations in the colon (46). The muscularis propria in the colon is thin, and colonic submucosal tumors (SMTs) are usually adherent to the serosa with an increased risk of perforation and failure to achieve R0 resection margins. EFTR is a novel method enabling resection of SMTs, which traditionally are managed by colonic resection. This new technique consists in resecting the tumor without interrupting the tumor capsule and with active perforation. At the end the defect will close with a nylon loop allowing the endoscopic closure of colonic wall mucosal defect. A prospective pilot study has shown the feasibility and safety of EFTR of colonic SMTs combined with standard metallic clips (47). Newly developed endoscopic clipping and sewing devices such as the over-the-scope clip and the OverStitch suturing device should increase the safety of the colonic EFTR procedure but needs to be investigated. EFTR could have a great impact in the management of intestinal GISTs that are more aggressive than gastric GISTs of same size and have a benign to malignant ratio of 1 to 2 (48). Recent guidelines by the National Comprehensive Cancer Network, in fact, recommend that all GISTs larger than 2 cm should be resected while the treatment options for incidental tumors smaller than 2 cm are resection or surveillance (49). Surveillance, however, may result in delayed diagnosis of malignancy. EFTR in these patients could offer an alternative option to traditional surgical management.
Magnetic compression anastomosis for minimally invasive colorectal surgery (MAGNAMOSIS)
MAGNAMOSIS system has proven to be effective in full-thickness porcine small-bowel anastomoses (50). MAGNAMOSIS forms a compression anastomosis using self-assembling magnetic rings that can be delivered via flexible endoscopy. The system allowed a hybrid endoscopic colorectal anastomosis (NOTES) with three abdominal trocars instead of conventional stapled anastomoses. It has the advantage over circular staplers of precise endoscopic delivery throughout the entire colon. The device is still undergoing design optimization but is a promising technology that enables both minimally invasive and NOTES approaches to colorectal surgery.
Conclusions
Endoscopy is a keystone in modern gastroenterology and thanks to its progress diagnosis and therapy of GI cancers completely changed in the last years. Endoscopy development is linked to different field such as technology, advances in knowledge of digestive diseases, evolution of disciplines such as radiology and oncology, and last but not least laws and costs. Since technology brings with it obsolescence in few times endoscopic technology becomes outmoded as well as the fibreoptic endoscope. Technology, especially if costly, improves outcomes but requires further and better data with statistical power. Evolution of technology in endoscopy will progressively eliminate the traditional boundaries between medicine and surgery. Endoscopy rooms will resemble operating rooms, the complexity of endoscopic procedures will progressively increase and the distinction between specialist and generalist endoscopist will become more definite.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Killian G.. The history of bronchoscopy and esophagoscopy. Laryngoscope 1911;21:891-7.
- Hopkins HH, Kapany NS. A flexible fiberscope using static scanning. Nature 1954;173:39-41.
- Hirschowitz BI, Curtiss LE, Peters CW, et al. Demonstration of a new gastroscope, the fiberscope. Gastroenterology 1958;35:50; discussion51-3.
- Dimagno EP, Buxton JL, Regan PT, et al. Ultrasonic endoscope. Lancet 1980;1:629-31. [PubMed]
- Bories E, Caillol F, Pesenti C, et al. Frontiers in interventional endoscopy. Bull Cancer 2007;94:1091-8. [PubMed]
- Sivak MV Jr. EUS: past, present, and the future of endoscopy. Gastrointest Endosc 2002;55:446-8. [PubMed]
- Kiesslich R, Burg J, Vieth M, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal Cancer in vivo. Gastroenterology 2004;127:706-13. [PubMed]
- Neumann H, Kiesslich R, Wallace MB, et al. Confocal laser endomicroscopy: technical advances and clinical applications. Gastroenterology 2010;139:388-92, 392.e1.
- Kiesslich R, Goetz M, Neurath MF. Confocal laser endomicroscopy for gastrointestinal diseases. Gastrointest Endosc Clin N Am 2008;18:451-66. [PubMed]
- Polglase AL, Mclaren WJ, Skinner SA, et al. A fluorescence confocal endomicroscope for in vivo microscopy of the upper- and the lower-GI tract. Gastrointest Endosc 2005;62:686-95. [PubMed]
- Kuiper T, van den Broek FJ, van Eeden S, et al. New classification for probe-based confocal laser endomicroscopy in the colon. Endoscopy 2011;43:1076-81. [PubMed]
- Li CQ, Xie XJ, Yu T, et al. Classification of inflammation activity in ulcerative colitis by confocal laser endomicroscopy. Am J Gastroenterol 2010;105:1391-6. [PubMed]
- Kiesslich R, Goetz M, Lammersdorf K, et al. Chromoscopy-guided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology 2007;132:874-82. [PubMed]
- Günther U, Kusch D, Heller F, et al. Surveillance colonoscopy in patients with inflammatory bowel disease: comparison of random biopsy vs. targeted biopsy protocols. Int J Colorectal Dis 2011;26:667-72. [PubMed]
- Marion JF, Waye JD, Present DH, et al. Chromoendoscopy-targeted biopsies are superior to standard colonoscopic surveillance for detecting dysplasia in inflammatory bowel disease patients: a prospective endoscopic trial. Am J Gastroenterol 2008;103:2342-9. [PubMed]
- Ang TL, Pittayanon R, Ang D, et al. 420 a multi-center randomized controlled trial of high definition white light endoscopy versus high definition narrow band imaging for the detection of focal gastric lesions. Gastrointest Endosc 2013;77:AB152.
- Bashar J, Qumseya BJ, White DL, et al. The diagnostic yield for detection of Barrett’s dysplasia by advanced imaging with targeted biopsies compared to conventional imaging with random biopsies: meta analysis and systematic review. Gastrointest Endosc 2013;77:AB11.
- Kumar S, Fioritto A, Mitani A, et al. Optical biopsy of sessile serrated adenomas: do these lesions resemble hyperplastic polyps under narrow-band imaging? Gastrointest Endosc 2013;78:902-9. [PubMed]
- Backman V, Roy HK. Light-scattering technologies for field carcinogenesis detection: a modality for endoscopic prescreening. Gastroenterology 2011;140:35-41. [PubMed]
- Roy HK, Mutyal NN, Goldberg MJ, et al. Microvascular biomarkers in the endoscopically normal rectal mucosa for colon neoplasia risk stratification: results of a training/validation dataset. Gastroenterology 2013;144:S116.
- Gralnek IM, Segol O, Suissa A, et al. Mo1672 A prospective feasibility study in human subjects evaluating a novel colonoscope featuring “full spectrum view”. Gastrointest Endosc 2013;77:AB466-7.
- Ohta H.. Mo1669 the dawn of internet linked robotic capsule endoscopy; a radio-controlled and motor-driven capsule endoscope that can be controlled via the internet. Gastrointest Endosc 2013;77:AB465-6.
- Wani S, Drahos J, Cook MB, et al. Comparison of endoscopic therapies and surgical resection in patients with early esophageal cancer: a population-based study. Gastrointest Endosc 2014;79:224-232.e1.
- Palta R, Wu BU, Mehta S, et al. 194 Evaluation of the revised Sendai 2012 guidelines for EUS-based investigation of pancreatic cysts: a multi-center study. Gastrointest Endosc 2013;77:AB133.
- Lawson RD, Hunt GC, Master SS, et al. 195 Validation of 2012 modified sendai criteria for predicting risk of Cancer development in pancreatic cysts. Gastrointest Endosc 2013;77:AB133-4.
- De la Serna C, Diez-Redondo P, Peñas I, et al. 142 Diagnostic yield and safety of EUS-guided single-incision needle-knife (SINK) biopsy for cyto-histological tissue diagnosis in upper gastrointestinal subepithelial lesions. Gastrointest Endosc 2013;77:AB128.
- Binmoeller KF, Kato M, Shah JN, et al. 60 “Suck-Ligate-Unroof-Biopsy” (Slub) using a mini-detachable loop for the diagnosis and therapy of small broad-based subepithelial tumors. Gastrointest Endosc 2013;77:AB124-5.
- Ko CW, Kalloo AN. Per-oral transgastric abdominal surgery. Chin J Dig Dis 2006;7:67-70. [PubMed]
- Swanstrom LL, Kozarek R, Pasricha PJ, et al. Development of a new access device for transgastric surgery. J Gastrointest Surg 2005;9:1129-36; discussion1136.
- Song TJ, Seo DW, Kim SH, et al. Endoscopic gastrojejunostomy with a natural orifice transluminal endoscopic surgery technique. World J Gastroenterol 2013;19:3447-52. [PubMed]
- Park SJ, Lee KY, Choi SI, et al. Pure NOTES rectosigmoid resection: transgastric endoscopic IMA dissection and transanal rectal mobilization in animal models. J Laparoendosc Adv Surg Tech A 2013;23:592-5. [PubMed]
- Cauche N, Hiernaux M, Chau A, et al. Endomina: the endoluminal universal robotized triangulation system: description and preliminary results in isolated pug stomach. Gastrointest Endosc 2013;77:AB204-5.
- Beccani M, Di Natali C, Valdastri P, et al. 705 Restoring tactile perception in natural orifice transluminal endoscopic surgery (NOTES) through wireless tissue palpation. Gastrointest Endosc 2013;77:AB163-4.
- Kantsevoy S, Bitner M, Turnbough L, et al. Sa1389 Endoscopic closure of large mucosal defects post endoscopic submucosal dissection (ESD) eliminates the need for hospital admission. Gastrointest Endosc 2013;77:AB187-8.
- Wang XY, Xu MD, Yao LQ, et al. Sa1396 Submucosal tunnel endoscopic resection for submucosal tumors of the esophagogastric junction originating from the muscularis propria layer: a feasibility study. Gastrointest Endosc 2013;77:AB190-1.
- Xu MD, Chen T, Yao L, et al. Sa1407 submucosal tunneling endoscopic resection for Upper-GI submucosal tumors: a feasibility study of 152 consecutive cases. Gastrointest Endosc 2013;77:AB194-5.
- Das KM, Kochhar R, Gupta NM, et al. Ultrasound-guided fine needle aspiration cytology of carcinoma involving the intra-abdominal oesophagus. Clin Radiol 1992;45:185-6. [PubMed]
- Repaka A, Poplin EA, August DA, et al. 350 Phase I Trial of Endoscopic Ultrasound (EUS) Guided Intratumoral Vaccination With Recombinant Panvac-F and Systemic Panvac-V in Patients With Locally Advanced Pancreatic Cancer. Gastrointest Endosc 2013;77:AB143.
- Artifon EL, Cunha MA, Da Silveira EB, et al. 349 EUS-Guided or interventional radiology to hepatic Intra-Arterial chemotherapy: a prospective trial. Gastrointest Endosc 2013;77:AB142-3.
- Kahaleh M, Sharaiha RZ, Widmer JL, et al. Radiofrequency ablation of malignant biliary strictures: results of a collaborative registry. Gastrointest Endosc 2013;77:AB141.
- Kallis Y, Phillips N, Steel A, et al. OC-075 analysis of long-term outcomes after endoscopic radiofrequency ablation for bile duct strictures in pancreatic malignancy suggests potential survival benefit. Gut 2013;62:A32.
- Strand DS, Cosgrove N, Patrie JT, et al. 290 ERCP-Directed radiofrequency ablation and photodynamic therapy are associated with comparable survival in the treatment of unresectable cholangiocarcinoma. Gastrointest Endosc 2013;77:AB140-1.
- Pai M, Senturk H, Lakhtakia S, et al. 351 endoscopic ultrasound guided radiofrequency ablation (EUS-RFA) for cystic neoplasms and neuroendocrine tumors of the pancreas. Gastrointest Endosc 2013;77:AB143-4.
- Spechler SJ, Souza RF. eds. Barrett esophagus and esophageal adenocarcinoma. Textbook of Gastroenterology. Vol. 1. West Sussex, UK: Wiley-Blackwell, 2009:826-48.
- Phoa N, Pouw R, Bisschops R, et al. 282 Radiofrequency ablation combined with endoscopic resection is highly effective for eradication of early Barrett’s neoplasia: final results of a large prospective European multicenter study (EURO-II). Gastrointest Endosc 2013;AB137.
- Matsuda T, Gotoda T, Saito Y, et al. Our perspective on endoscopic resection for colorectal neoplasms. Gastroenterol Clin Biol 2010;34:367-70. [PubMed]
- Xu M, Wang XY, Zhou PH, et al. Endoscopic full-thickness resection of colonic submucosal tumors originating from the muscularis propria: an evolving therapeutic strategy. Endoscopy 2013;45:770-3. [PubMed]
- Lai EC, Lau SH, Lau WY. Current management of gastrointestinal stromal tumors--a comprehensive review. Int J Surg 2012;10:334-40. [PubMed]
- Demetri GD, von Mehren M, Antonescu CR, et al. NCCN task force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 2010;8:S1-41; quizS42.
- Wall J, Diana M, Leroy J, et al. MAGNAMOSIS IV: magnetic compression anastomosis for minimally invasive colorectal surgery. Endoscopy 2013;45:643-8. [PubMed]


