The role of the gastrointestinal tract in the control of energy balance
Introduction
The human body is endowed with a complex physiological system that maintains a relatively constant body weight, despite wide variations in daily energy intake and energy expenditure. Signals from peripheral tissues and organs that participate in this regulatory mechanism usually originated from the gastrointestinal tract [(GIT), including pancreas], and adipose tissue. The hypothalamus—particularly the arcuate nucleus (ARC), and the brain—particularly the nucleus of the solitary tract (NTS), are the main sites of convergence and integration of central and peripheral signals that regulate energy balance (1-3). Traditionally, the primary role of GIT peptides was considered to be limited to “satiety signals”, delivering short-term messages to the brain for the beginning and end of a meal and for the physiological events occurring between meals. However, GIT peptides also influence gastric-emptying, gut motility and nutrient utilization, all contributing to long-term body weight control (4,5). The GIT hormones can act directly in the ARC nucleus by crossing the blood-brain barrier (BBB) or interfering with brain endothelial cells, or they can act indirectly, by binding to vagal and spinal nerves, giving rise to afferent signals that reach the NTS before transmission to the ARC nucleus (1-5).
The global obesity epidemic and its associated burden of morbidities have brought greater urgency to understanding the processes of energy balance. Appetite and metabolic regulation by GIT hormones and intestinal microbiota offer an integrated model of the brain-gut connection involving both endocrine and neuronal systems. In this article, we review the current knowledge on the main gut hormones, their role in energy and glucose homeostasis, and their potential utility in the treatment of obesity and metabolic syndrome (MS). In addition, we highlight new findings on the potential implications of intestinal microbiota on energy homeostasis.
GIT-brain axis
As part of a major endocrine system, the GIT secretes several hormones responsible for different physiological actions, such as GIT functioning, appetite and satiety regulation, nutrient digestion, absorption and distribution, and energy homeostasis.
Central effects of gut hormones are mainly due to their actions in two distinct neuron populations within ARC nucleus of the hypothalamus: (I) anorexigenic neurons that express pro-opiomelanocortin and cocaine- and amphetamine-related transcript (POMC/CART); and (II) orexigenic neurons that express neuropeptide Y and Agouti-related peptide (NPY/AgRP). These primary targets communicate with second-order neurons in other hypothalamic nuclei [especially paraventricular nucleus (PVN), dorso-medial (DMN), ventro-medial (VMN) and lateral hypothalamus (LH)], where peripheral information is integrated with behavioral, hormonal and nutritional inputs coming from the periphery and higher cortical centers. Several hypothalamic neurotransmitters produced in these regions, including the corticotropin releasing hormone (CRH), thyrotropin releasing hormone (TRH), the melanin concentrating hormone (MCH), brain-derived neurotrophic factor (BDNF), oxytocin and orexins, are all strongly involved in energy homeostasis. These orexigenic and anorexigenic neuronal groups are connected at several locations in the central nervous system (CNS), in such a manner that the activation of one group inhibits the other and vice-versa (3-6). The BBB is critical for adequate function of the GIT-brain axis, but the molecular mechanisms involved with the GIT-derived hormones transportation into the brain are still largely unknown (6). Gut hormones can also bind to receptors in gastric vagal afferent neurons, generating signals to the NTS and, from there, to the ARC nucleus. Besides the hypothalamic circuits, some GIT-derived hormones, such as ghrelin, have been found to affect mesolimbic pathways related to reward, feeding behavior and food preference as part of the hedonic system (7).
The GIT-derived hormones cholecystokinin (CCK), peptide YY (PYY), pancreatic polypeptide (PP), oxyntomodulin (OXM), gastric inhibitory polypeptide (GIP) and glucagon-like peptide 1 (GLP-1) act as anorexigenic factors, reducing food intake and increasing energy expenditure. They are released in the postprandial periods and suppressed during fasting or before meals. In contrast, ghrelin is the only GIT-derived hormone with orexigenic properties, promoting meal initiation and increasing food intake (3-6). Table 1 summarizes the main GIT-derived hormones affecting energy balance with their sites of production, receptors, and main biological effects.
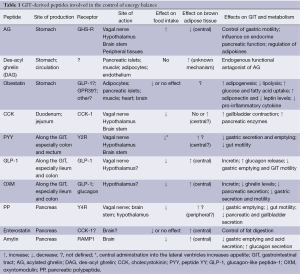
Full table
Gastrointestinal and pancreatic peptides
Cholecystokinin (CCK)
Studies from the seventies in the last century were the first to correlate gastrointestinal peptides with the regulation of energy balance (8). CCK, a peptide predominantly produced in the duodenum and jejunum during the immediate postprandial period, was shown to be involved in the control of appetite by acting on the afferent vagal nerve fibers and/or directly on hypothalamic nuclei. CCK reduces food intake, meal size and meal duration when administered to both rodents and humans, by binding to CCK-1 (CCK-A) receptors expressed along the GIT. Moreover, CCK is also locally produced in the brain, where it exerts its effect by binding to CCK-2 (CCK-B) receptors on cerebral areas involved in reward, memory and satiety (4,5). In the GIT, CCK simulates gallbladder contraction and secretion of pancreatic enzymes involved in the digestive process (6). There are also indications that CCK might also stimulate brown adipose tissue (BAT) activity via central mechanisms (9,10). However, the potential role of CCK as a therapeutic target for obesity has been questioned by studies that report that animals do compensate the reduction in food intake by increasing the number of meals, without showing a change in body weight (11). In addition, studies in obese subjects with CCK1R agonists have failed to show any weight loss (5,12). Nevertheless, the initial findings with CCK paved the way to investigate a system that recognizes the presence of food in the GIT, signaling to the brain via neuronal and endocrine mechanisms to regulate appetite and satiety. Since then, several gastrointestinal peptides have been discovered and their therapeutic potential in physiological or pharmacological concentrations has been tested in both animal and humans studies.
Ghrelin
Ghrelin is a 28-amino acid peptide predominantly produced by oxyntic cells of the stomach, and in smaller amounts, in the intestine, pancreas and other tissues (13). Discovered in 1999 by Kojima et al. (14) as the natural ligand of the growth hormone secretagogue receptor (GHS-R), ghrelin stimulates growth hormone (GH) secretion by the pituitary and exerts other neuroendocrine activities. In the energy balance, ghrelin plays an important role as the only known peripheral hormone with orexigenic properties. In healthy volunteers, intravenous and subcutaneous administration of ghrelin induces a 30% increase in food intake (15). Ghrelin and GHS-R knock-out animals have no significant changes in food intake and body weight when fed with standard chow (16,17). On the other hand, chronic administration of ghrelin causes hyperphagia and obesity in rodents (18). In addition, lack of ghrelin and GHS-R make the animals resistant to diet-induced obesity and favors the use of fat as energy substrate, when these animals are subjected to a high-fat diet (17,19). In ob/ob mice, the absence of ghrelin attenuates the diabetic phenotype, but not the obesity, supporting a role for ghrelin in glucose metabolism (20). Central actions of ghrelin include inhibition of BAT activity (10).
In circulation, ghrelin is present in two forms: (I) acylated ghrelin (AG) that contains an n-octanoic acid at serine-3 position, which is essential for binding and activation of the GHS-R and for the modulation of its neuroendocrine and orexigenic effects; (II) des-acyl ghrelin (DAG) that is the most abundant form in circulation and that is unable to bind and activate GHS-R at physiological concentrations; ghrelin is acylated by the enzyme ghrelin o-acyltransferase (GOAT), which is co-expressed together with the ghrelin gene in the stomach. The GHS-R is widely distributed in the body, with high expression levels in the hypothalamus (especially in the ARC, PVN and LHA) and pituitary, and low expression levels in other brain areas as well as in peripheral tissues, particularly in the endocrine pancreas, GIT, immune cells and the heart (3-5,13). GHS-R present in brain areas is involved in reward, emotion and memory, indicating that ghrelin plays also a role in increasing desire for food intake and non-homeostatic feeding behaviors (7).
Ghrelin induces an increase in food intake by acting at the ARC nucleus, working as a functional antagonist of leptin actions, which results in a stimulation of NPY neurons and an inhibition of POMC neurons activity. The orexigenic actions of ghrelin occur independently of its effects on GH secretion. Ghrelin reaches the hypothalamus via the blood stream and the brain stem through vagal innervation (3-5,13). While ghrelin suppression after meal seems not to be related to vagal signaling, it has been reported that the increase in ghrelin levels during fasting can be completely abolished by subdiaphragmatic vagotomy, suggesting that the integrity of the vagal nerve is crucial for the orexigenic effects of ghrelin (21).
Serum ghrelin concentrations vary widely throughout the day, with higher values during sleep, and elevations in the pre-prandial periods followed by falls after meals. This pattern of secretion gave ghrelin its nickname as a “hunger hormone”, responsible for the meals initiation (22-24). However, it is more likely that the physiological role of ghrelin is to prepare the body for an influx of metabolic energy (5). The ghrelin secretion differs between lean and obese, which might have some relevance for the pathogenesis of obesity. Plasma ghrelin levels are decreased in obese individuals and the postprandial dips in ghrelin concentrations are proportional to energy intake in lean, but not in obese subjects (25,26). Moreover, obesity is associated with a much lower reduction of postprandial ghrelin levels and absence of nocturnal elevation as seen in subjects of normal weight (27). An exception to this rule is the Prader-Willi syndrome, in which obesity is associated with increased plasma ghrelin concentrations (28).
Gastric secretion of ghrelin may be influenced by numerous factors, such as administration of glucose and insulin, activation of somatostatin receptors, the cholinergic system, GLP-1, PYY, OXM, thyroid hormones (TH) and testosterone (13). Changes in body weight are accompanied by changes in ghrelin levels, which increase after weight loss and decrease after weight gain. These changes seem to be controlled by mTOR phosphorylation in gastric tissue, which might function as a peripheral fuel sensor to modulate ghrelin expression (29). Interestingly, it has recently been suggested that the intracellular mTOR pathway might be a link between ghrelin and the endogenous cannabinoid system (CB1), an important physiological system involved in energy balance regulation. Pharmacological blockade of CB1 receptors expressed in gastric cells is sensed as a satiety signal comparable to food intake, resulting in mTOR pathway activation, which in turn decreases ghrelin secretion with a subsequent inhibition of the orexigenic message communicated to the brain through the vagal nerve (30). Taken together, these data show a great potential for targeting the stomach-brain communication axis for future drug developments. In this way, ghrelin antagonists and receptor blockers have received special attention as anti-obesity agents as well as for therapeutic use in other nutritional disorders such as anorexia nervosa and cachexia (4).
DAG and obestatin
As previously mentioned, DAG does not bind and activate the GHS-R and in virtue of that, it has long been considered a physiologically inactive degradation product of AG. However, recent studies have called attention to AG-independent effects of DAG through a specific, unidentified receptor, or via another unknown ghrelin receptor. There is also evidence that DAG could act as a endogenous functional AG-antagonist (31). DAG transgenic mice show a decrease in body weight, food intake, fat pad mass, and gastric emptying, along with a moderately decreased linear growth (32). In rodent models, DAG has been associated with restoration of endothelial progenitor cell function, increased muscle regeneration and vascular remodeling (31). In both humans and animal models, a relative DAG deficiency, characterized either by lower DAG levels or increased AG/DAG ratios, has been linked to obesity, diabetes and MS. Intravenous DAG administration improves insulin sensitivity and displays hypoglycemic properties in both healthy volunteers and overweight diabetic patients (31). Moreover, BAT is activated by DAG via an uncertain mechanism (10).
The gene encoding ghrelin also encodes another peptide called obestatin (33). As for DAG, obestatin exhibits antidiabetogenic activities, its serum levels are decreased in the MS and an obestatin receptor has not been identified yet. The receptor for GLP-1 and the orphan G-protein-coupled receptor GPR39 have been suggested as possible candidates, but additional studies are required to sustain their roles in the obestatin effects (33). In the adipose tissue, obestatin stimulates adipogenesis, inhibits lipolysis, and promotes glucose and fatty acid uptake. Mice treated with obestatin show elevated adiponectin, reduced leptin levels and reduced proinflammatory cytokine release from fat, muscle and liver. Obestatin exhibits survival effects in different cell types, such as pancreatic β-cells, muscle and cardiomyocytes. The central actions of obestatin are more controversial, with some studies reporting on suppression of food intake and reduced body weight, while others could not demonstrate these effects (33).
Peptide YY (PYY)
PYY is structurally related to PP and NPY and all belong to the polypeptide-fold protein family, which is characterized by a hairpin turn within the 36-amino acid backbone, and a C-terminal amidation. PYY is produced by entero-endocrine L-cells throughout the GIT, with tissue concentrations that increase along the GIT, reaching higher levels in the colon and rectum. It is also expressed in neurons located in the gigantocellular reticular nucleus of the rostral medulla. In the GIT itself, PYY inhibits secretion, gut motility and gastric emptying (5,34,35).
PYY is present in two forms: PYY1-36 and PYY3-36. The predominant form of PYY, as it is stored in intestinal cells (together with GLP-1), is PYY1-36, which is released into circulation and cleaved by the enzymatic action of dipeptidyl peptidase (DPP-IV) to give rise to the active form PYY3-36, which is truncated at the N-terminus. PYY secretion is proportional to the caloric content, macronutrient composition and consistency of the food, with serum concentrations increasing rapidly in the first 2 hours after meal and remaining high up to 6 hours. PYY secretion is mediated by neural reflexes and by direct contact to nutrients (12,34,35). In humans, high-carbohydrate, low-fat diet was associated with the highest levels of PYY3-36, whereas in rodents, protein intake provided the most potent stimulus for its release (4). Obese subjects have reduced basal PYY3-36 levels and show a blunted response after meal. In contrast, fasting PYY3-36 levels are elevated in anorexia nervosa, after gastric bypass surgery for obesity, and in other conditions associated with reduced appetite (34,35).
Peripheral administration of physiological doses of PYY3-36 leads to a significant reduction in food intake in rodents. In contrast, central administration of PYY3-36 into the lateral ventricles strikingly increases appetite, showing that PYY3-36 exerts both anorectic and orexigenic actions depending on the receptor type expressed in different regions of the brain (35). In normal weight volunteers, intravenous PYY3-36 reduces energy intake by 30% (36). In obesity, circulating levels of PYY3-36 are relatively low and its postprandial secretion is deficient in comparison with lean individuals. However, administration of PYY3-36 in obese individuals may also result in reduced appetite and caloric intake. It is possible that the reduction in food intake by PYY3-36 in humans might be, at least in part, secondary to its side effects as nausea and aversion to food (35-37).
PYY3-36 crosses the BBB and its mechanism of action involves binding to the Y2-pre-synaptic inhibitory receptors expressed in both vagal afferent neurons and NPY/AgRP neurons in the ARC nucleus (6,34,35). Intact vagal afferent signaling from the gut to the brain stem is essential for the anorectic effects of systemically administered PYY3-36, as evidenced by the lack of such an effect in vagotomized rats (38,39). Direct actions of PYY3-36 on hypothalamic neurons inhibit NPY production and result in increased activity of POMC/CART neurons. However, PYY3-36 acts even in the absence of a melanocortin system, as observed in genetically modified animals that do not express POMC or MC4R, suggesting that melanocortin signaling is not essential for the anorectic effects of PYY3-36 (40). Additionally, administration of PYY3-36 reduces ghrelin levels, which might be an additional factor contributing to its anorectic effects (41). Several animal and human studies performed in the last years have demonstrated an association between PYY overexpression or PYY infusion and increased thermogenesis, increased heart rate and altered substrate partitioning in favor of fat oxidation. The effects of PYY on BAT are not completely clear, but from the scarcely available data, it seems that PYY may activate BAT via central mechanisms (10,35).
For therapeutic purposes in obesity, the half-life of native PYY has to be extended and several approaches have been employed to reach this goal. Long-acting analogues of PYY for subcutaneous administration have been developed and phase I trials are in progress to look at safety, tolerability and efficacy of some of these compounds. Moreover, some research groups are investigating alternative methods for PYY delivery, such as a nasal route, the buccal mucosa, and even by genetic manipulation to up-regulate PYY expression in the salivary glands through a viral vector-mediated gene transfer. In rats, oral PYY administration was achieved by conjugating PYY to vitamin B12 and using the B12 uptake pathway to facilitate peptide delivery (4).
PP, enterostatin and amylin
PP is produced by the F-cells of the endocrine pancreas, which are located at the islets periphery. Small amounts are also secreted from the distal GIT. PP is released into circulation after meals in proportion to their caloric content, reaching the lowest blood levels in the early hours of the morning and the highest at night, with postprandial elevations that last up to 6 hours after meal. Its effects on GIT include reduction of gastric emptying, inhibition of pancreatic and gallbladder secretion and reduction of gut motility (5,6,34).
The mechanism of PP action involves binding and activation of Y4 receptors in the vagal nerve and in cerebral areas with incomplete BBB, such as the hypothalamus and the brainstem, as PP is unable to cross the BBB (6). Knockout models of the Y4 receptor have high plasma PP levels and increased feeding behavior, while mice overexpressing PP show reduced food intake and weight gain (42,43). Moreover, vagotomy attenuates PP’s inhibitory effect on food intake (4). Systemic administration of PP promotes reduced food intake in rodents and humans, but this is not observed after intra-cerebro-ventricular (ICV) injection. Obese animals are less sensitive to the effects of PP than those of normal weight (4). In humans, intravenous PP infusion decreases food intake by 21.8% at a free-choice buffet, without affecting gastric emptying (44). Patients with Prader-Willi syndrome exhibit a blunted PP response to meals, and PP infusion was shown to promote 12% reduction of food intake in these individuals, by mechanisms not yet elucidated (45,46). In anorexia nervosa and in patients with advanced malignant disease, basal PP levels are elevated (46). It has been postulated that PP could also increase energy expenditure (47). Like PYY, PP has a short half-life and long-acting analogues are needed to allow its future therapeutic application. Results of a phase I study with PP1420, a peptidase resistant-PP analogue with altered amino acid sequence of native PP, were recently published, showing an augmented half-life of 2.42-2.61 hours, and good tolerability (48).
The exocrine pancreas is also responsible for the production of enterostatin, a peptide secreted in response to fat intake to facilitate its digestion. Enterostatin effects seem to be dependent on CCK-1 receptors (10). Although the administration of enterostatin in animals reduces fat intake, no significant effects have been observed in humans. In contrast, animal studies have shown that enterostatin can induce BAT activity via a central effect (10). Amylin, a peptide co-secreted with insulin from pancreatic β-cells in a ratio of 100:1, inhibits gastric emptying, gastric acid secretion and glucagon secretion, and reduces food intake and meal size in animals (5,10). Central actions of amylin have been shown to acutely stimulate BAT activity via receptor activity-modifying protein 1 (RAMP1) (10). The synthetic analogue of amylin, pramlintide, significantly reduces hemoglobin-A1c and body weight in patients with both type 1 and type 2 diabetes mellitus (49).
Glucagon-like peptide-1 (GLP-1)
The pro-glucagon gene product is cleaved in different parts by the enzymes convertase 1 and convertase 2, and this process varies among the tissues. In the pancreas, the main product of this cleavage is glucagon, whereas in the intestine the main products are GLP-1, GLP-2 and OXM (4-6,50,51). In physiological conditions, GLP-1 is released into circulation after meal, acting as an “incretin”, and promoting increased glucose-mediated pancreatic insulin secretion after an oral nutrient load and, consequently, influencing glucose homeostasis. Other GLP-1 actions include suppression of glucagon release, delaying gastric emptying, and inhibition of GIT motility. The half-life time of GLP-1 is very short, being rapidly degraded by the DPP-IV (51).
Central and peripheral actions of GLP-1 are important in the regulation of energy balance. GLP-1 receptors are present in hypothalamic nuclei and brain stem areas and are known to be involved in the control of energy balance, but binding of GLP-1 to POMC neurons in the ARC nucleus has not been demonstrated (6). Thus, GLP-1 anorectic effects appear to be substantially mediated by the afferent vagal nerve projecting to the NTS, which, in turn, signals to the ARC. Long-term ICV administration of GLP-1 in rodents leads to anorexia, induces satiety and increases energy expenditure, leading to a reduced body weight (4-6,50,51). Systemic administration of GLP-1 was found to decrease hepatic and peripheral insulin resistance in high-fat fed mice via central mechanisms. Some studies show that circulating levels of GLP-1 are lower in obese subjects and increase after weight loss. In lean and obese subjects, systemic administration of GLP-1 causes a dose-dependent reduction of caloric intake (4,51).
GLP-1 was the first GIT hormone to be successfully used for therapeutic purposes in humans. Several preparations of GLP-1 receptor agonists and DPP-IV inhibitors are currently available for the treatment of type 2 diabetes (52). However, DPP-IV inhibitors are neutral and GLP-1 receptor agonists show limited efficacy in promoting weight loss in the doses used for diabetes control (53). Nausea is a frequent side-effect of GLP-1 receptor agonists that prevents application of higher dosages that might be more efficacious in the fight against obesity. Another hesitation to approve these drugs for treating obesity is related to safety issues. Recently, it has been suggested that both DPP-4 inhibitors and GLP-1 receptor agonists would increase the potential risk of pancreatitis, pancreatic cancer, hyperplasia of the exocrine pancreas, and thyroid C-cell hyperplasia (54). To date, these findings remain under investigation, and no causality relationship has been proven thus far; physicians are only advised to individually evaluate the risk-benefit ratio of incretin-based therapies (54).
Oxyntomodulin (OXM)
OXM is a 37-amino acid peptide product of the pro-glucagon gene, which is released from intestinal L-cells into circulation in proportion to caloric intake (4-6). OXM inhibits gastric motility and pancreatic and gastric secretion, while it stimulates glycolysis and exerts an incretin-like effect. It also reduces food intake when administered ICV in rodents or systemically in both rodents and humans, and reduces body weight and fat mass when injected chronically. In humans, OXM administration also reduces the levels of circulating ghrelin, which may contribute to its effects on appetite. However, the weight loss observed in animal models treated with OXM is greater than what would be expected by the reduction in food intake alone, suggesting that OXM promotes increased energy expenditure (4).
OXM binds to both the GLP-1 and the glucagon receptor. While the effects of OXM on energy expenditure are mediated by the glucagon receptor, the majority of its effects on appetite seem to occur via binding to the GLP-1 receptor. GLP-1 and OXM are equally effective in inducing anorexia, despite the fact that OXM binds GLP-1 receptor with a lower affinity. Thus, differences in the biological effects of OXM and GLP-1 might be due to variations in tissue penetration, degradation or intracellular signaling pathways (4-6).
Subcutaneous self-administration of OXM over a 4-week period resulted in a 2.4% reduction in body weight in overweight and obese volunteers (55). From another study in healthy overweight and obese subjects, the same investigators showed that OXM increased energy expenditure while reduced energy intake, resulting in negative energy balance (56,57). These very preliminary results from short-term clinical studies open up a new and exciting potential way to treat obesity. The dual effects of OXM, reducing food intake and at the same time increasing energy expenditure, show that one single drug can initiate a two-pronged attack against obesity (4). Indeed, a combination approach employing a peptide analogue dual agonist of these two systems, has been already successfully used in animal models (58,59).
IM, obesity and metabolic disorders
The human intestine harbors more than 100 trillion bacteria and archaea that constitute a complex and diverse intestinal microbiota. This community is estimated to consist of approximately 1,100 prevalent species, with an estimated average number of 160 to 500 bacterial species per individual. Each human being has its own microbiota composition, which is established before the age of two and which remains stable throughout life under healthy conditions (60,61). More recent metagenomic techniques have estimated that the microbial gene content is a 150-fold larger than the human genome, and most of these genes are of unknown function (62). Mammalian microbiota predominantly consists out of four bacterial phyla: the Gram-negative Bacteroidetes and Proteobacteria and the Gram-positive Actinobacteria and Firmicutes. Apparently, the human host provides a nutrient rich-environment for this microbiota. The intestinal microbiota, in turn, exert metabolic, protective and structural functions, including colonic fermentation, digestion of otherwise indigestible plant polysaccharides, mucosal and systemic immunologic defense and regeneration of the intestinal epithelium (60,61).
Obesity has been associated with important changes in the composition and function of the intestinal microbiota, known as dysbiosis. Obese animals and humans may present reduced number of Bacteroidetes and a proportional increase of the Firmicutes. However, these findings are largely unconfirmed, and some researchers claim that phylum differences are likely less important than metagenomic-based functional aspects (60). Possible confounding factors among the studies include diet, age, previous use of antibiotics, and the genetic background. For instance, high-fat diets may affect intestinal epithelial integrity, resulting in increased permeability for small compounds and endotoxins, leading to systemic inflammation. However, multiple interactions between certain dietary factors, the microbiota, the innate immune system and the host per se may take place to promote barrier dysfunction (60).
Intestinal microbiota might modulate body composition mainly by increased energy extraction from food and by regulating fat storage. Moreover, dysbiosis can also influence the GIT-brain axis, affecting intestinal L-cells differentiation, nutrient signaling pathways, GIT-derived hormone secretion, brain functions, and host behavior, all potentially contributing to increased energy intake, weight gain and metabolic disorders (60,61). Although these novel insights are interesting, there are still many controversial issues on the physiological and pathological implications of the gut microbiota and host relationships that need to be addressed to establish the precise role of intestinal microbiota in human health.
Conclusions
Many hormones derived from GIT are involved in energy homeostasis and body weight regulation. The hypothalamus, particularly the ARC nucleus, is the main site responsible for integrating the GIT signals with those arising from other peripheral tissues and from the external milieu. The central analysis of all these input signals triggers several compensatory responses to maintain a balance between energy intake and expenditure (Figure 1). A malfunctioning of one or more components of this complex machinery might result in energy imbalance and significant changes in body weight and metabolic profile. The outstanding scientific advances obtained in the knowledge of these body’s communication systems, including the GIT-brain axis, renew our hopes for the future development of safer and more effective therapeutic approaches against obesity, diabetes and metabolic disorders.
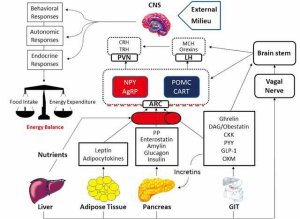
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Badman MK, Flier JS. The gut and energy balance: visceral allies in the obesity wars. Science 2005;307:1909-14. [PubMed]
- Guyenet SJ, Schwartz MW. Clinical review: Regulation of food intake, energy balance, and body fat mass: implications for the pathogenesis and treatment of obesity. J Clin Endocrinol Metab 2012;97:745-55. [PubMed]
- Boguszewski CL, Paz-Filho G, Velloso LA. Neuroendocrine body weight regulation: integration between fat tissue, gastrointestinal tract, and the brain. Endokrynol Pol 2010;61:194-206. [PubMed]
- Troke RC, Tan TM, Bloom SR. The future role of gut hormones in the treatment of obesity. Ther Adv Chronic Dis 2014;5:4-14. [PubMed]
- Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest 2007;117:13-23. [PubMed]
- Schaeffer M, Hodson DJ, Mollard P. The blood-brain barrier as a regulator of the gut-brain axis. Front Horm Res 2014;42:29-49. [PubMed]
- Menzies JR, Skibicka KP, Leng G, et al. Ghrelin, reward and motivation. Endocr Dev 2013;25:101-11. [PubMed]
- Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol 1973;84:488-95. [PubMed]
- Yoshimatsu H, Egawa M, Bray GA. Effects of cholecystokinin on sympathetic activity to interscapular brown adipose tissue. Brain Res 1992;597:298-303. [PubMed]
- van den Beukel JC, Grefhorst A. Interactions between the gut, the brain and brown adipose tissue function. Front Horm Res 2014;42:107-22. [PubMed]
- West DB, Fey D, Woods SC. Cholecystokinin persistently suppresses meal size but not food intake in free-feeding rats. Am J Physiol 1984;246:R776-87. [PubMed]
- Moran TH, Dailey MJ. Minireview: Gut peptides: targets for antiobesity drug development? Endocrinology. 2009;150:2526-30. [PubMed]
- van der Lely AJ, Tschöp M, Heiman ML, et al. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev 2004;25:426-57. [PubMed]
- Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656-60. [PubMed]
- Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 2001;86:5992. [PubMed]
- Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol 2003;23:7973-81. [PubMed]
- Zigman JM, Nakano Y, Coppari R, et al. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest 2005;115:3564-72. [PubMed]
- Wren AM, Small CJ, Abbott CR, et al. Ghrelin causes hyperphagia and obesity in rats. Diabetes 2001;50:2540-7. [PubMed]
- Wortley KE, Anderson KD, Garcia K, et al. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci U S A 2004;101:8227-32. [PubMed]
- Sun Y, Asnicar M, Saha PK, et al. Ablation of ghrelin improves the diabetic but not obese phenotype of ob/ob mice. Cell Metab 2006;3:379-86. [PubMed]
- Williams DL, Grill HJ, Cummings DE, et al. Vagotomy dissociates short- and long-term controls of circulating ghrelin. Endocrinology 2003;144:5184-7. [PubMed]
- Cummings DE, Frayo RS, Marmonier C, et al. Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. Am J Physiol Endocrinol Metab 2004;287:E297-304. [PubMed]
- Cummings DE, Purnell JQ, Frayo RS, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001;50:1714-9. [PubMed]
- Drazen DL, Vahl TP, D’Alessio DA, et al. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology 2006;147:23-30. [PubMed]
- Shiiya T, Nakazato M, Mizuta M, et al. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab 2002;87:240-4. [PubMed]
- le Roux CW, Patterson M, Vincent RP, et al. Postprandial plasma ghrelin is suppressed proportional to meal calorie content in normal-weight but not obese subjects. J Clin Endocrinol Metab 2005;90:1068-71. [PubMed]
- English PJ, Ghatei MA, Malik IA, et al. Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab 2002;87:2984. [PubMed]
- Feigerlová E, Diene G, Conte-Auriol F, et al. Hyperghrelinemia precedes obesity in Prader-Willi syndrome. J Clin Endocrinol Metab 2008;93:2800-5. [PubMed]
- Folgueira C, Seoane LM, Casanueva FF. The brain-stomach connection. Front Horm Res 2014;42:83-92. [PubMed]
- Senin LL, Al-Massadi O, Folgueira C, et al. The gastric CB1 receptor modulates ghrelin production through the mTOR pathway to regulate food intake. PLoS One 2013;8:e80339. [PubMed]
- Delhanty PJ, Neggers SJ, van der Lely AJ. Should we consider des-acyl ghrelin as a separate hormone and if so, what does it do? Front Horm Res 2014;42:163-74. [PubMed]
- Asakawa A, Inui A, Fujimiya M, et al. Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut 2005;54:18-24. [PubMed]
- Trovato L, Gallo D, Settanni F, et al. Obestatin: is it really doing something? Front Horm Res 2014;42:175-85. [PubMed]
- Holzer P, Reichmann F, Farzi A. Neuropeptide Y, peptide YY and pancreatic polypeptide in the gut-brain axis. Neuropeptides 2012;46:261-74. [PubMed]
- Price SL, Bloom SR. Protein PYY and its role in metabolism. Front Horm Res 2014;42:147-54. [PubMed]
- Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature 2002;418:650-4. [PubMed]
- Sloth B, Holst JJ, Flint A, et al. Effects of PYY1-36 and PYY3-36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol Endocrinol Metab 2007;292:E1062-8. [PubMed]
- Koda S, Date Y, Murakami N, et al. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology 2005;146:2369-75. [PubMed]
- Abbott CR, Monteiro M, Small CJ, et al. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 2005;1044:127-31. [PubMed]
- Challis BG, Coll AP, Yeo GS, et al. Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3-36). Proc Natl Acad Sci U S A 2004;101:4695-700. [PubMed]
- Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med 2003;349:941-8. [PubMed]
- Ueno H, Yamaguchi H, Mizuta M, et al. The role of PYY in feeding regulation. Regul Pept 2008;145:12-6. [PubMed]
- Edelsbrunner ME, Painsipp E, Herzog H, et al. Evidence from knockout mice for distinct implications of neuropeptide-Y Y2 and Y4 receptors in the circadian control of locomotion, exploration, water and food intake. Neuropeptides 2009;43:491-7. [PubMed]
- Batterham RL, Le Roux CW, Cohen MA, et al. Pancreatic polypeptide reduces appetite and food intake in humans. J Clin Endocrinol Metab 2003;88:3989-92. [PubMed]
- Berntson GG, Zipf WB, O’Dorisio TM, et al. Pancreatic polypeptide infusions reduce food intake in Prader-Willi syndrome. Peptides 1993;14:497-503. [PubMed]
- Tauber M, Diene G, Mimoun E, et al. Prader-Willi syndrome as a model of human hyperphagia. Front Horm Res 2014;42:93-106. [PubMed]
- Asakawa A, Inui A, Yuzuriha H, et al. Characterization of the effects of pancreatic polypeptide in the regulation of energy balance. Gastroenterology 2003;124:1325-36. [PubMed]
- Tan TM, Field BC, Minnion JS, et al. Pharmacokinetics, adverse effects and tolerability of a novel analogue of human pancreatic polypeptide, PP 1420. Br J Clin Pharmacol 2012;73:232-9. [PubMed]
- Younk LM, Mikeladze M, Davis SN. Pramlintide and the treatment of diabetes: a review of the data since its introduction. Expert Opin Pharmacother 2011;12:1439-51. [PubMed]
- Holst JJ. Glucagon and glucagon-like peptides 1 and 2. Results Probl Cell Differ 2010;50:121-35. [PubMed]
- Dailey MJ, Moran TH. Glucagon-like peptide 1 and appetite. Trends Endocrinol Metab 2013;24:85-91. [PubMed]
- Umpierrez GE, Meneghini L. Reshaping diabetes care: the fundamental role of dipeptidyl peptidase-4 inhibitors and glucagon-like peptide-1 receptor agonists in clinical practice. Endocr Pract 2013;19:718-28. [PubMed]
- Holst JJ, Deacon CF. Is there a place for incretin therapies in obesity and prediabetes? Trends Endocrinol Metab 2013;24:145-52. [PubMed]
- Butler PC, Elashoff M, Elashoff R, et al. A critical analysis of the clinical use of incretin-based therapies: Are the GLP-1 therapies safe? Diabetes Care 2013;36:2118-25. [PubMed]
- Nauck MA. A critical analysis of the clinical use of incretin-based therapies: The benefits by far outweigh the potential risks. Diabetes Care 2013;36:2126-32. [PubMed]
- Wynne K, Park AJ, Small CJ, et al. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double-blind, randomized, controlled trial. Diabetes 2005;54:2390-5. [PubMed]
- Wynne K, Park AJ, Small CJ, et al. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. Int J Obes (Lond) 2006;30:1729-36. [PubMed]
- Pocai A, Carrington PE, Adams JR, et al. Glucagon-like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes 2009;58:2258-66. [PubMed]
- Day JW, Ottaway N, Patterson JT, et al. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Nat Chem Biol 2009;5:749-57. [PubMed]
- Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest 2011;121:2126-32. [PubMed]
- Duca F, Gérard P, Covasa M, et al. Metabolic interplay between gut bacteria and their host. Front Horm Res 2014;42:73-82. [PubMed]
- Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010;464:59-65. [PubMed]


